Synthesis of New Modified with Rhodamine B Peptides for Antiviral Protection of Textile Materials
Abstract
1. Introduction
2. Results and Discussion
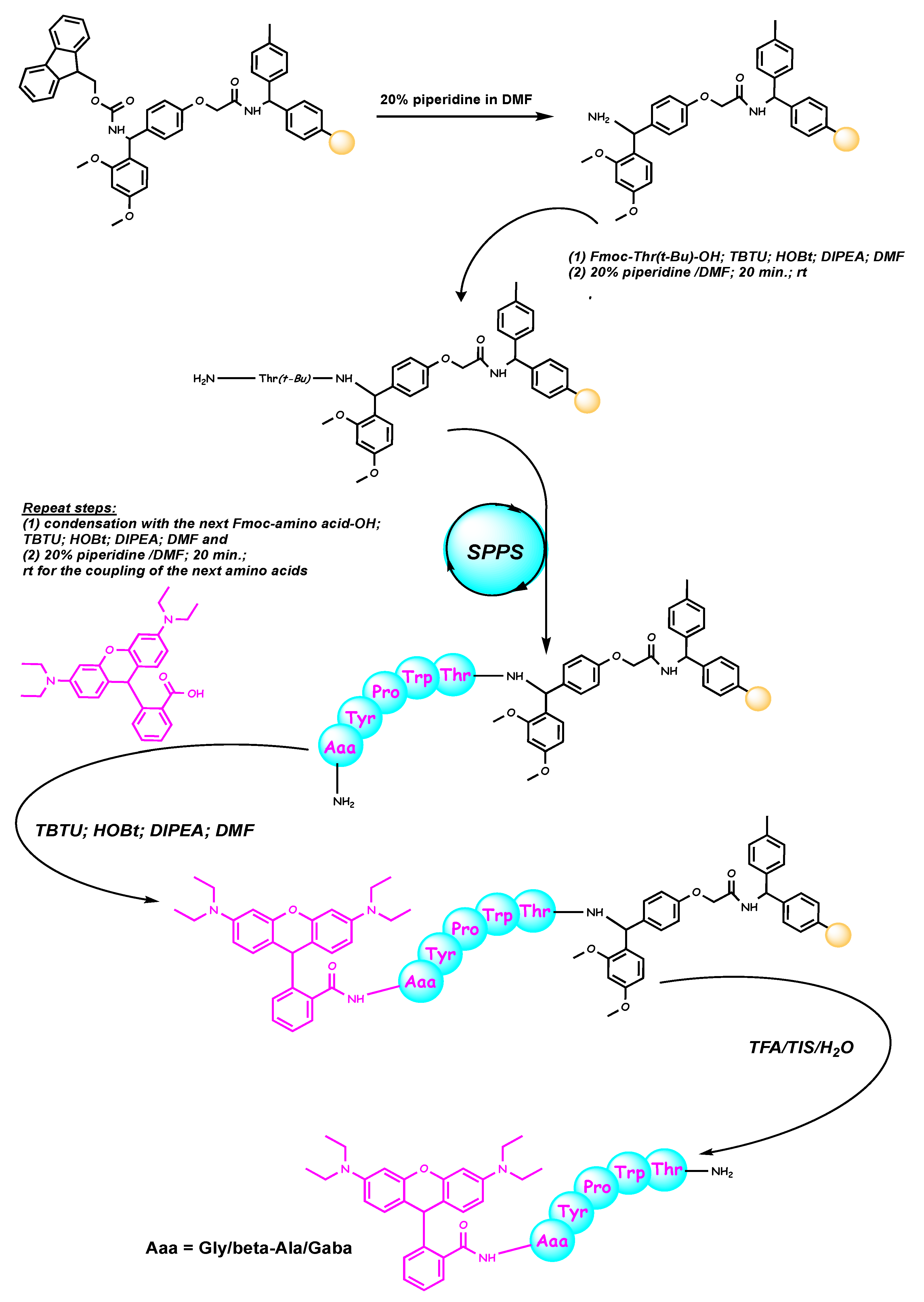
2.1. Chemistry
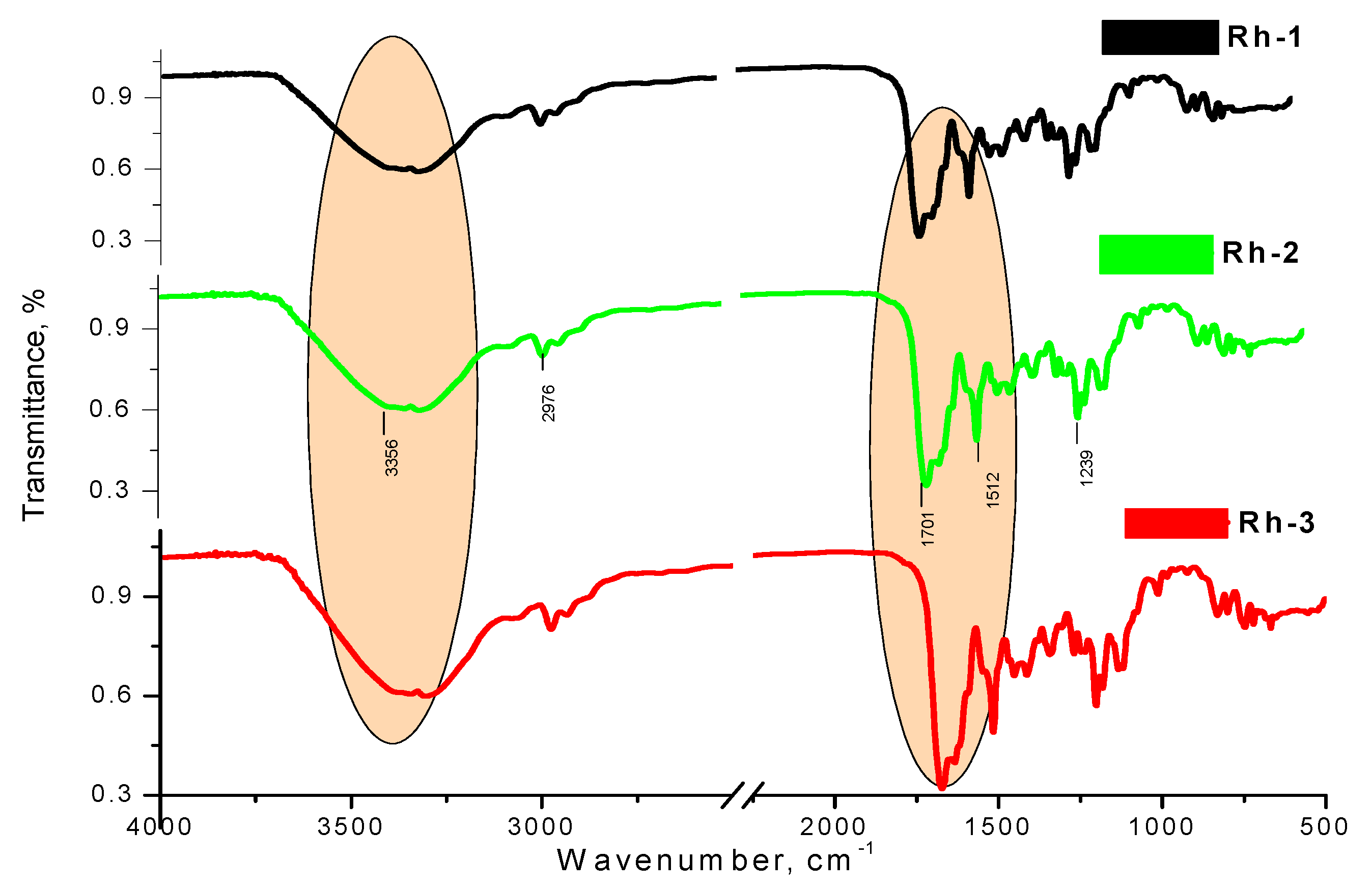
2.2. Physicochemical Characterizations
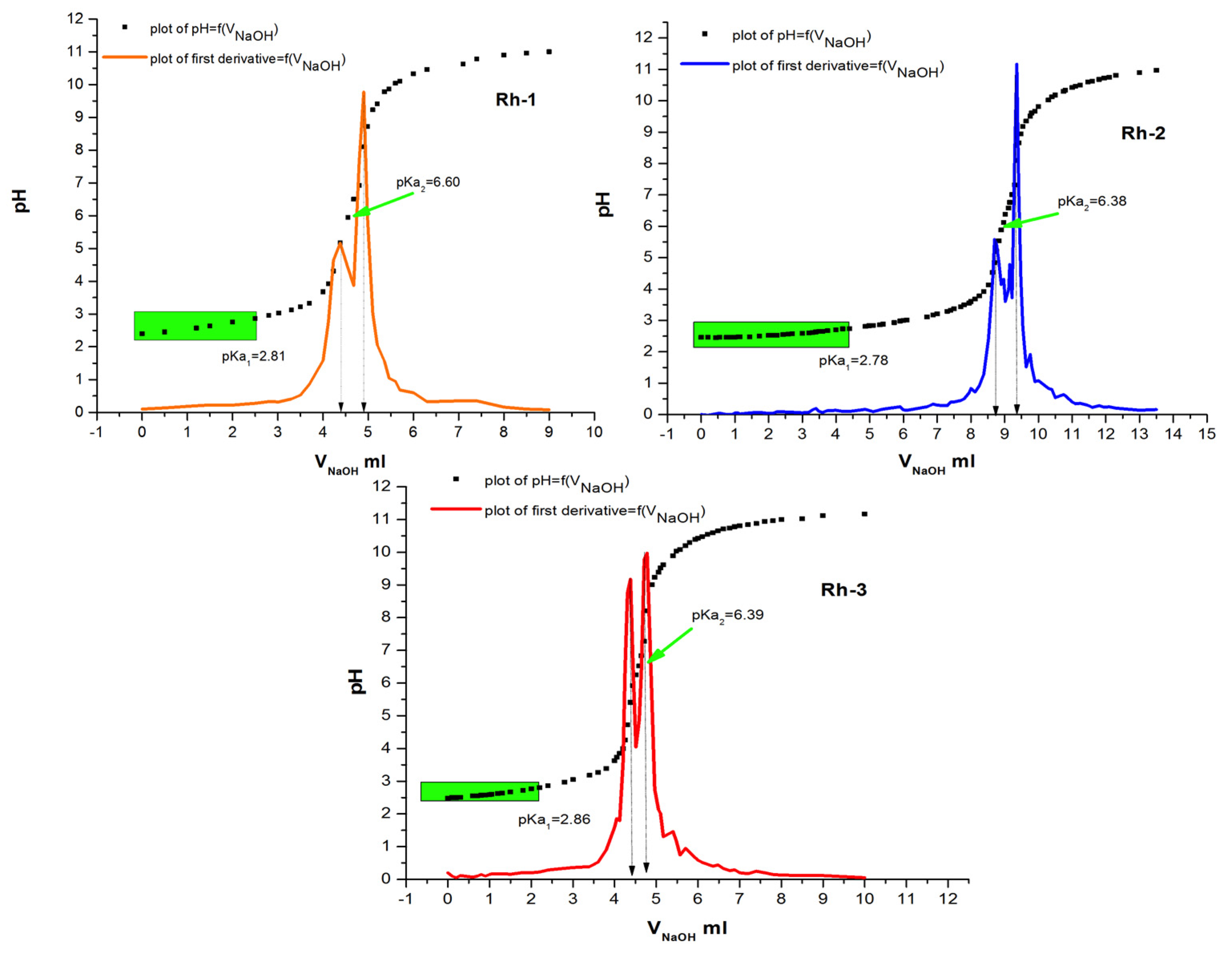
Determination of Physicochemical Constants
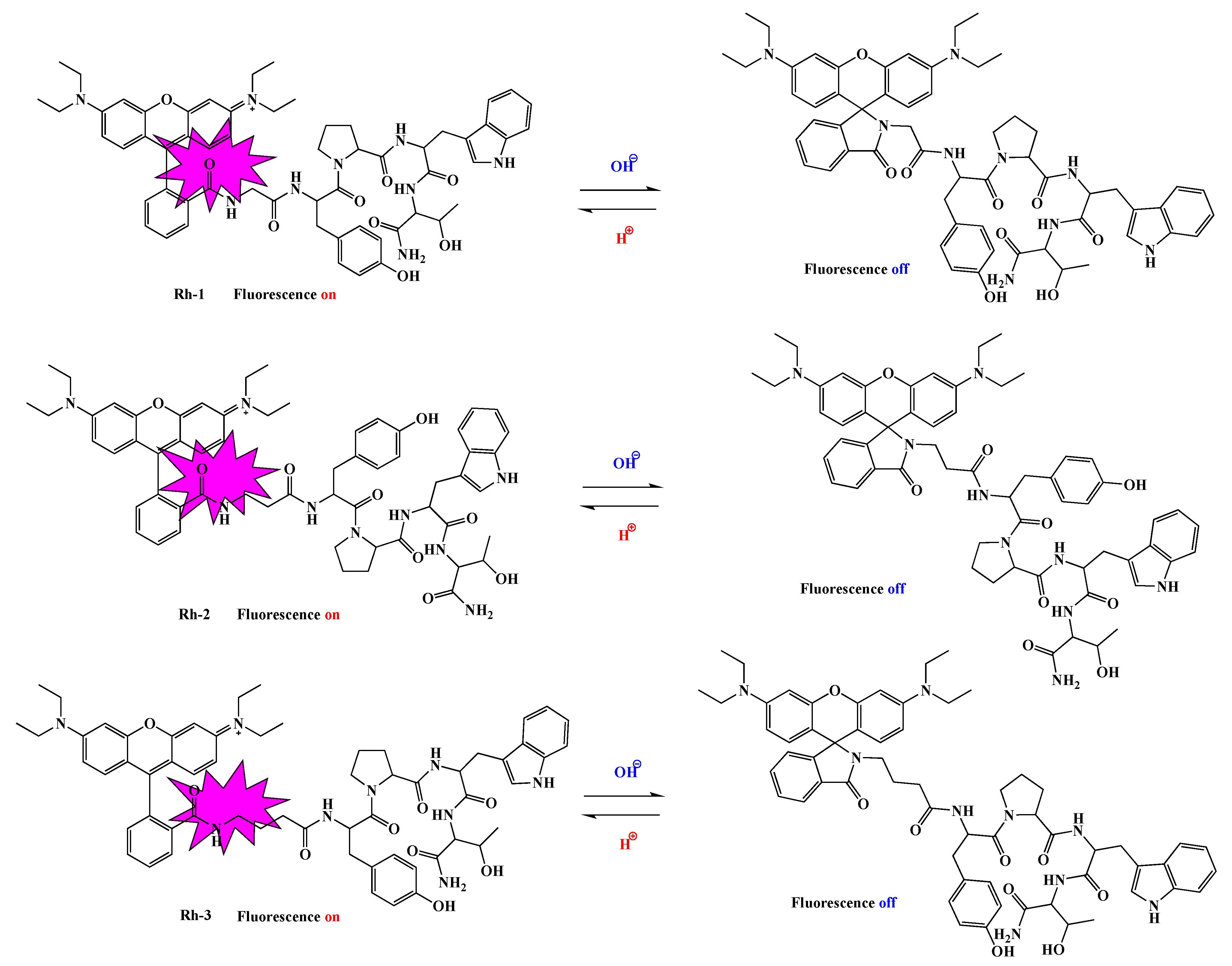
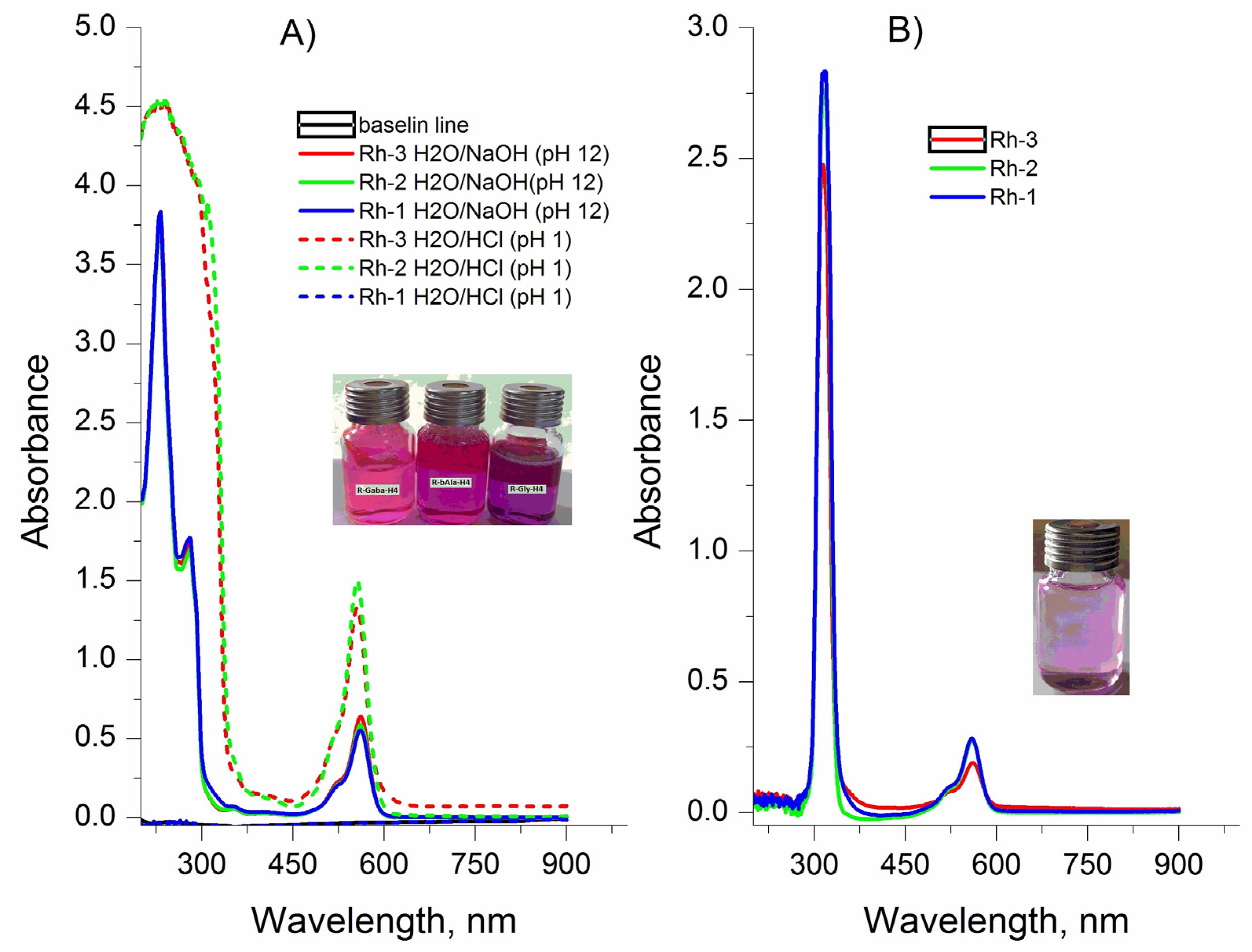
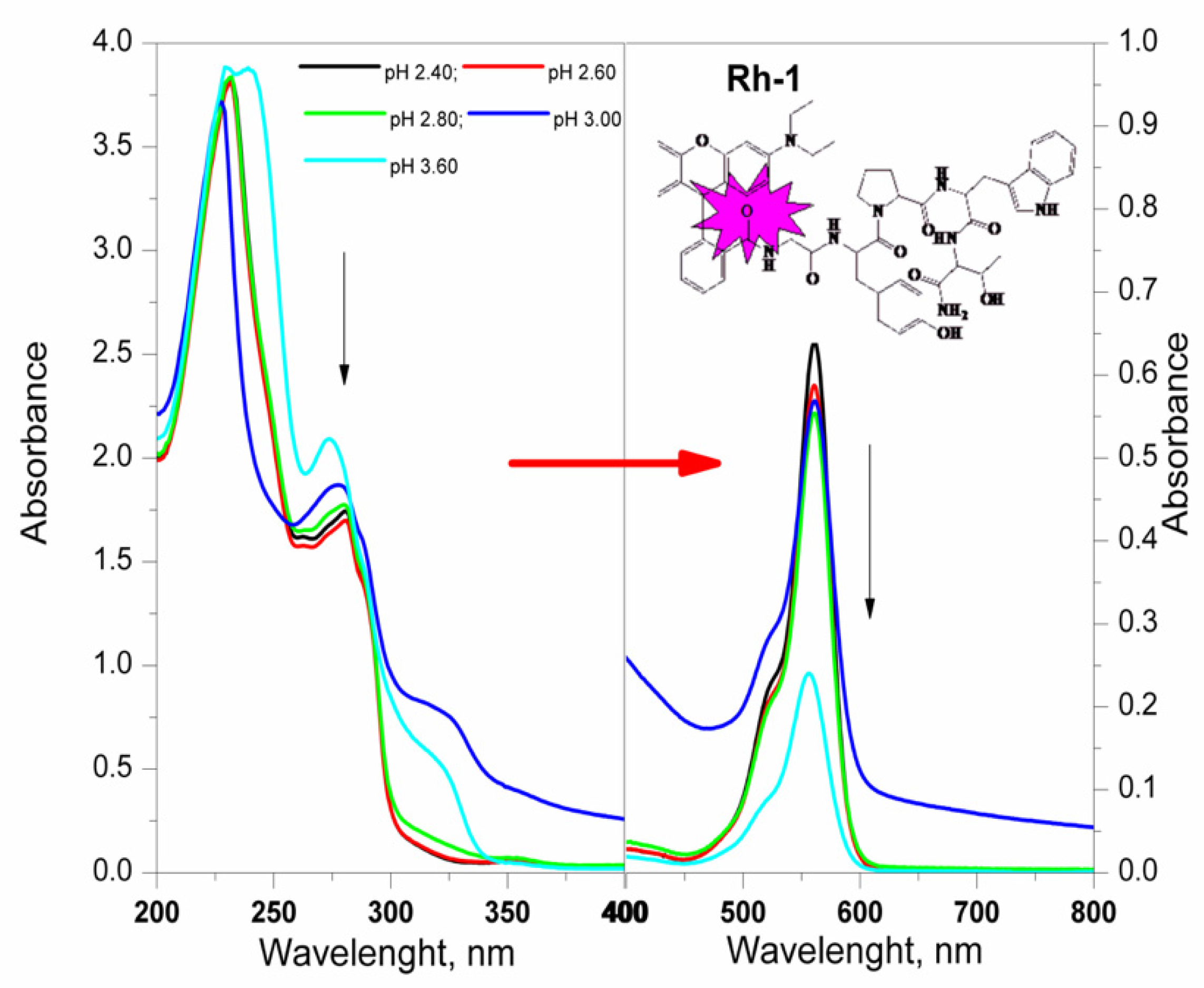
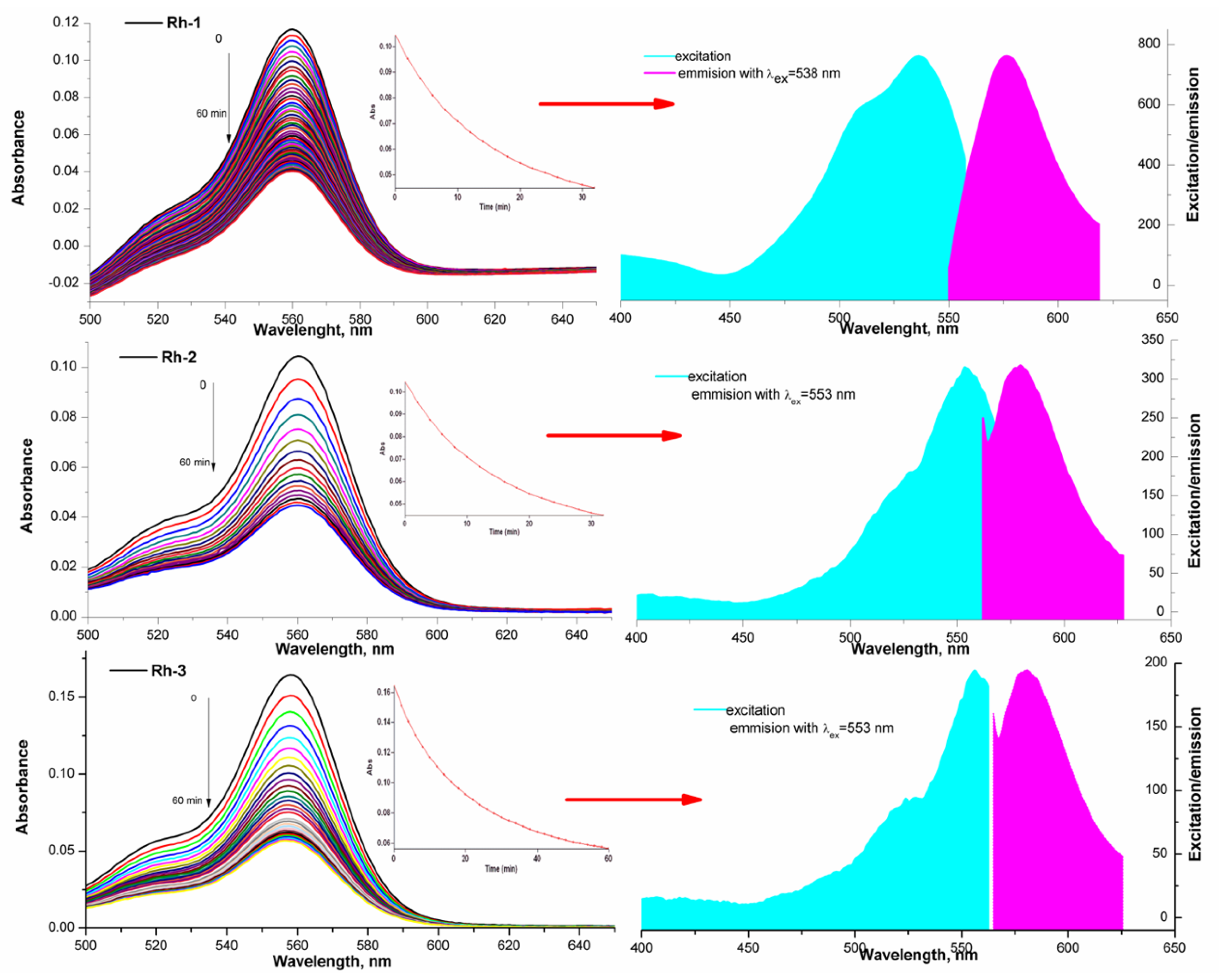
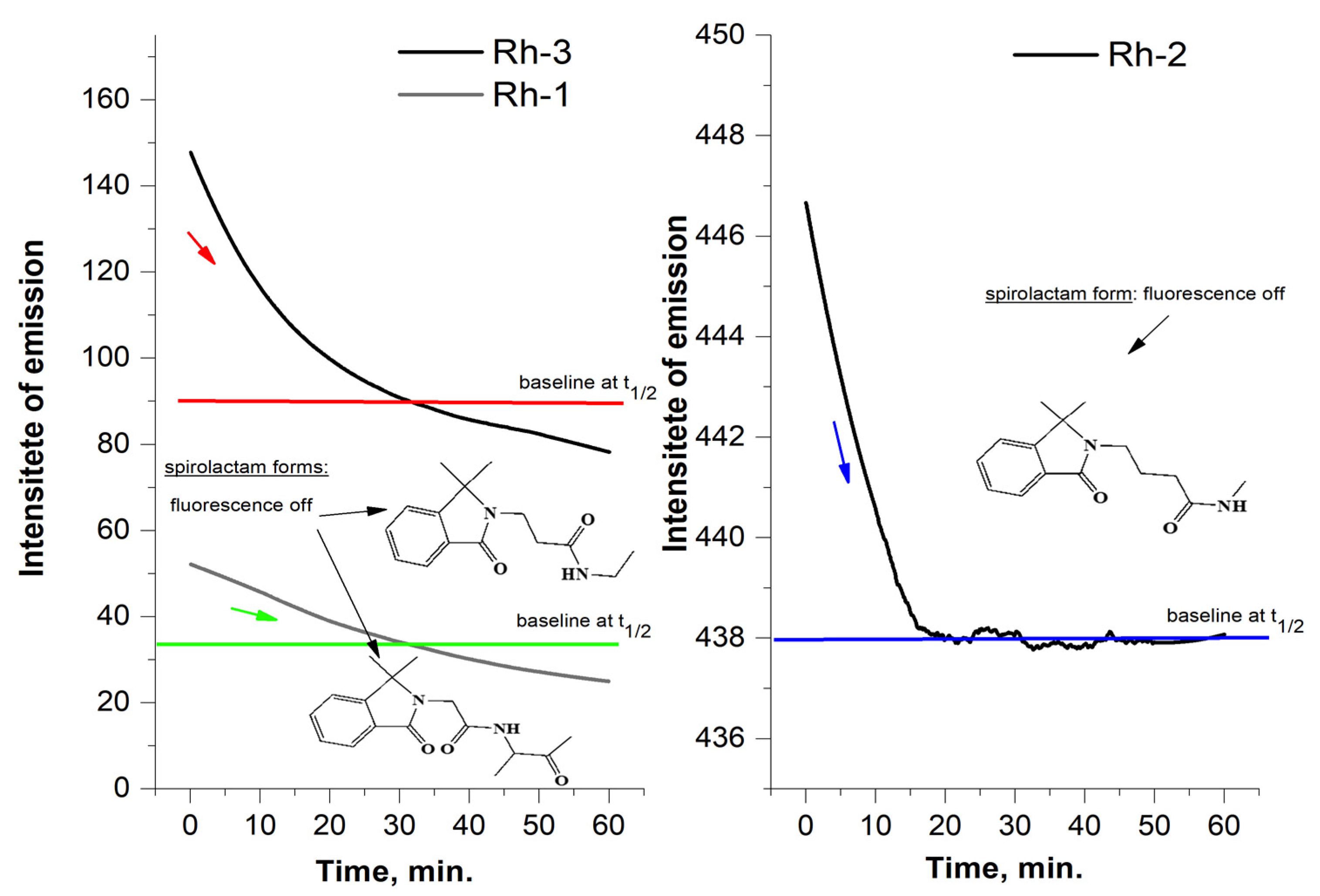
2.3. Spectral Characterizations
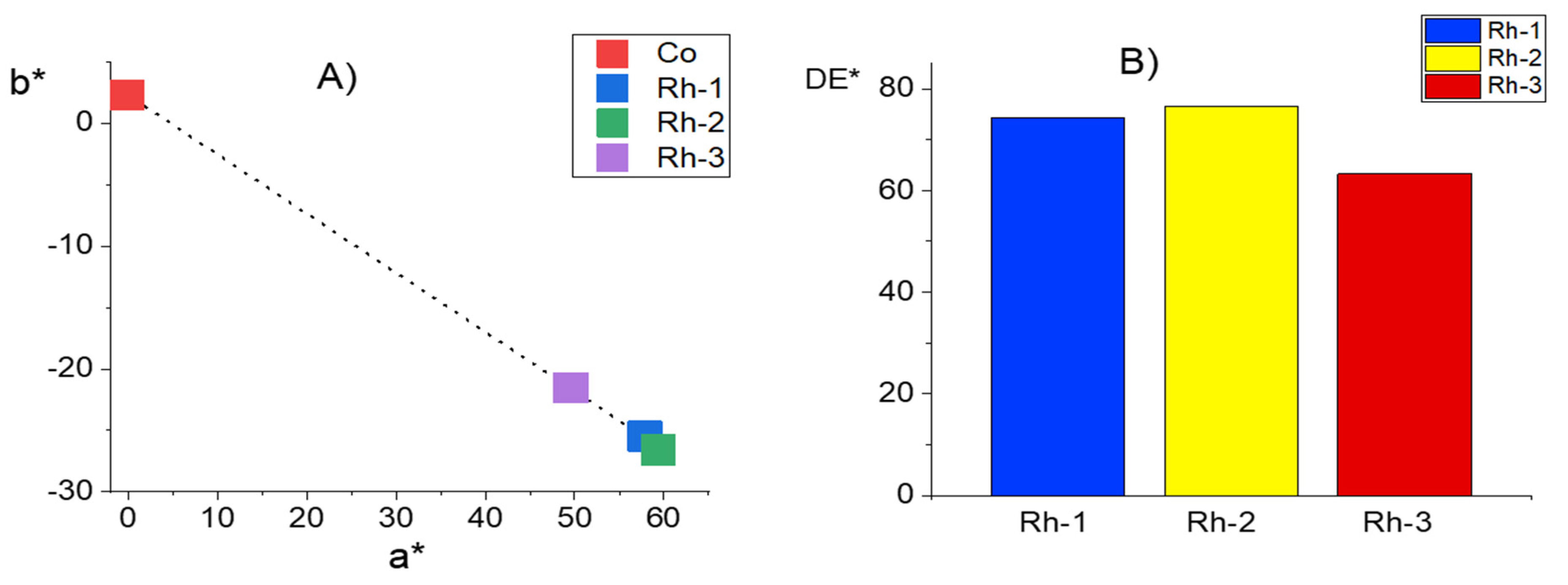
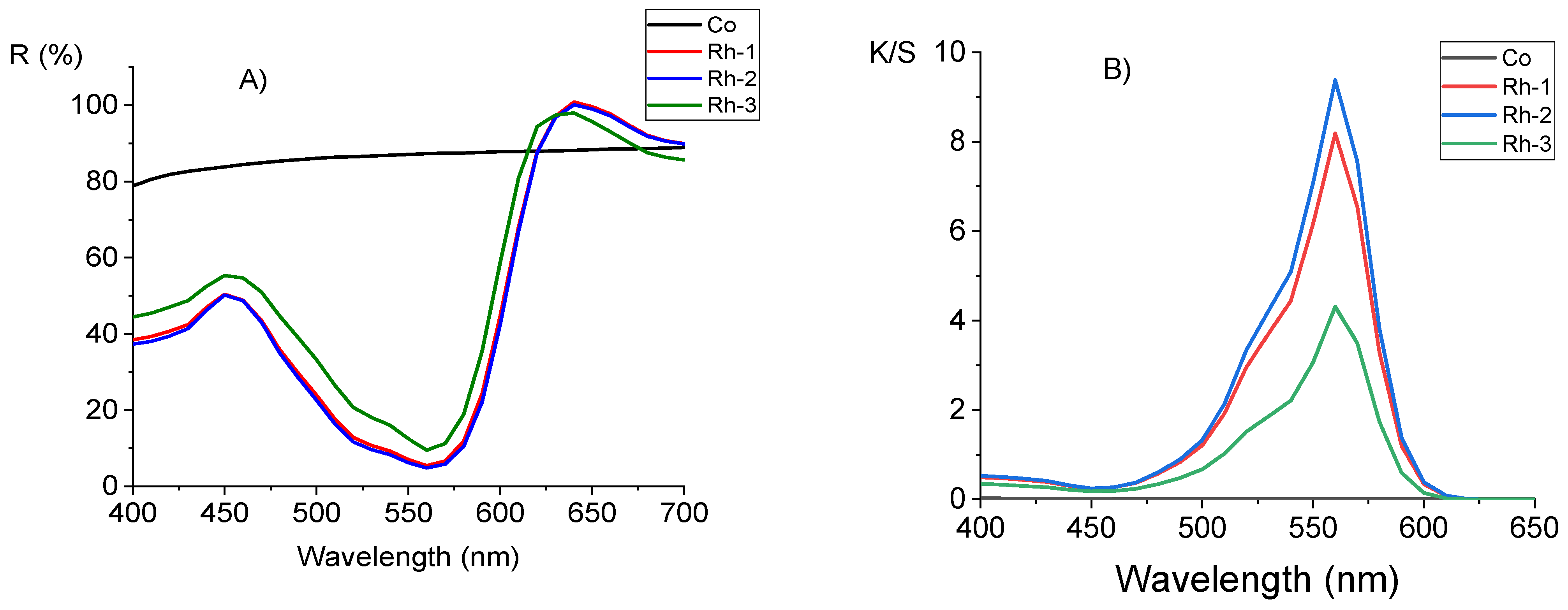
2.4. Color Characterisation of Cotton Fabrics
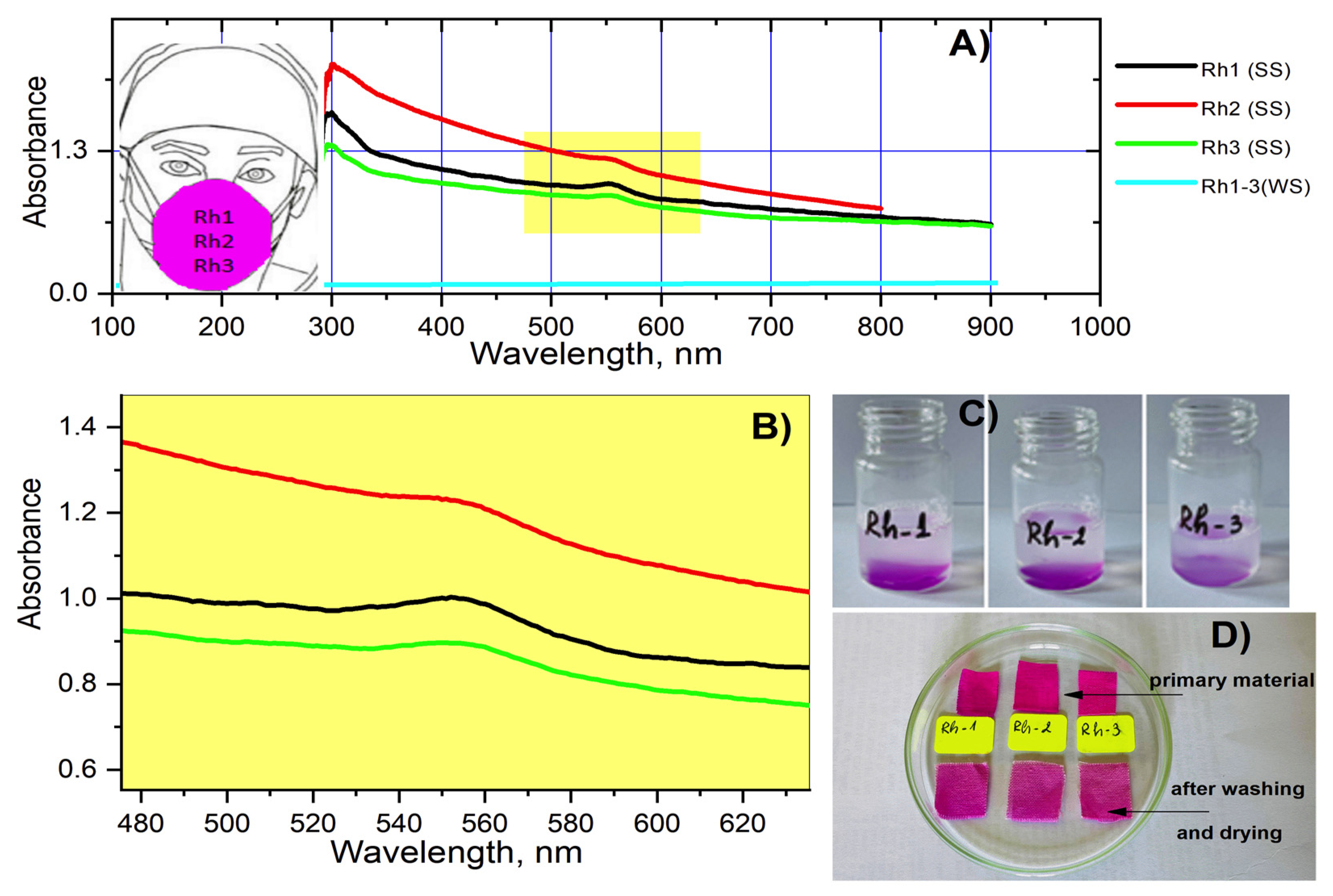
2.5. Fastness Testing
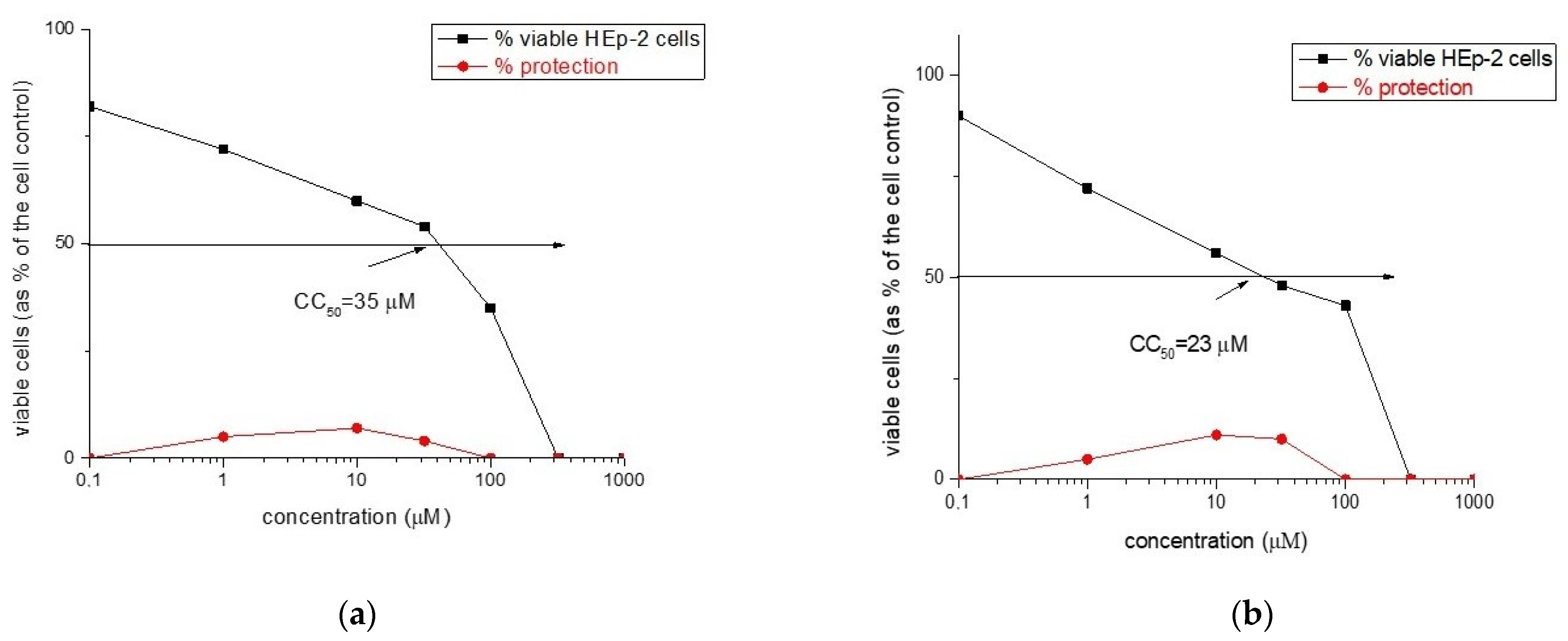
2.6. Virological Activity
3. Materials and Methods
3.1. Synthesis of the Peptides (Rh-1, Rh-2, and Rh-3)
3.2. Physicochemical Characterization
3.2.1. Spectral Measurements
3.2.2. Potentiometric Titration
3.3. Cotton Fabric Modification and Characterization
3.3.1. Functionalization of Cotton Fabric with Chloroacetyl Chloride
3.3.2. Dyeing of Functionalized Cotton Fabric with Rh-1, Rh-2, and Rh-3
3.3.3. Characterization of Cotton Fabrics
3.3.4. Fastness Testing
3.4. Virology
3.4.1. Cytotoxicity Assay
3.4.2. Antiviral Activity Assay
3.4.3. Virucidal Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jamison, J.M.; Krabill, K.; Hatwalkar, A.; Jamison, E.; Tsai, C.C. Potentiation of the antiviral activity of poly r (AU) by xanthene dyes. Cell Biol. Int. Rep. 1990, 14, 1075–1084. [Google Scholar] [CrossRef]
- Lavis, L.D.; Raines, R.T. Bright ideas for chemical biology. ACS Chem. Biol. 2008, 3, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yuan, L.; Chen, Y.; Ma, W.; Ran, F.; Zhang, L.; Zhou, D.; Xiao, S. Rhodamine B-based fluorescent probes for molecular mechanism study of the anti-influenza activity of pentacyclic triterpenes. Eur. J. Med. Chem. 2020, 205, 112664. [Google Scholar] [CrossRef]
- Hwaná Kwak, J.; He, Y.; Yoon, B.; Juá Kang, E.; Koo, S.; Yang, Z.; Kang, E.J.; Lee, B.H.; Han, S.-Y.; Yoo, Y.C.; et al. Synthesis of rhodamine-labelled dieckol: Its unique intracellular localization and potent anti-inflammatory activity. Chem. Commun. 2014, 50, 13045–13048. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.; Martinho, J.M. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef]
- Birtalan, E.; Rudat, B.; Kölmel, D.K.; Fritz, D.; Vollrath, S.B.; Schepers, U.; Bräse, S. Investigating rhodamine B-labeled peptoids: Scopes and limitations of its applications. Pept. Sci. 2011, 96, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Tintori, C.; Civra, A.; Cavalli, R.; Tiberi, M.; Botta, L.; Brai, A.; Poli, G.; Tapparel, C.; Lembo, D.; et al. Novel broad spectrum virucidal molecules against enveloped viruses. PLoS ONE 2018, 13, e0208333. [Google Scholar] [CrossRef]
- Carravilla, P.; Cruz, A.; Martin-Ugarte, I.; Oar-Arteta, I.R.; Torralba, J.; Apellaniz, B.; Pérez-Gil, J.; Requejo-Isidro, J.; Huarte, N.; Nieva, J.L. Effects of HIV-1 gp41-derived virucidal peptides on virus-like lipid membranes. Biophys. J. 2017, 113, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Pazos, E.; Vazquez, O.; Mascarenas, J.L.; Vazquez, M.E. Peptide-based fluorescent biosensors. Chem. Soc. Rev. 2009, 38, 3348–3359. [Google Scholar] [CrossRef]
- Wang, L.; Xie, J.; Schultz, P.G. Expanding the genetic code. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 225–249. [Google Scholar] [CrossRef]
- Thurley, S.; Röglin, L.; Seitz, O. Hairpin peptide beacon: Dual-labeled PNA-peptide-hybrids for protein detection. J. Am. Chem. Soc. 2007, 129, 12693–12695. [Google Scholar] [CrossRef]
- Chersi, A.; Giommi, S.; Rosanò, L. Selective ‘in synthesis’ labeling of peptides with biotin and rhodamine. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 196–200. [Google Scholar] [CrossRef]
- Blishchenko, E.Y.; Sazonova, O.V.; Kalinina, O.A.; Yatskin, O.N.; Philippova, M.M.; Surovoy, A.Y.; Karelin, A.A.; Ivanov, V.T. Family of hemorphins: Co-relations between amino acid sequences and effects in cell cultures. Peptides 2002, 23, 903–910. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Yoshikawa, M. Release of Hemorphin-5 from Human Hemoglobin by Pancreatic Elastase. Biosci. Biotechnol. Biochem. 2002, 66, 1130–1132. [Google Scholar] [CrossRef][Green Version]
- Ali, A.; Alzeyoudi, S.A.R.; Almutawa, S.A.; Alnajjar, A.N.; Vijayan, R. Molecular basis of the therapeutic properties of hemorphins. Pharmacol. Res. 2020, 158, 104855. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Vijayan, R. Hemorphins Targeting G. Protein-Coupled Receptors. Pharmaceuticals 2021, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhao, L.; Jing, Y. Hemoglobin-derived peptides and mood regulation. Peptides 2020, 127, 170268. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, P.; Hartman, K.; Drabik, A.; Hung, H.Y.; Huang, E.Y.; Gibula-Tarlowska, E.; Kotlinska, J.H.; Silberring, J. Hemorphins—From discovery to functions and pharmacology. Molecules 2021, 26, 3879. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Chiu, T.H.; Chen, C.L. Structure-activity relationships of naturally occurring and synthetic opioid tetrapeptides acting on locus coeruleus neurons. Eur. J. Pharmacol. 1999, 372, 229–236. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Pechlivanova, D.; Georgieva, S.; Dzhambazova, E. Synthesis, characterization and nociceptive screening of new VV-hemorphin-5 analogues. Bioorg. Med. Chem. Lett. 2018, 28, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Rangelov, M.; Peneva, P.; Todorova, N.; Tchekalarova, J. Anticonvulsant evaluation and docking analysis of VV-Hemorphin-5 analogues. Drug Dev. Res. 2019, 80, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Georgieva, S.; Tchekalarova, J.; Vitkova, V.; Antonova, K.; Georgiev, A. Synthesis, characterization and anticonvulsant activity of new azobenzene-containing VV-hemorphin-5 bio photoswitch. Amino Acids 2019, 51, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Rangelov, M.; Georgieva, S.; Todorova, N. Synthesis, characterization and anticonvulsant activity of new series of N-modified analogues of VV-hemorphin-5 with aminophosphonate moiety. Amino Acids 2019, 51, 1527–1545. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S. Potential anticonvulsant activity of novel VV-hemorphin-7 analogues containing unnatural amino acids: Synthesis and characterization. Amino Acids 2020, 52, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Georgieva, S.; Peneva, P.; Tchekalarova, J. Spectral and electrochemical solvatochromic investigations of newly synthesized peptide-based chemosensor bearing azobenzene side chain bio photoswitch. Dyes Pigm. 2021, 191, 109348. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.; Zhuang, Q. Highly sensitive and selective biosensor for heparin detection with rhodamine B-labelled peptides as fluorescent bioreceptors. Sens. Actuators. B Chem. 2019, 299, 126873. [Google Scholar] [CrossRef]
- Ali, A.; Baby, B.; Soman, S.S.; Vijayan, R. Molecular insights into the interaction of hemorphin and its targets. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Srivastava, N.; Garg, P.; Srivastava, P.; Seth, P.K. A molecular dynamics simulation study of the ACE2 receptor with screened natural inhibitors to identify novel drug candidate against COVID-19. Peer J. 2021, 9, e11171. [Google Scholar] [CrossRef] [PubMed]
- Sauperl, O. Textiles for protection against microorganism. AIP Conf. Proc. 2016, 1727, 020021. [Google Scholar] [CrossRef]
- Iyigundogdu, Z.U.; Demir, O.; Asutay, A.B.; Sahin, F. Developing novel antimicrobial and antiviral textile products. Appl. Biochem. Biotechnol. 2017, 181, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Abed, Y.; Boivin, G. Influenza drug resistance. Semin. Respir. Crit. Care Med. 2011, 32, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Stevenazzi, A.; Marchini, M.; Sandrone, G.; Vergani, B.; Lattanzio, M. Amino acidic scaffolds bearing unnatural side chains: An old idea generates new and versatile tools for the life sciences. Bioorg. Med. Chem. Lett. 2014, 24, 5349–5356. [Google Scholar] [CrossRef]
- Fornander, L.H.; Feng, B.; Beke-Somfai, T.; Nordén, B. UV transition moments of tyrosine. J. Phys. Chem. B 2014, 118, 9247–9257. [Google Scholar] [CrossRef] [PubMed]
- Yashchuk, V.M.; Kudrya, V.Y.; Levchenko, S.M.; Tkachuk, Z.Y.; Hovorun, D.M.; Mel’nik, V.I.; Vorob’yov, V.P.; Klishevich, G.V. Optical response of the polynucleotides-proteins interaction. Mol. Cryst. Liq. Cryst. 2011, 535, 93–110. [Google Scholar] [CrossRef]
- Xia, M.C.; Cai, L.; Zhang, S.; Zhang, X. Cell-penetrating peptide spirolactam derivative as a reversible fluorescent pH probe for live cell imaging. Anal. Chem. 2017, 89, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Oberlinner, A. Rhodamine dyes. U.S. Patent 4647675, 3 March 1987. [Google Scholar]
- El Seoud, O.A.; Baader, W.J.; Bastos, E.L. Practical chemical kinetics in solution. In Encyclopedia of Physical Organic Chemistry; Wiley: Hoboken, NJ, USA, 2016; Volume 21, pp. 1–68. [Google Scholar] [CrossRef]
- Zheng, H.; Shang, G.-Q.; Yang, S.-Y.; Gao, X.; Xu, J.-G. Fluorogenic and chromogenic rhodamine spirolactam based probe for nitric oxide by spiro ring opening reaction. Org. Lett. 2008, 10, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Grabchev, I.; Betcheva, R. Sensor potential of 1, 8-naphthalimide and its dyeing ability of cotton fabric. Dyes Pigm. 2013, 98, 64–70. [Google Scholar] [CrossRef]
- Becerir, B. An approach for estimating the relation between K/S values and dye uptake. Colourage 2003, 50, 39–48. [Google Scholar]
- Robinson, W.E., Jr.; McDougall, B.; Tran, D.; Selsted, M.E. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 1998, 63, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of use of antiviral peptides against influenza virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Sarin, V.K.; Kent, S.B.H.; Tam, J.P.; Merrifield, R.B. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal. Biochem. 1986, 117, 147–157. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. Chemical modification of cotton fabric with 1, 8-naphthalimide for use as heterogeneous sensor and antibacterial textile. J. Photochem. Photobiol. A Chem. 2019, 382, 111924. [Google Scholar] [CrossRef]
- Becerir, B. Color concept in textiles: A review. J. Text. Eng. Fash. Technol. 2017, 1, 240–244. [Google Scholar] [CrossRef][Green Version]
- Mohini, K.; Tejashree, L.; Vijay, N. Data set on analysis of dyeing property of natural dye from Thespesia populnea bark on different fabrics. Data Brief 2018, 16, 401–410. [Google Scholar] [CrossRef]

| Compound | pK | pI | k, s−1 | τ1/2 | |
|---|---|---|---|---|---|
| pK1 | pK2 | ||||
| Rh-1 | 2.81 | 6.6 | 4.7 | 4.65 × 10−2 ± 0.0046 | 14.9 |
| Rh-2 | 2.78 | 6.38 | 4.58 | 5.53 × 10−2 ± 0.0012 | 12.5 |
| Rh-3 | 2.86 | 6.39 | 4.63 | 8.12 × 10−2 ± 0.0014 | 8.54 |
| Virus | Δlog 30 min | Δlog 60 min | ||||
|---|---|---|---|---|---|---|
| Rh-1 | Rh-2 | Rh-3 | Rh-1 | Rh-2 | Rh-3 | |
| HRSV-2 | 0.2 | 0.4 | 0.2 | 1.3 | 1.3 | 1.7 |
| HAdV-5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Virus | Δlog 30 min | Δlog 60 min | ||||
|---|---|---|---|---|---|---|
| Rh-1-Textile | Rh-2-Textile | Rh-3-Textile | Rh-1-Textile | Rh-2-Textile | Rh-3-Textile | |
| HRSV-2 | − | − | − | 0.2 | 0.2 | 0.1 |
| HAdV-5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Compound | Cytotoxicity | Antiviral Activity | |||
|---|---|---|---|---|---|
| CC50 (µM/mL) in HEp-2 Cells | HRSV-S2 | HAdV-5 | |||
| IC50 (µM/mL) | SI | IC50 (µM/mL) | SI | ||
| Rh-1 | 35 | NA | - | NA | - |
| Rh-2 | 23 | NA | - | NA | - |
| Rh-3 | 113 | NA | - | NA | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Grabchev, I. Synthesis of New Modified with Rhodamine B Peptides for Antiviral Protection of Textile Materials. Molecules 2021, 26, 6608. https://doi.org/10.3390/molecules26216608
Todorov P, Georgieva S, Staneva D, Peneva P, Grozdanov P, Nikolova I, Grabchev I. Synthesis of New Modified with Rhodamine B Peptides for Antiviral Protection of Textile Materials. Molecules. 2021; 26(21):6608. https://doi.org/10.3390/molecules26216608
Chicago/Turabian StyleTodorov, Petar, Stela Georgieva, Desislava Staneva, Petia Peneva, Petar Grozdanov, Ivanka Nikolova, and Ivo Grabchev. 2021. "Synthesis of New Modified with Rhodamine B Peptides for Antiviral Protection of Textile Materials" Molecules 26, no. 21: 6608. https://doi.org/10.3390/molecules26216608
APA StyleTodorov, P., Georgieva, S., Staneva, D., Peneva, P., Grozdanov, P., Nikolova, I., & Grabchev, I. (2021). Synthesis of New Modified with Rhodamine B Peptides for Antiviral Protection of Textile Materials. Molecules, 26(21), 6608. https://doi.org/10.3390/molecules26216608











