Marine-Derived Biowaste Conversion into Bioceramic Membrane Materials: Contrasting of Hydroxyapatite Synthesis Methods
Abstract
:1. Introduction
2. Results and Discussion
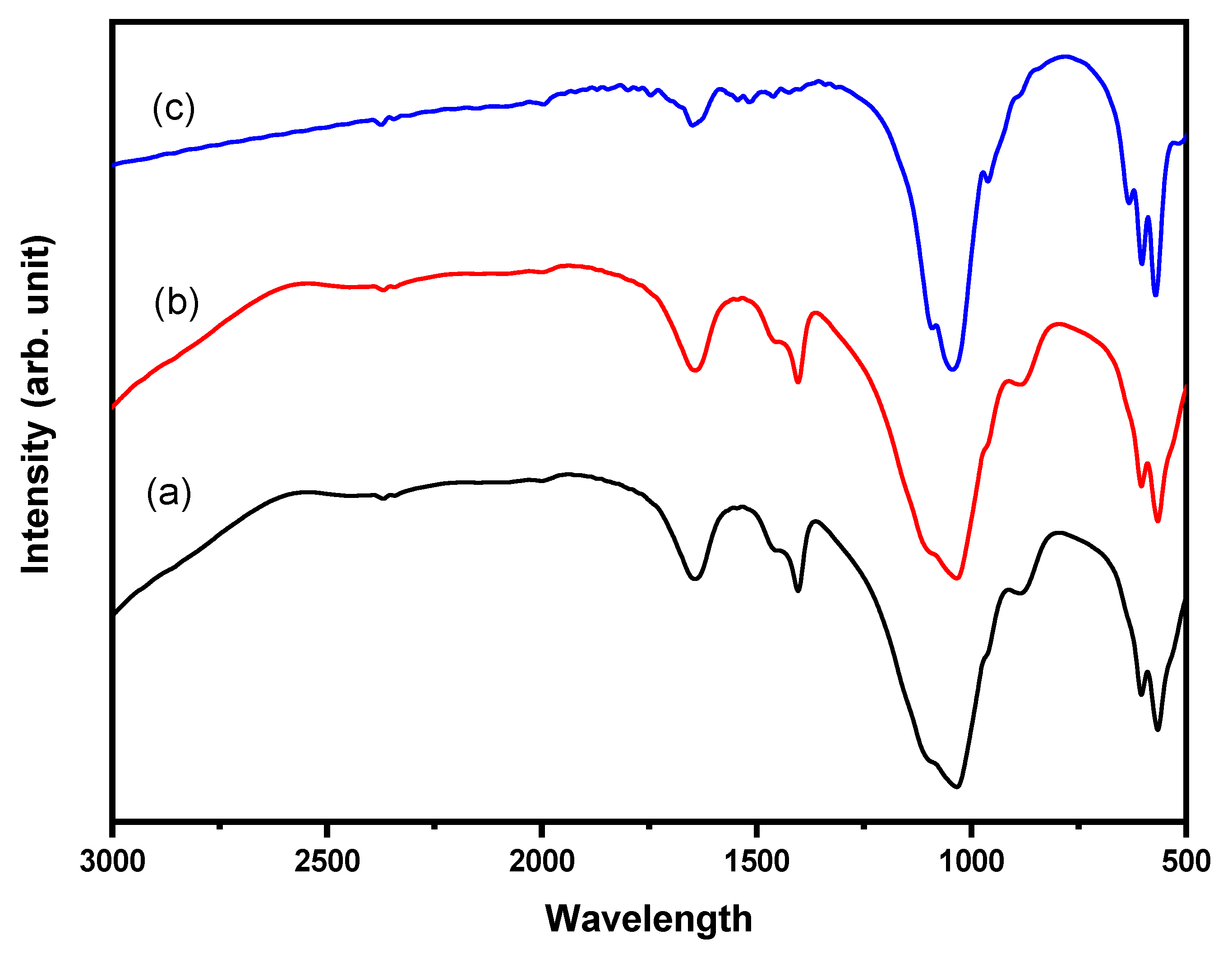
2.1. Molecular Structure
2.2. Crystallinity and Chemical Composition
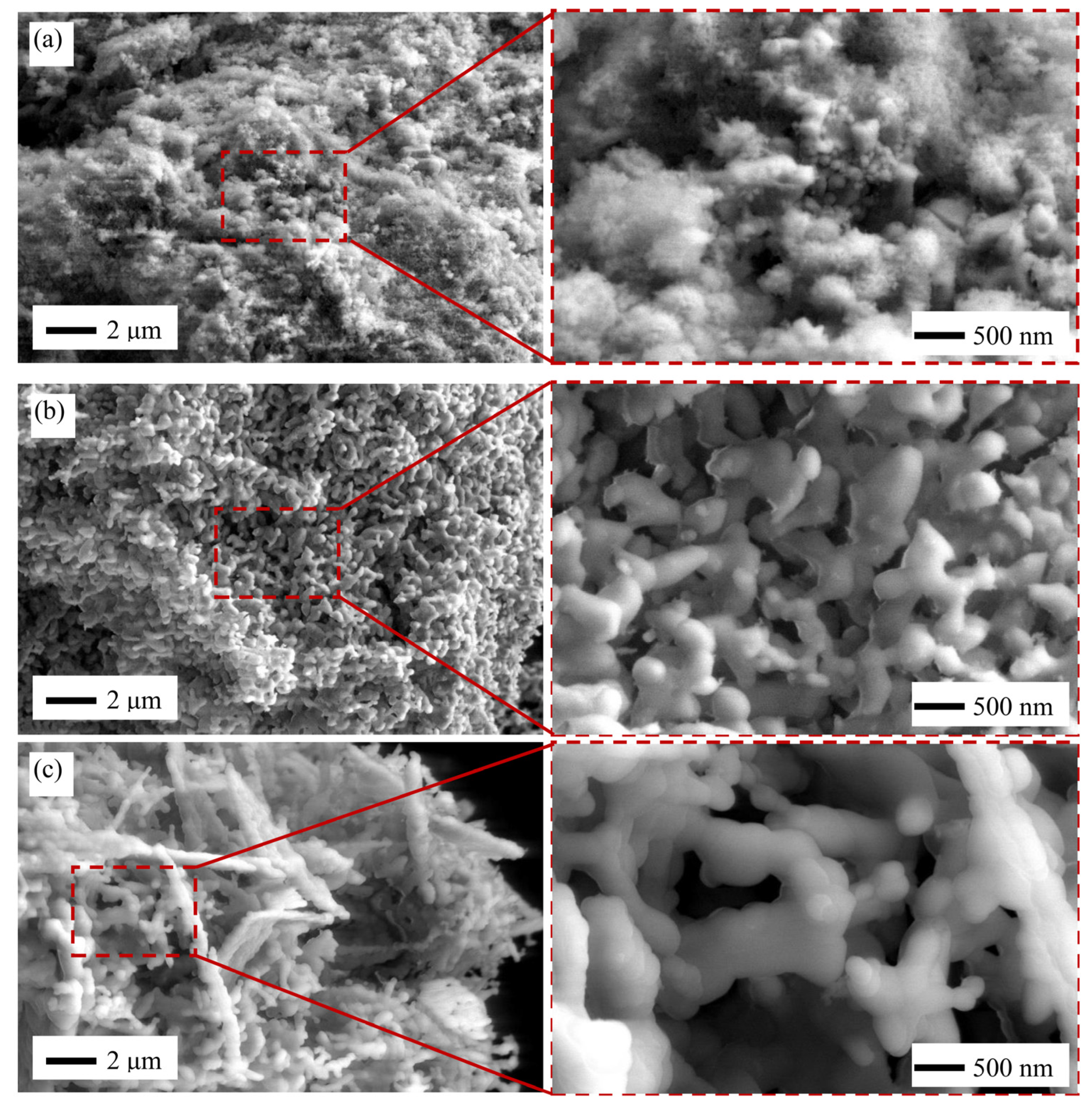
2.3. HAp Microstructure
2.4. An Outlook: Bioceramic Ultrafiltration Membrane Application
3. Materials and Methods
3.1. Materials
3.2. Preparation of Calcium Oxide
3.3. Synthesis of Hydroxyapatite: Microwave
3.4. Synthesis of Hydroxyapatite: Coprecipitation
3.5. Synthesis of Hydroxyapatite: Sol-Gel
3.6. Characterization of Hydroxyapatite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef]
- Rubini, D.; Farisa Banu, S.; Veda Hari, B.N.; Ramya Devi, D.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Chitosan extracted from marine biowaste mitigates staphyloxanthin production and biofilms of Methicillin-resistant Staphylococcus aureus. Food Chem. Toxicol. 2018, 118, 733–744. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of marine-derived biowaste to develop chitin/fish gelatin products as bioactive carriers and moisture scavengers. Sci. Total Environ. 2020, 706, 135747. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Dabiri, S.M.H.; Rezaie, A.A.; Moghimi, M.; Rezaie, H. Extraction of Hydroxyapatite from Fish Bones and Its Application in Nickel Adsorption. BioNanoScience 2018, 8, 823–834. [Google Scholar] [CrossRef]
- Wibisono, Y.; Yuliani, R.; Kamilia, N.; Ardian, D.; Lastriyanto, A.; Rafianto, V.; Diniardi, E.; Sandra. Hybridization of nitrogen compounds and hydroxyapatite: A slowly released fertiliser for water sustainability. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012005. [Google Scholar] [CrossRef]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balázsi, C.; Wéber, F.; Kövér, Z.; Horváth, E.; Németh, C. Preparation of calcium–phosphate bioceramics from natural resources. J. Eur. Ceram. Soc. 2007, 27, 1601–1606. [Google Scholar] [CrossRef]
- Zakharov, N.A.; Polunina, I.A.; Polunin, K.E.; Rakitina, N.M.; Kochetkova, E.I.; Sokolova, N.P.; Kalinnikov, V.T. Calcium Hydroxyapatite for Medical Applications. Inorg. Mater. 2004, 40, 641–648. [Google Scholar] [CrossRef]
- Kumta, P.N.; Sfeir, C.; Lee, D.-H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Xiong, Z.-C.; Yang, R.-L.; Zhu, Y.-J.; Chen, F.-F.; Dong, L.-Y. Flexible hydroxyapatite ultralong nanowire-based paper for highly efficient and multifunctional air filtration. J. Mater. Chem. A 2017, 5, 17482–17491. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Park, N.; Jang, Y.; Lim, H.; Kim, W. Application of the hydroxyapatite crystallization-filtration process to recover phosphorus from wastewater effluents. Water Sci. Technol. 2020, 81, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.; Binner, J.G.P. An evaluation of hydroxyapatite-based filters for removal of heavy metal ions from aqueous solutions. J. Mater. Sci. 1996, 31, 1231–1241. [Google Scholar] [CrossRef]
- Abo-Almaged, H.H.; Gaber, A.A. Synthesis and characterization of nano-hydroxyapatite membranes for water desalination. Mater. Today Commun. 2017, 13, 186–191. [Google Scholar] [CrossRef]
- Kuiper, M.; Sanches, R.M.; Walford, J.A.; Slater, N.K.H. Purification of a functional gene therapy vector derived from Moloney murine leukaemia virus using membrane filtration and ceramic hydroxyapatite chromatography. Biotechnol. Bioeng. 2002, 80, 445–453. [Google Scholar] [CrossRef]
- Yang, L.; Ning, X.; Chen, K.; Zhou, H. Preparation and properties of hydroxyapatite filters for microbial filtration. Ceram. Int. 2007, 33, 483–489. [Google Scholar] [CrossRef]
- Zhang, C.; Uchikoshi, T.; Liu, L.; Kikuchi, M.; Ichinose, I. Effect of Surface Modification with TiO2 Coating on Improving Filtration Efficiency of Whisker-Hydroxyapatite (HAp) Membrane. Coatings 2020, 10, 670. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.H.; Shin, D.-G.; Noviyanto, A.; Lee, H.-M.; Nishimura, T.; Jang, B.-K.; Kwon, W.-T.; Kim, Y.; Kim, S.; et al. Phase transformation on spark plasma sintered dense polycarbosilane-derived SiC without additive. Scr. Mater. 2018, 143, 188–190. [Google Scholar] [CrossRef]
- Noviyanto, A.; Yoon, D.-H. Metal oxide additives for the sintering of silicon carbide: Reactivity and densification. Curr. Appl. Phys. 2013, 13, 287–292. [Google Scholar] [CrossRef]
- Qureshi, H.F.; Nijmeijer, A.; Winnubst, L. Influence of sol–gel process parameters on the micro-structure and performance of hybrid silica membranes. J. Membr. Sci. 2013, 446, 19–25. [Google Scholar] [CrossRef]
- Wibisono, Y.; Dwijaksara, N.L.B.; Widayatno, W.B.; Wismogroho, A.S.; Amal, M.I.; Rochman, N.T.; Nishimura, T.; Noviyanto, A. Synthesis and Sinterability of Hydroxyapatite from Fishery by-products. J. Korean Ceram. Soc. 2018, 55, 570–575. [Google Scholar] [CrossRef] [Green Version]
- Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Pandele, A.M.; Comanici, F.E.; Carp, C.A.; Miculescu, F.; Voicu, S.I.; Thakur, V.K.; Serban, B.C. Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Sivasankari, S.; Kalaivizhi, R.; Gowriboy, N.; Ganesh, M.R.; Shazia Anjum, M. Hydroxyapatite integrated with cellulose acetate/polyetherimide composite membrane for biomedical applications. Polym. Compos. 2021, 42, 5512–5526. [Google Scholar] [CrossRef]
- Alrafai, H.A.; Ali Al-Ahmed, Z.; Ahmed, M.K.; Afifi, M.; Shoueir, K.R.; Abu-Rayyan, A. The degradation of methylene blue dye using copper-doped hydroxyapatite encapsulated into polycaprolactone nanofibrous membranes. New J. Chem. 2021, 45, 16143–16154. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Zhu, Y.-J.; Wu, J.; Dong, L.-Y. Nanofiltration Filter Paper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers/Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 17198–17209. [Google Scholar] [CrossRef]
- Kallem, P.; Bharath, G.; Rambabu, K.; Srinivasakannan, C.; Banat, F. Improved permeability and antifouling performance of polyethersulfone ultrafiltration membranes tailored by hydroxyapatite/boron nitride nanocomposites. Chemosphere 2021, 268, 129306. [Google Scholar] [CrossRef]
- Nor Suhaida Rasman, S.; Riduan Jamalludin, M.; Najieha Kamarudin, S.; Khadijah Hubadillah, S.; Arif Budiman Pauzan, M.; Hafiz Dzarfan Othman, M. Fabrication and characterization of hydroxyapatite based cow bone polysulfone mixed matrix membrane. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1142, 012010. [Google Scholar] [CrossRef]
- Luo, J.; Nordvang, R.T.; Morthensen, S.T.; Zeuner, B.; Meyer, A.S.; Mikkelsen, J.D.; Pinelo, M. An integrated membrane system for the biocatalytic production of 3′-sialyllactose from dairy by-products. Bioresour. Technol. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Guadix, A.; Guadix, E.M. Recovery of caprine milk oligosaccharides with ceramic membranes. J. Membr. Sci. 2006, 276, 23–30. [Google Scholar] [CrossRef]
- Urashima, T.; Taufik, E.; Fukuda, K.; Asakuma, S. Recent Advances in Studies on Milk Oligosaccharides of Cows and Other Domestic Farm Animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.-Y.; Zhang, C.-B.; Huang, J.-F. Synthesis of hydroxyapatite nanoparticles in ultrasonic precipitation. Ceram. Int. 2005, 31, 1041–1044. [Google Scholar] [CrossRef]
- Xu, J.; Khor, K.; Dong, Z.; Gu, Y.W.; Kumar, R.R.; Cheang, P. Preparation and characterization of nano-sized hydroxyapatite powders produced in a radio frequency (rf) thermal plasma. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2004, 374, 101–108. [Google Scholar] [CrossRef]
- Masuda, Y.; Matubara, K.; Sakka, S. Synthesis of Hydroxyapatite from Metal Alkoxides through Sot-Gel Technique. J. Ceram. Soc. Jpn. 1990, 98, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Nathanael, A.J.; Han, S.S.; Oh, T.H. Polymer-Assisted Hydrothermal Synthesis of Hierarchically Arranged Hydroxyapatite Nanoceramic. J. Nanomater. 2013, 2013, 962026. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Manivasagan, P.; Kang, K.-H.; Chalisserry, E.P.; Anil, S.; Kim, D.G.; Kim, S.-K. Isolation and Characterization of Nano-Hydroxyapatite from Salmon Fish Bone. Materials 2015, 8, 5426–5439. [Google Scholar] [CrossRef]
- Haas, R.; Donath, K.; Födinger, M.; Watzek, G. Bovine hydroxyapatite for maxillary sinus grafting: Comparative histomorphometric findings in sheep. Clin. Oral Implant. Res. 1998, 9, 107–116. [Google Scholar] [CrossRef]
- Ozawa, M.; Suzuki, S. Microstructural Development of Natural Hydroxyapatite Originated from Fish-Bone Waste through Heat Treatment. J. Am. Ceram. Soc. 2002, 85, 1315–1317. [Google Scholar] [CrossRef]
- Ulfyana, D.; Anugroho, F.; Sumarlan, S.H.; Wibisono, Y. Bioceramics synthesis of hydroxyapatite from red snapper fish scales biowaste using wet chemical precipitation route. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012038. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wu, Y.-N.; Ho, W.-F. Hydroxyapatite synthesized from oyster shell powders by ball milling and heat treatment. Mater. Charact. 2011, 62, 1180–1187. [Google Scholar] [CrossRef]
- Hu, J.; Russell, J.J.; Ben-Nissan, B.; Vago, R. Production and analysis of hydroxyapatite from Australian corals via hydrothermal process. J. Mater. Sci. Lett. 2001, 20, 85–87. [Google Scholar] [CrossRef]
- Ho, W.-F.; Hsu, H.-C.; Hsu, S.-K.; Hung, C.-W.; Wu, S.-C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Raya, I.; Mayasari, E.; Yahya, A.; Syahrul, M.; Latunra, A.I. Shynthesis and Characterizations of Calcium Hydroxyapatite Derived from Crabs Shells (Portunus pelagicus) and Its Potency in Safeguard against to Dental Demineralizations. Int. J. Biomater. 2015, 2015, 469176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, J.-Y.; Jung, M.-J.; Jeong, I.-H.; Kim, G.-W.; Sim, J.-M.; Nam, S.-Y.; Kim, B.-M. Effects of crab shell extract as a coagulant on the textural and sensorial properties of tofu (soybean curd). Food Sci. Nutr. 2019, 7, 547–553. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.-H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic–inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, I.; Itoh, S.; Suzuki, M.; Osaka, A.; Tanaka, J. The chitosan prepared from crab tendons: II. The chitosan/apatite composites and their application to nerve regeneration. Biomaterials 2003, 24, 3285–3292. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Itoh, S.; Suzuki, M.; Sakane, M.; Osaka, A.; Tanaka, J. The chitosan prepared from crab tendon I: The characterization and the mechanical properties. Biomaterials 2003, 24, 2031–2036. [Google Scholar] [CrossRef]
- Dal Sasso, G.; Asscher, Y.; Angelini, I.; Nodari, L.; Artioli, G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci. Rep. 2018, 8, 12025. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Stephen Sipaut@ Mohd Nasri, C.; Bin Arshad, S.E. Hydrothermal synthesis of hydroxyapatite powders using Response Surface Methodology (RSM). PLoS ONE 2021, 16, e0251009. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Chen, L.; Lin, K.; Chang, J. Dental enamel-like hydroxyapatite transformed directly from monetite. J. Mater. Chem. 2012, 22, 22637–22641. [Google Scholar] [CrossRef]
- Dhanaraj, K.; Suresh, G. Conversion of waste sea shell (Anadara granosa) into valuable nanohydroxyapatite (nHAp) for biomedical applications. Vacuum 2018, 152, 222–230. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef] [PubMed]
- Kostov-Kytin, V.V.; Dyulgerova, E.; Ilieva, R.; Petkova, V. Powder X-ray diffraction studies of hydroxyapatite and β-TCP mixtures processed by high energy dry milling. Ceram. Int. 2018, 44, 8664–8671. [Google Scholar] [CrossRef]
- Han, J.-K.; Song, H.-Y.; Saito, F.; Lee, B.-T. Synthesis of high purity nano-sized hydroxyapatite powder by microwave-hydrothermal method. Mater. Chem. Phys. 2006, 99, 235–239. [Google Scholar] [CrossRef]
- Silva, C.C.; Graça, M.P.F.; Valente, M.A.; Góes, J.C.; Sombra, A.S.B. Microwave preparation, structure and electrical properties of calcium–sodium–phosphate biosystem. J. Non-Cryst. Solids 2006, 352, 3512–3517. [Google Scholar] [CrossRef]
- Siddharthan, A.; Seshadri, S.K.; Kumar, T.S.S. Influence of microwave power on nanosized hydroxyapatite particles. Scr. Mater. 2006, 55, 175–178. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Wang, Y.; Ma, L.; Li, F. Preparation and thermal stability analysis of hydroxyapatite derived from the precipitation process and microwave irradiation method. Mater. Lett. 2004, 58, 3586–3590. [Google Scholar] [CrossRef]
- Jenkins, R.; Fawcett, T.G.; Smith, D.K.; Visser, J.W.; Morris, M.C.; Frevel, L.K. JCPDS—International Centre for Diffraction Data Sample Preparation Methods in X-Ray Powder Diffraction. Powder Diffr. 1986, 1, 51–63. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Noviyanto, A.; Nishimura, T.; Kitano, M.; Ohashi, N. Pulverization of oxide powders utilizing thermal treatment in ammonia-based atmosphere. J. Eur. Ceram. Soc. 2016, 36, 4083–4088. [Google Scholar] [CrossRef]
- Al-Qasas, N.S.; Rohani, S. Synthesis of Pure Hydroxyapatite and the Effect of Synthesis Conditions on its Yield, Crystallinity, Morphology and Mean Particle Size. Sep. Sci. Technol. 2005, 40, 3187–3224. [Google Scholar] [CrossRef]
- Büyüksağiş, A.; Çiftçi, N. HAP Coatings for Biomedical Applications: Biocompatibility and Surface Protection against Corrosion of Ti, Ti6Al4V and AISI 316L SS. Prot. Met. Phys. Chem. Surf. 2020, 56, 834–843. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [Green Version]
- Miskufova, A.; Havlik, T.; Bitschnau, B.; Kielski, A.; Pomadowski, H. Properties of CaO Sintered with Addition of Active Alumina. Ceramics-Silikáty 2015, 59, 115–124. [Google Scholar]
- Chen, G.-H. Effect of replacement of MgO by CaO on sintering, crystallization and properties of MgO–Al2O3–SiO2 system glass-ceramics. J. Mater. Sci. 2007, 42, 7239–7244. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bose, S.; Hosick, H.L.; Bandyopadhyay, A. CaO–P2O5–Na2O-based sintering additives for hydroxyapatite (HAp) ceramics. Biomaterials 2004, 25, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Guan, K.; Gao, P.; Peng, C.; Wu, J. A preparation method for the highly permeable ceramic microfiltration membrane—Precursor film firing method. RSC Adv. 2018, 8, 2906–2914. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Yang, Y.; Chang, Q.; Liu, F.; Wang, Y.; Rao, J. Preparation of a High-Performance Porous Ceramic Membrane by a Two-Step Coating Method and One-Step Sintering. Appl. Sci. 2019, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Azaman, F.; Muhamad Nor, M.A.A.; Wan Abdullah, W.R.; Razali, M.H.; Che Zulkifli, R.; Ahmad Zaini, M.A.; Ali, A. Review on natural clay ceramic membrane: Fabrication and application in water and wastewater treatment. Malays. J. Fundam. Appl. Sci. 2021, 17, 62–78. [Google Scholar] [CrossRef]
- Onoda, H.; Sasaki, K. Iron, Copper, and Nickel Removal with Calcium Hydrogen Phosphate and Calcium Pyrophosphates in Solution. Univers. J. Mater. Sci. 2017, 5, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Runge, K.; Blumberga, A.; Blumberga, D. Bioeconomy Growth in Latvia. System-dynamics Model for High-value Added Products in Fisheries. Energy Procedia 2017, 113, 339–345. [Google Scholar] [CrossRef]
- Piccirillo, C.; Rocha, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. A hydroxyapatite–Fe2O3 based material of natural origin as an active sunscreen filter. J. Mater. Chem. B 2014, 2, 5999–6009. [Google Scholar] [CrossRef]
- Rozaini, M.Z.H.; Wai, H.H.C.P.; Razali, M.H.; Osman, U.M.; Anuar, S.T.; Soh, S.K.C.; Ghazali, S.R.B.; Ibrahim, N.H.; Fei, L.C.; Rahmah, S. Calcium Hydroxyapatite-Based Marine Origin: Novel Sunscreen Materials for Cosmeceutical Treatments. Orient. J. Chem. 2018, 34, 2770–2776. [Google Scholar] [CrossRef] [Green Version]
- Wibisono, Y.; Rafianto, V.; Alvianto, D.; Bagus Hermanto, M. Prediction of size reduction by batch ball milling process for crab shell powder prior hydroxyapatite conversion. IOP Conf. Ser. Earth Environ. Sci. 2020, 542, 012011. [Google Scholar] [CrossRef]
- Sajahan, N.A.; Wan Ibrahim, W.M.A. Microwave Irradiation of Nanohydroxyapatite from Chicken Eggshells and Duck Eggshells. Sci. World J. 2014, 2014, 275984. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, T. Synthesis and Characterization of Hydroxypatite Derived from Asian Green Mussel Shell by Sol-Gel Method (in Bahasa Indonesia); IPB University: Bogor, Indonesia, 2013. [Google Scholar]


| Methods | Elements (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | P | Ti | Mn | Fe | Cu | Sr | Zr | Others | Ca/P | |
| Microwave | 74.35 | 21.3 | 0.074 | 0.27 | 1.09 | 0.066 | 2.3 | 0.3 | 0.250 | 2.69 |
| Coprecipitation | 78.02 | 17.3 | 0.063 | 0.28 | 1.10 | 0.068 | 2.5 | 0.3 | 0.369 | 2.73 |
| Sol–Gel | 79.34 | 16.4 | 0.068 | 0.26 | 1.08 | 0.065 | 2.2 | 0.3 | 0.287 | 4.84 |
| Methods | Crystallite Size (nm) | Strain (%) | Particle Size (nm) |
|---|---|---|---|
| Microwave | 10.3 | 0.98 | 100 ± 29 |
| Coprecipitation | 179 | 0.09 | 465 ± 107 |
| Sol–Gel | 96.7 | 0.11 | 522 ± 206 |
| Methods | Thermal-Induced Reaction | Time (min) |
|---|---|---|
| Microwave | 600 W; 800 W | 15 |
| Coprecipitation | 1000 °C | 360 |
| Sol–Gel | 1000 °C | 360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibisono, Y.; Pratiwi, A.Y.; Octaviani, C.A.; Fadilla, C.R.; Noviyanto, A.; Taufik, E.; Uddin, M.K.H.; Anugroho, F.; Rochman, N.T. Marine-Derived Biowaste Conversion into Bioceramic Membrane Materials: Contrasting of Hydroxyapatite Synthesis Methods. Molecules 2021, 26, 6344. https://doi.org/10.3390/molecules26216344
Wibisono Y, Pratiwi AY, Octaviani CA, Fadilla CR, Noviyanto A, Taufik E, Uddin MKH, Anugroho F, Rochman NT. Marine-Derived Biowaste Conversion into Bioceramic Membrane Materials: Contrasting of Hydroxyapatite Synthesis Methods. Molecules. 2021; 26(21):6344. https://doi.org/10.3390/molecules26216344
Chicago/Turabian StyleWibisono, Yusuf, Alien Yala Pratiwi, Christine Ayu Octaviani, Cut Rifda Fadilla, Alfian Noviyanto, Epi Taufik, Muhammad K.H. Uddin, Fajri Anugroho, and Nurul Taufiqu Rochman. 2021. "Marine-Derived Biowaste Conversion into Bioceramic Membrane Materials: Contrasting of Hydroxyapatite Synthesis Methods" Molecules 26, no. 21: 6344. https://doi.org/10.3390/molecules26216344
APA StyleWibisono, Y., Pratiwi, A. Y., Octaviani, C. A., Fadilla, C. R., Noviyanto, A., Taufik, E., Uddin, M. K. H., Anugroho, F., & Rochman, N. T. (2021). Marine-Derived Biowaste Conversion into Bioceramic Membrane Materials: Contrasting of Hydroxyapatite Synthesis Methods. Molecules, 26(21), 6344. https://doi.org/10.3390/molecules26216344










