Defensive Mechanisms in Cucurbits against Melon Fly (Bactrocera cucurbitae) Infestation through Excessive Production of Defensive Enzymes and Antioxidants
Abstract
:1. Introduction
2. Results
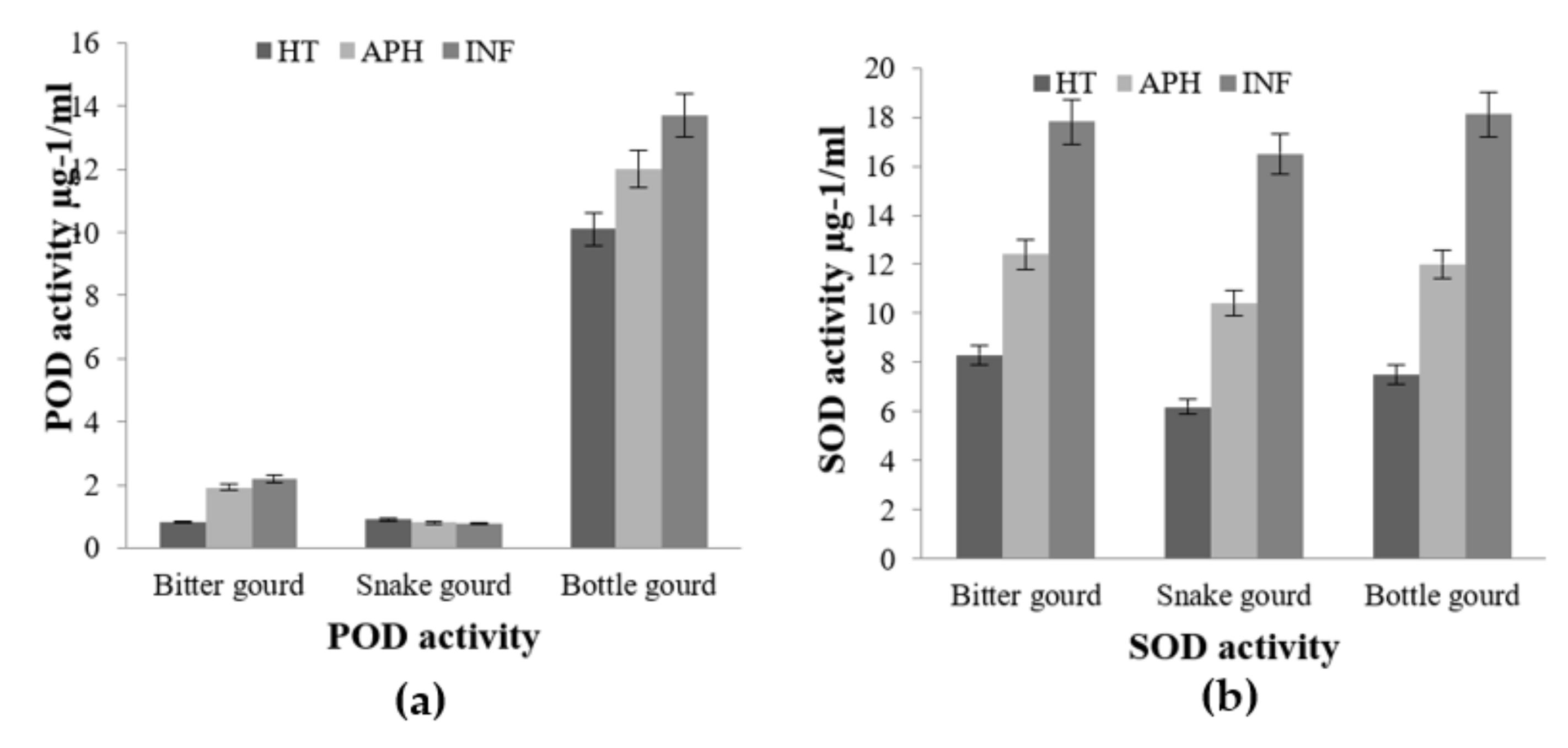
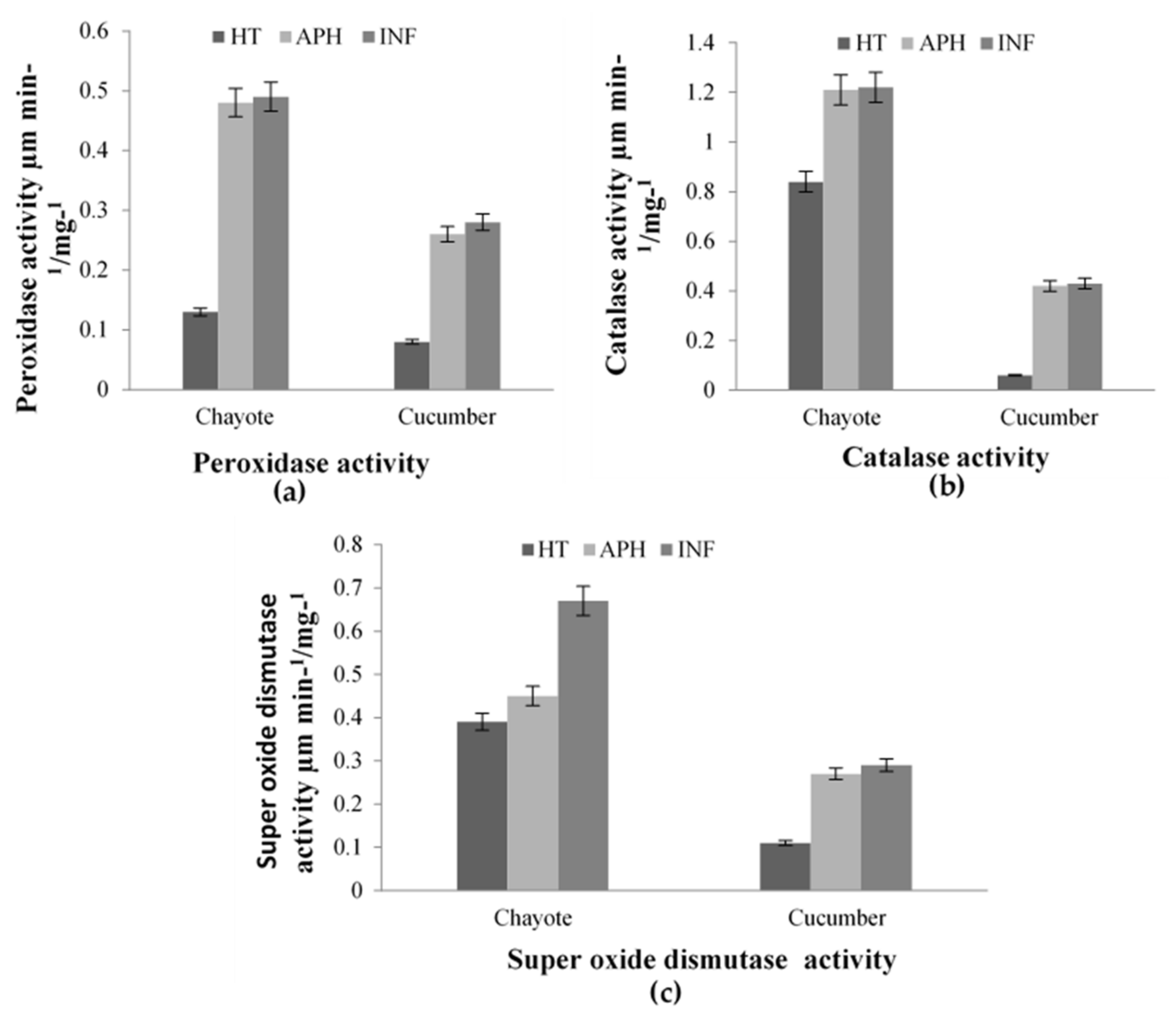
2.1. Accelerated ROS Detoxifying Potential as A Defensive Response against Melon Fly Infection in Cucumber and Chayote
2.2. Non-Enzymatic Antioxidant Potential of Healthy and Infected Cucurbits
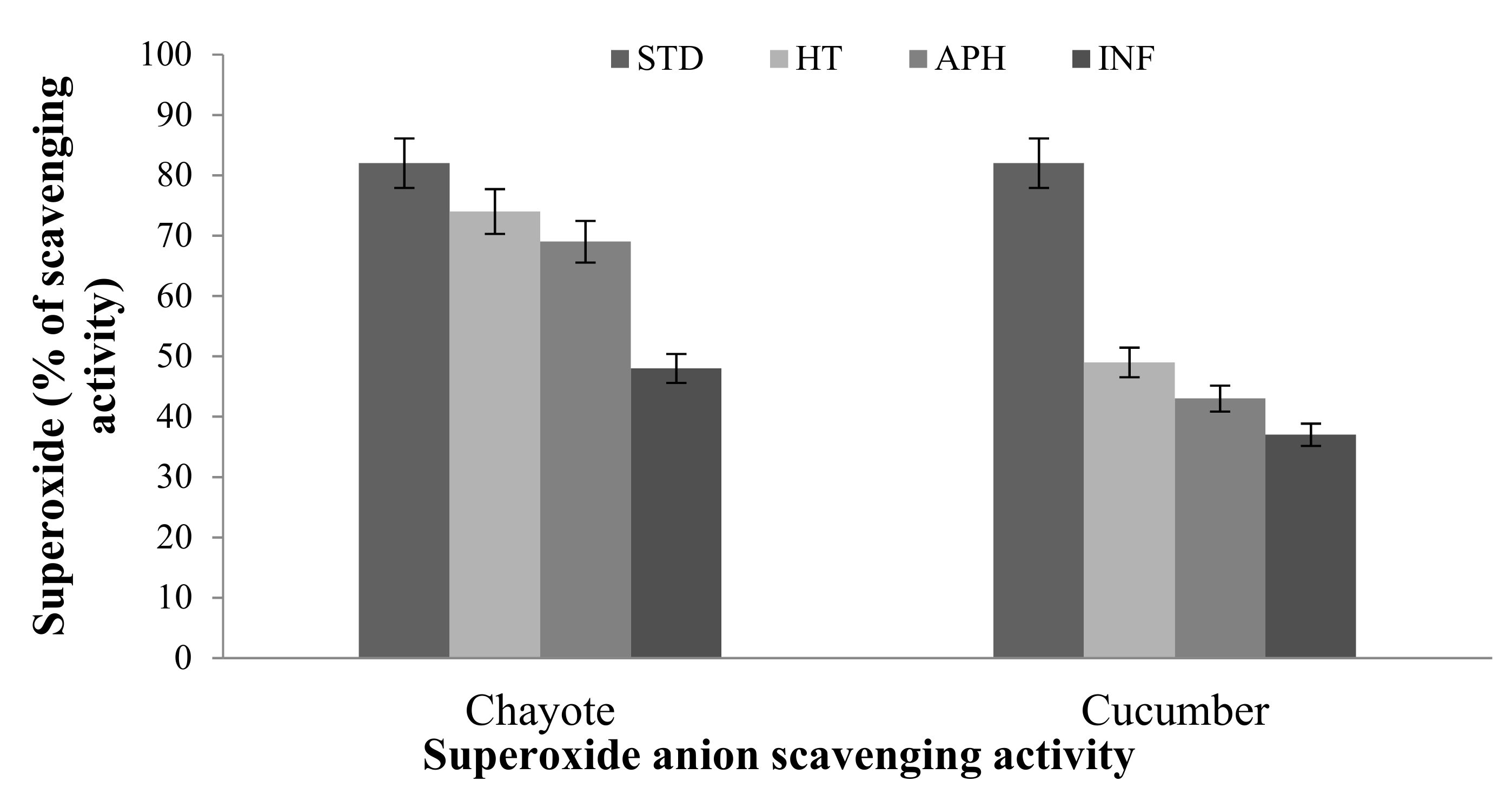
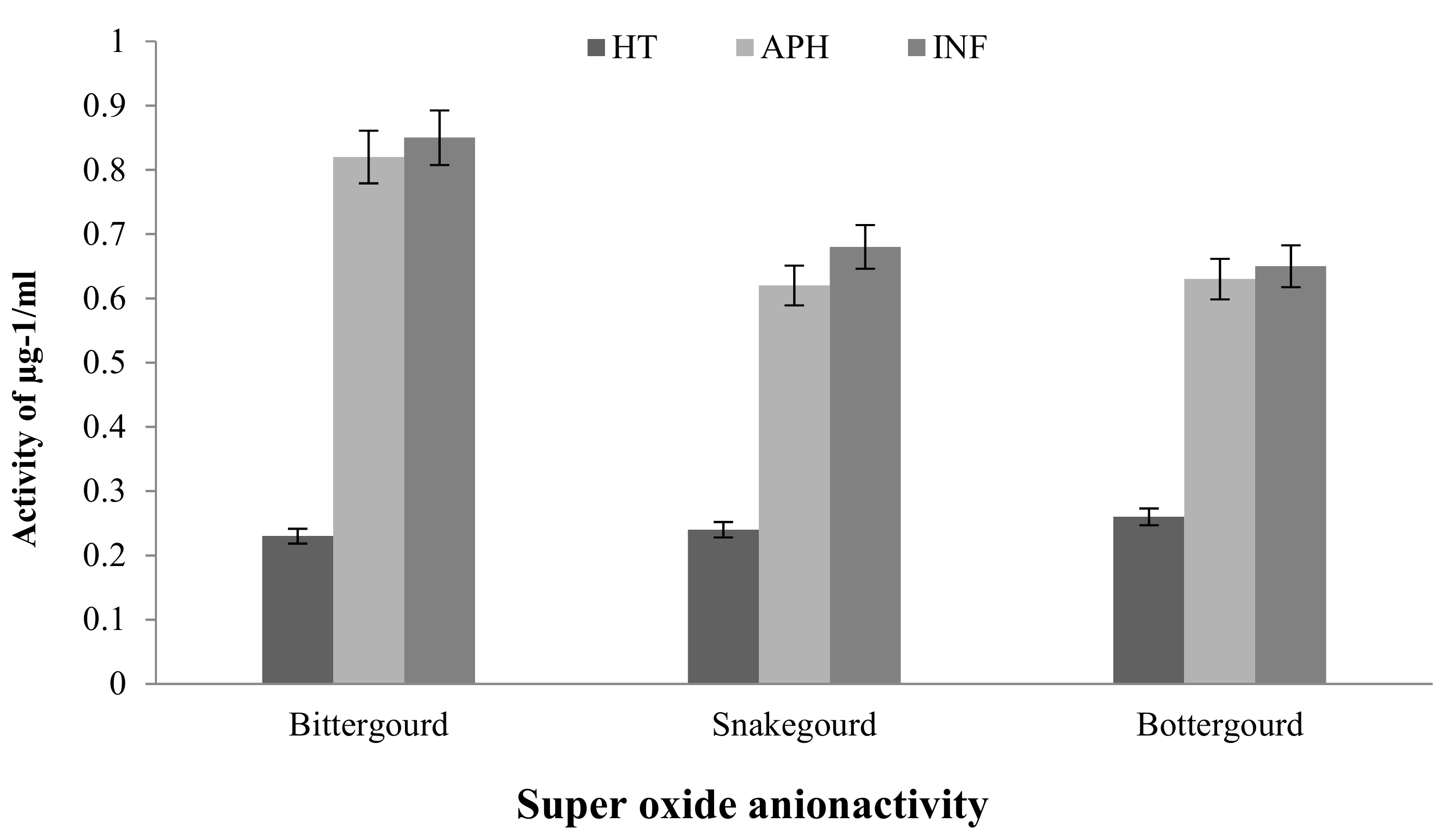
2.2.1. Superoxide Anion (o2–) Assay
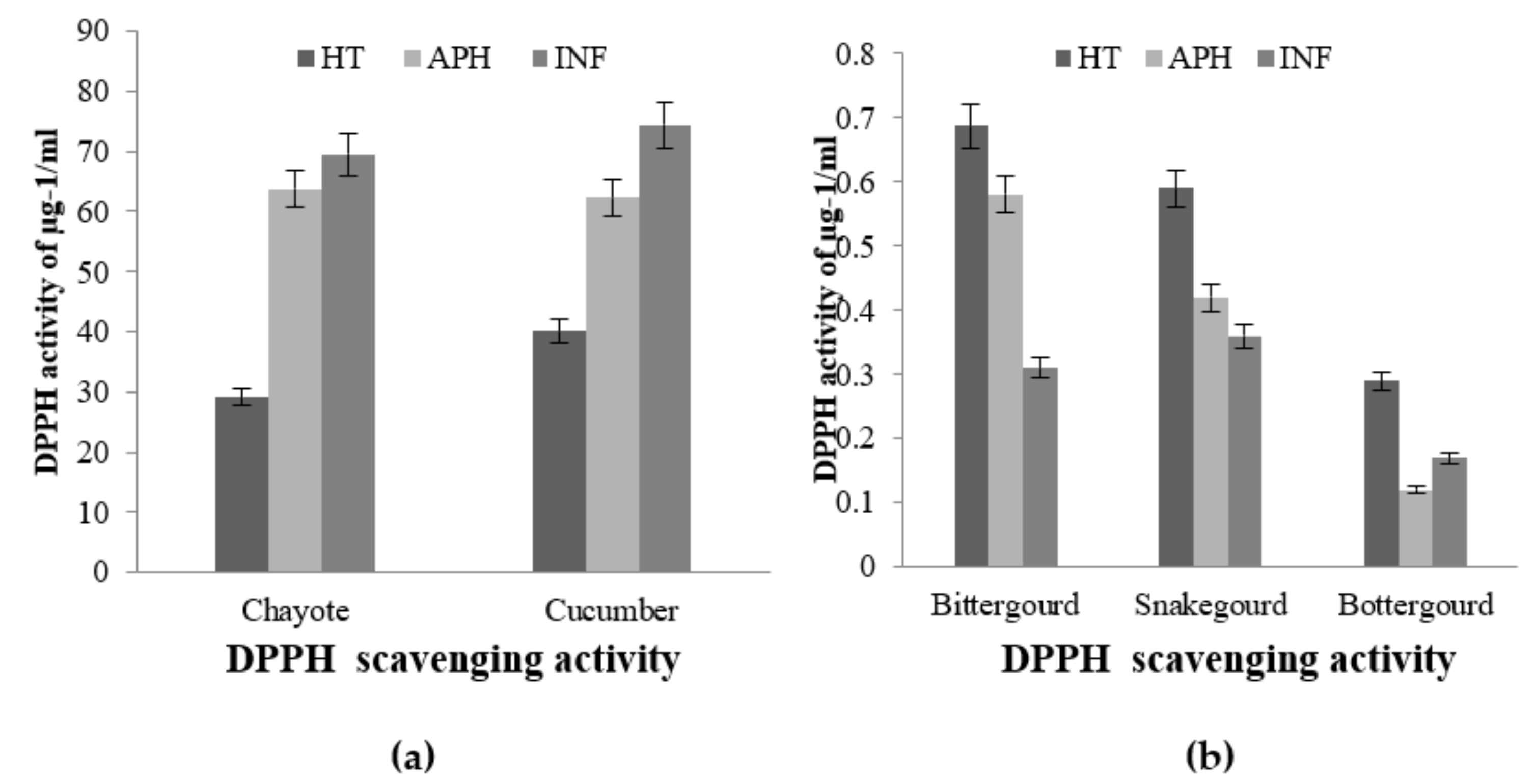
2.2.2. DPPH Free Radical Scavenging Assay
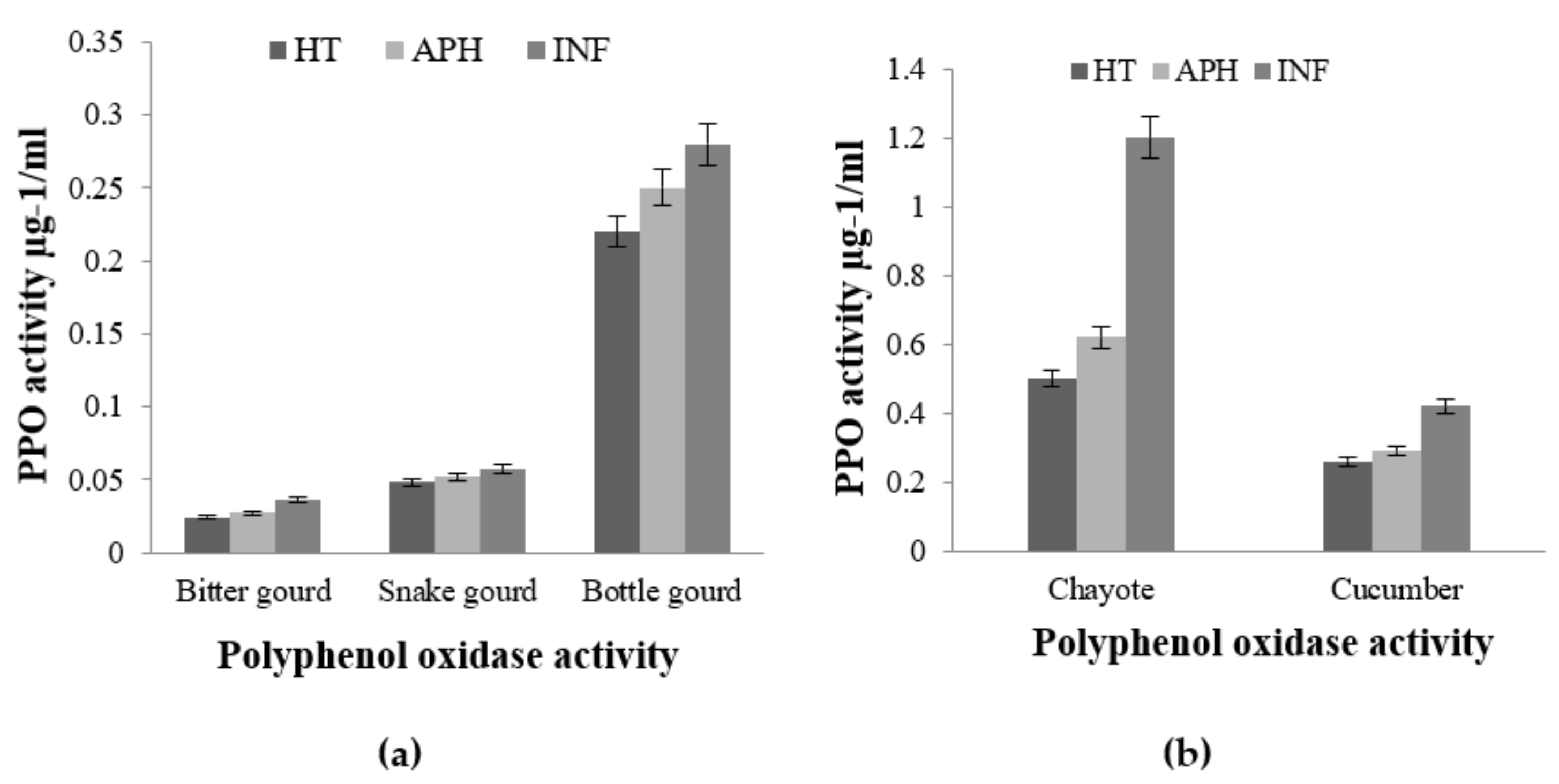
2.3. Enzymatic Antioxidant Potential through Expression of PPO, SOD, and POX in Infected and Healthy Cucurbit Fruits on Melon Fly Infestation
2.4. Assay of the Non-Enzymatic Antioxidant Potential of Cucurbit Fruit Extracts
Ferric-Reducing Antioxidant Power (FRAP) and Superoxide Anion (o2–) Assay
3. Discussion
4. Hypothetical Schematic Representation of the Antioxidant-Dependent Mechanism of Cucurbit Fruits against Melon Fly Infestation
5. Materials and Methods
5.1. Assessment of Melons Fly Infestation on Cucurbit Fruits
5.2. Estimation of Total Flavonoid and Phenolic Contents
5.3. Assay of Non-Enzymatic Antioxidant Potential of Cucurbit Fruit Extracts
5.3.1. FRAP Activity
5.3.2. Superoxide Anion (o2–) Activity
5.3.3. DPPH Activity
5.4. Activity of Defensive Enzymes Activity of Cucurbit Fruit Extracts
5.4.1. Polyphenol Oxidase (PPO) Assay
5.4.2. Peroxidase (POD) Assay
5.4.3. Superoxide Dismutase Activity (SOD)
5.4.4. Catalase Assay (CAT)
5.5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Satyanarayana, B.; Subhashini, D.P.; Arundhati, K.A.; Hemalatha, P.J. Studies on antioxidants and peroxidase isoenzymes in seedlings of twelve cultivars with four different duration of flowering time in pigeon pea (Cajanus cajan (L.) Millspaugh). Free Radic. Antioxid. 2013, 3, 67–72. [Google Scholar]
- Meenakshi, S.; Rashmi, V. Study of combine effect with fly ash and Cow dung Amendment on morphological status of Indian wheat plant (Triticum aestivum L.). Asian J. Plant Pathol. 2021, 11, 73–82. [Google Scholar]
- Shen, K.; Hu, J.; Wu, B.; An, K.; Zhang, J.; Liu, J.; Zhang, R. Competitive Interactions between Immature Stages of Bactrocera cucurbitae (Coquillett) and Bactrocera tau (Walker) (Diptera: Tephritidae) under Laboratory Conditions. Neotrop. Entomol. 2014, 43, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Rubinowska, K.; Górska-Drabik, E. Physiological and biochemical responses of fern Nephrolepsi sbiserrata (Sw.) Schott. to Coccus hesperidum L. infestation. Acta Biol. Crac. Ser. Bot. 2013, 55, 93–98. [Google Scholar]
- Ashry, N.A.; Mohamed, H.I. Impact of secondary metabolites and related enzymes in flax resistance and/or susceptibility to powdery mildew. Afr. J. Adv. Biotechnol. 2012, 11, 1073–1077. [Google Scholar]
- Fletcher, B.S. The biology of Dakine fruit flies. Annu. Rev. Entomol. 1987, 32, 115–144. [Google Scholar] [CrossRef]
- Aneta, W.; Jan, O.S.; Renata, C. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar]
- Bevan, M.; Shufflebottom, D.; Edwards, K.; Jefferson, R.; Schuch, W. Tissue-and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989, 8, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Goudarshivananavar, B.C.; Vigneshwaran, V.; Somegowda, M.; Dharmappa, K.K.; Pramod, S.N. Therapeutic potential of Polyalthiacerasoides stem bark extracts against oxidative stress and nociception. Anc. Sci. Life 2015, 35, 70–78. [Google Scholar] [PubMed]
- Espinosa, F.; Garrido, I.; Ortega, A.; Casimiro, I.; Álvarez-Tinaut, M.C. Redox Activities and ROS, NO and Phenylpropanoids Production by Axenically Cultured Intact Olive Seedling Roots after Interaction with a Mycorrhizal or a Pathogenic Fungus. PLoS ONE 2014, 9, 100132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.M.; Chakraborty, N.; Baidyanath, P. Foliar gall and antioxidant enzyme responses in Alstonia scholaris, R. Br. After psylloid herbivory-an experimental and statistical analysis. Glob. J. Bot. Sci. 2014, 2, 12–20. [Google Scholar]
- Madhusudana, S.; Vigneshwaran, V.; Rajeshwar, A.N.; Shivashankar, S.; Pramod, S.N. Biotic Stress Induced by Bacterocera cucurbitae (Melon Fly) Triggers DefenseRelated Phenylpropanoid Pathway (PPP) and ROS Detoxifying Enzymes in Cucurbits as Adaptation. Asian J. Plant Sci. Res. 2017, 7, 18–29. [Google Scholar]
- Domingo, J.C.; Cordobilla, B.; Ferrer, R.; Giralt, M.; Alegre-Martín, J.; Castro-Marrero, J. Are circulating fibroblast growth Factor -21 and N-terminal prohormone of brain natriuretic peptide promising novel biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome. Antioxid. Redox Sign. 2021, 34, 1420–1427. [Google Scholar] [CrossRef]
- Yamamura, K.; Kiritani, K. A simple method to estimate the potential increase in the number of generations under global warming in temperate zones. Appl. Èntomol. Zoöl. 1998, 33, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Shingo, K.; Tomoaki, I.; Taisei, K.; Kosho, A.; Hozumi, M.; Hideshi, I.; Takaaki, A. High Precision Sulfur metabolomics innovated by a new Specific probe for trapping reactive sulfur species. Antioxid. Redox Sign. 2020, 34, 1407–1419. [Google Scholar]
- Racchi, M.L. Antioxidant Defenses in Plants with Attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef]
- Weems, C.F.; Taylor, L.K.; Cannon, M.F.; Marino, R.C.; Romano, D.M.; Scott, B.G.; Perry, A.M.; Triplett, V. Post Traumatic Stress, Context, and the Lingering Effects of the Hurricane Katrina Disaster among Ethnic Minority Youth. J. Abnorm. Child Psychol. 2010, 38, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, S.; Sumathi, M.; Krishnakumar, N.; Rao, V. Role of phenolic acids and enzymes of phenylpropanoid pathway in resistance of chayote fruit (Sechium edule) against infestation by melon fly, Bactrocera cucurbitae. Ann. Appl. Biol. 2015, 166, 420–433. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef] [Green Version]
- Sapkota, R.; Dahal, K.C.; Thapa, R.B. Damage assessment and management of cucurbit fruit flies in spring-summer squash. J. Entomol. Nematol. 2010, 2, 007–012. [Google Scholar]
- Vishwanathreddy, H.; Bhat, G.G.; Inamdar, S.R.; Gudihal, R.K.; Swamy, B.M. Sclerotium rolfsii lectin extracts in-secticidal activity Spodoptera litura larvae by binding to membrane protein of midgut. Toxicon 2013, 8, 47–57. [Google Scholar]
- Fodor, J.; Gullner, G.; Adam, A.L.; Barna, B.; Komives, T.; Király, Z.J.P.P. Local and Systemic Responses of Antioxidants to Tobacco Mosaic Virus infection and to Salicylic Acid in Tobacco. Plant Physiol. 1997, 114, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Rekha, K.K.; Mukesh, K.; Guptha, R.N. Purification and characterization of trypsin inhibitor from Cicer arietinum L. and its efficiency against Helicoverpa armigera. Braz. J. Plant Physiol. 2008, 20, 313–322. [Google Scholar]
- Allan, A.C.; Fluhr, R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 1997, 9, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Deepak, M.K.; Surendra, S.K.; Mahabaleshwar, V.H.; Hanzhong, B. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2000; p. 936. [Google Scholar]
- Vranová, E.; Inzé, D.; Van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.J.; Orlandi, E.W. Active oxygen in plant/pathogen interactions. Annu. Rev. Phytopathol. 1995, 33, 299–321. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Com-pounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa L. Leaves Extracts and their Radical Scavenging Activity. Z. Für Nat. C 2007, 62, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Shivashankar, S.; Sumathi, M.; Ranganath, H.R. Roles of reactive oxygen species and antioxidant systems in the resistance response of chayote fruit (Sechium edule) to melon fly [Bactrocera cucurbitae (Coquillett)]. J. Hortic. Sci. Biotechnol. 2012, 87, 391–397. [Google Scholar] [CrossRef]
- Alafiatayo, A.A.; Syahida, A.; Mahmood, M. Total Antioxidant Capacity, Flavonoid, Phenolic Acid And Polyphenol Content In Ten Selected Species Of Zingiberaceae Rhizomes. Afr. J. Tradit. Complement. Altern. Med. 2014, 1, 7–13. [Google Scholar]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and an-ti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 83–198. [Google Scholar] [CrossRef]
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant. Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Anwesha, B.A.G. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar]
- Liu, Y.C.; Lin, J.S. The response of melon fly, Dacuscucurbitae Coquillett to the attraction of 10% MC. Plant Prot. Bull. 2005, 35, 79–88. [Google Scholar]
- Putter, J.; Becker, R. Bergmeyer HU Methods of Enzymatic Anayalysis; Verlagchemieweinheim: Berlin, Germany, 1998; p. 286. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with increased leaves of mem-brane permeability and lipid peroxidation, and decreased levels of superoxide dismutase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Ashima, S.G.; Robert, P.; Webb, A.; Holaday, S.; Randy, D. Allen Over expression of Superoxide Dismutase Protects Plants from Oxidative Stress. Plant Physiol. 1993, 103, 1067–1073. [Google Scholar]


| Parameter | Chayote | Cucumber | Bitter Gourd | Snake Gourd | Bottle Gourd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HT | APH | INF | HT | APH | INF | HT | APH | INF | HT | APH | INF | HT | APH | INF | |
| TPC GAE (µg/mL) | 0.731 ± 0.01 | 0.683 ± 0.01 | 0.657 ± 0.01 | 0.462 ± 0.05 | 0.418 ± 0.05 | 0.408 ± 0.05 | 0.537 ± 0.01 | 0.483 ± 0.01 | 0.371 ± 0.01 | 0.485 ± 0.05 | 0.425 ± 0.05 | 0.340 ± 0.02 | 0.824 ± 0.02 | 0.715 ± 0.05 | 0.695 ± 0.01 |
| TFC GAE (µg/mL) | 0.937 ± 0.01 | 0.913 ± 0.01 | 0.894 ± 0.01 | 0.375 ± 0.11 | 0.328 ± 1.01 | 0.276 ± 0.01 | 0.408 ± 1.01 | 0.353 ± 0.01 | 0.327 ± 0.01 | 0.27 ± 0.11 | 0.248 ± 1.06 | 0.196 ± 0.03 | 0.686 ± 0.01 | 0.652 ± 0.01 | 0.637 ± 0.01 |
| IC50 DPPH | 81.35 ± 0.01 | 78.61 ± 0.01 | 78.14 ± 0.01 | 62.42 ± 0.02 | 58.17 ± 0.01 | 55.86 ± 0.02 | 63.24 ± 0.03 | 59.37 ± 0.05 | 56.51 ± 0.01 | 49.75 ± 0.03 | 42.67 ± 0.02 | 41.52 ± 0.02 | 73.86 ± 0.05 | 72.46 ± 0.05 | 71.52 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somegowda, M.; Raghavendra, S.; Sridhara, S.; Rajeshwara, A.N.; N. Pramod, S.; Shivashankar, S.; Lin, F.; El-Abedin, T.K.Z.; Wani, S.H.; Elansary, H.O. Defensive Mechanisms in Cucurbits against Melon Fly (Bactrocera cucurbitae) Infestation through Excessive Production of Defensive Enzymes and Antioxidants. Molecules 2021, 26, 6345. https://doi.org/10.3390/molecules26216345
Somegowda M, Raghavendra S, Sridhara S, Rajeshwara AN, N. Pramod S, Shivashankar S, Lin F, El-Abedin TKZ, Wani SH, Elansary HO. Defensive Mechanisms in Cucurbits against Melon Fly (Bactrocera cucurbitae) Infestation through Excessive Production of Defensive Enzymes and Antioxidants. Molecules. 2021; 26(21):6345. https://doi.org/10.3390/molecules26216345
Chicago/Turabian StyleSomegowda, Madhusudana, S. Raghavendra, Shankarappa Sridhara, Achur. N. Rajeshwara, Siddanakoppalu. N. Pramod, S. Shivashankar, Feng Lin, Tarek K Zin El-Abedin, Shabir Hussain Wani, and Hosam O. Elansary. 2021. "Defensive Mechanisms in Cucurbits against Melon Fly (Bactrocera cucurbitae) Infestation through Excessive Production of Defensive Enzymes and Antioxidants" Molecules 26, no. 21: 6345. https://doi.org/10.3390/molecules26216345
APA StyleSomegowda, M., Raghavendra, S., Sridhara, S., Rajeshwara, A. N., N. Pramod, S., Shivashankar, S., Lin, F., El-Abedin, T. K. Z., Wani, S. H., & Elansary, H. O. (2021). Defensive Mechanisms in Cucurbits against Melon Fly (Bactrocera cucurbitae) Infestation through Excessive Production of Defensive Enzymes and Antioxidants. Molecules, 26(21), 6345. https://doi.org/10.3390/molecules26216345








