Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels
Abstract
1. Introduction
2. Results
2.1. Selection of Biocompatible Components
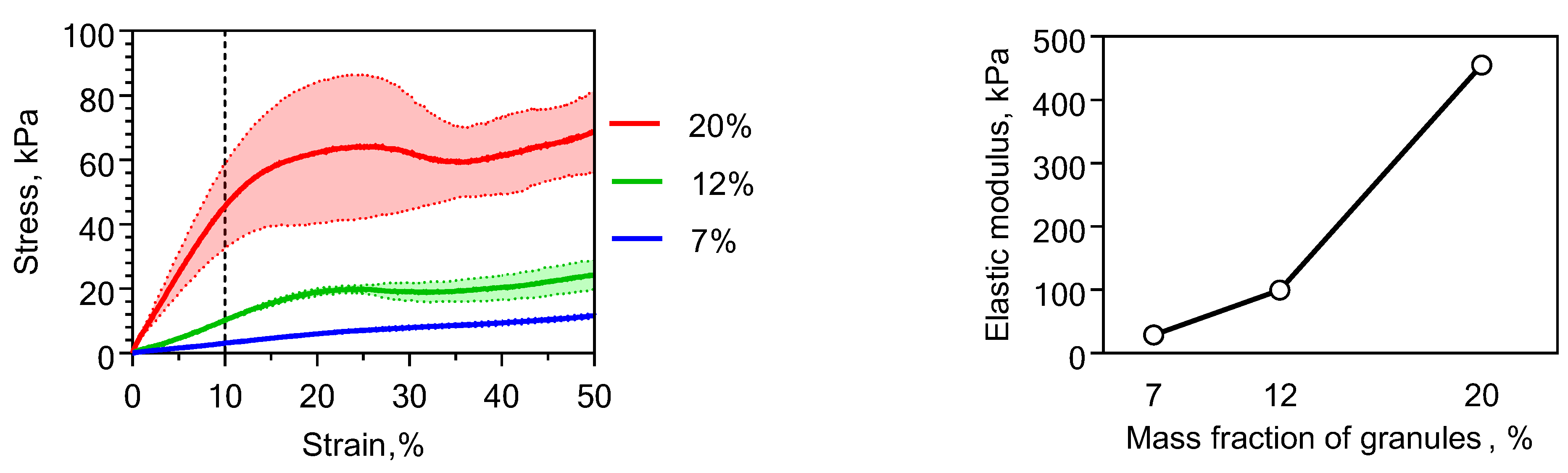
2.2. Physical Properties of Chitosan-Based Materials
2.3. Induction of Orthotopic Bone Formation
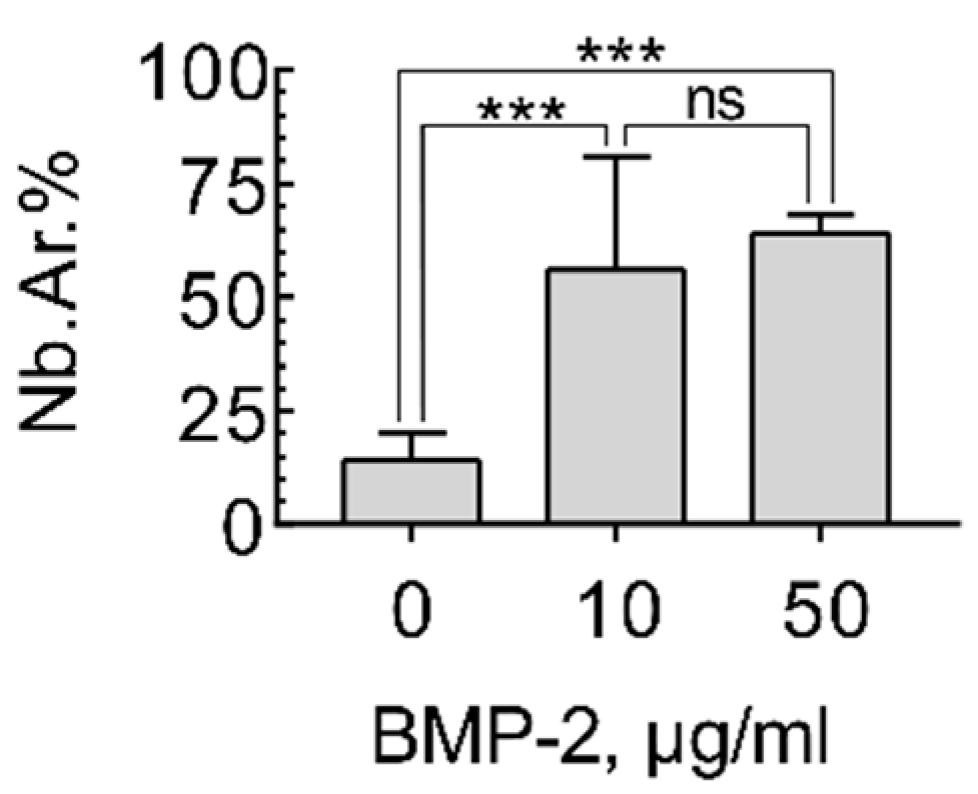
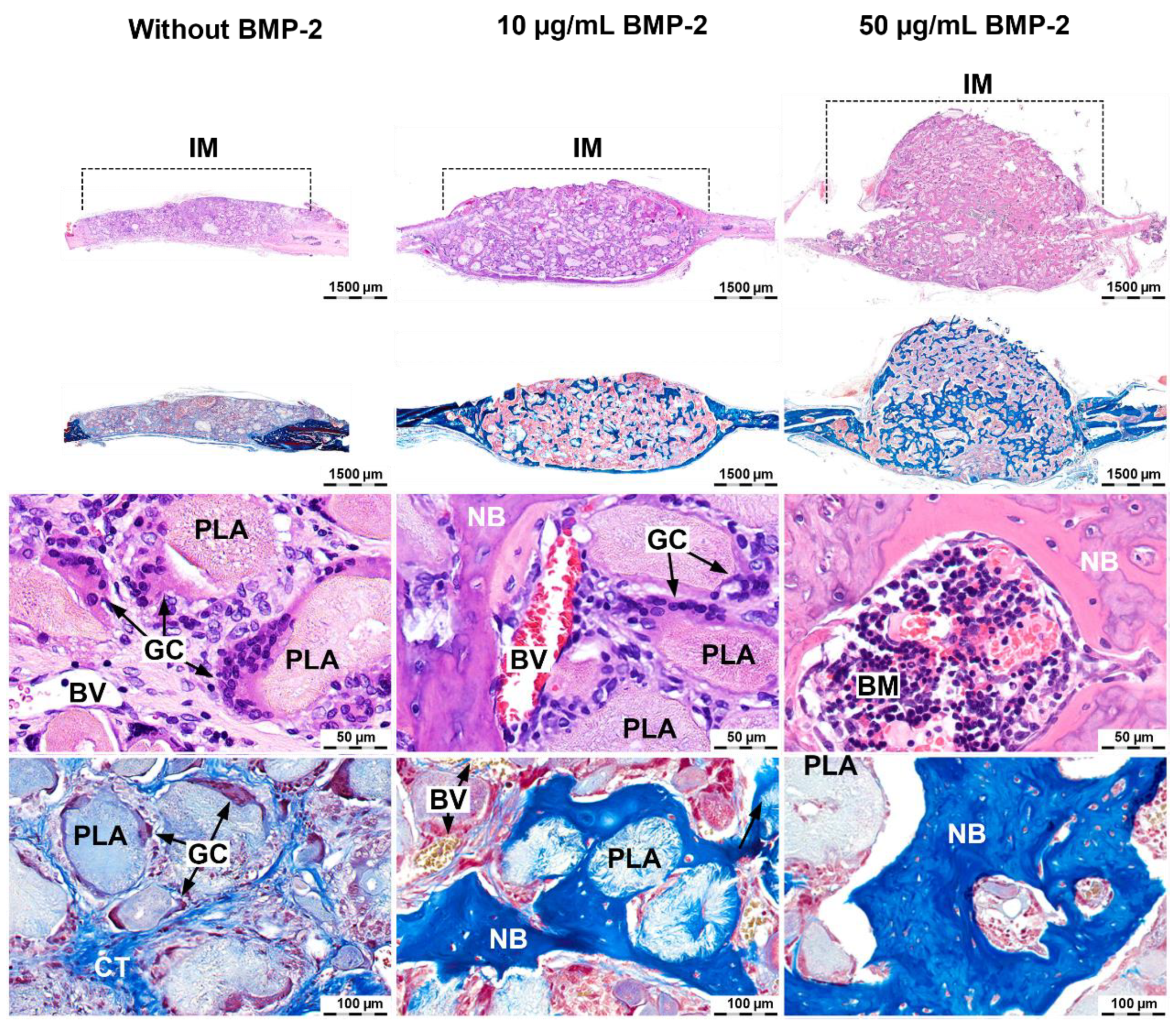
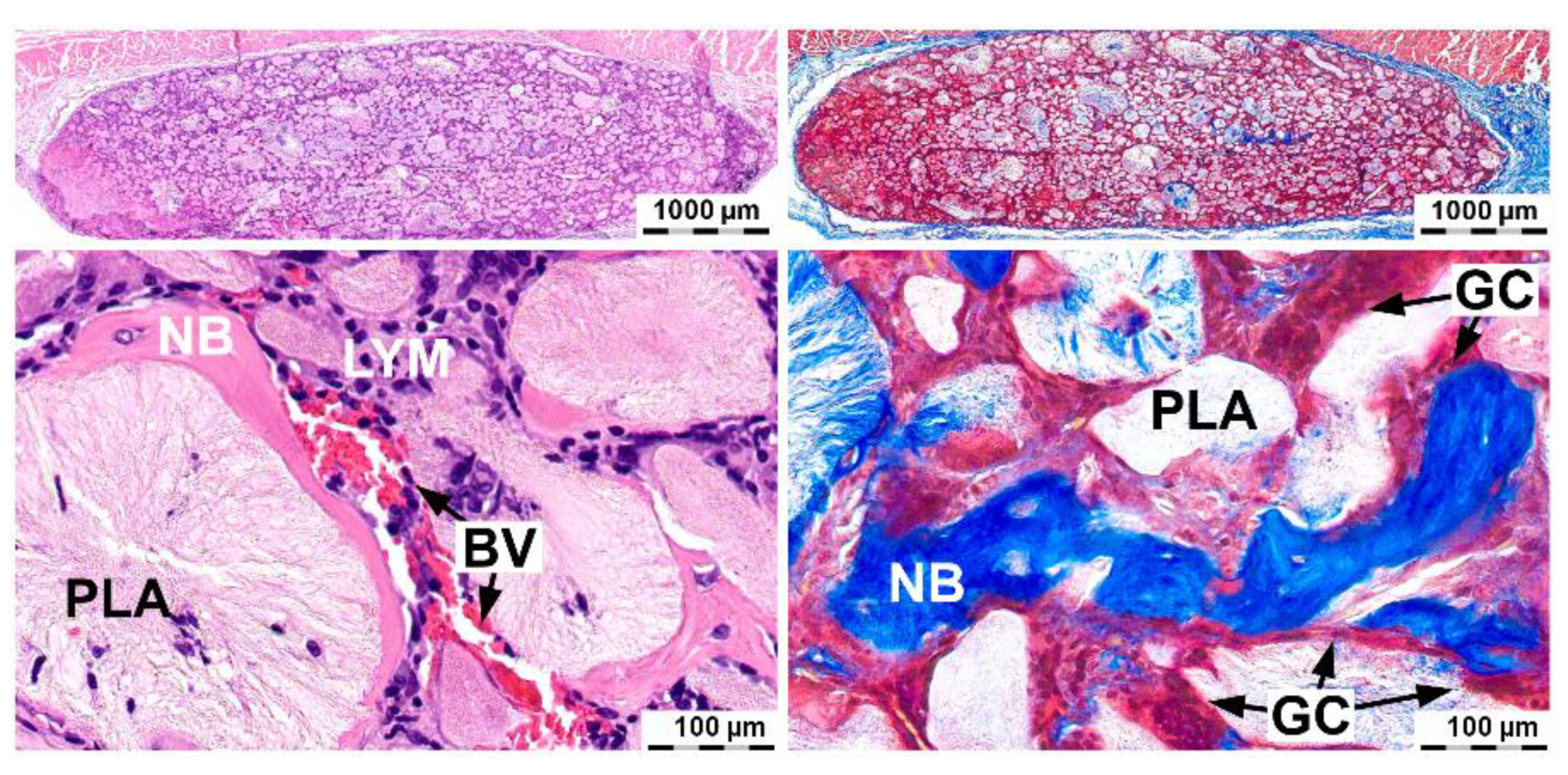
2.4. Induction of Ectopic Bone Formation
3. Discussion
4. Materials and Methods
4.1. Chitosan Hydrogel
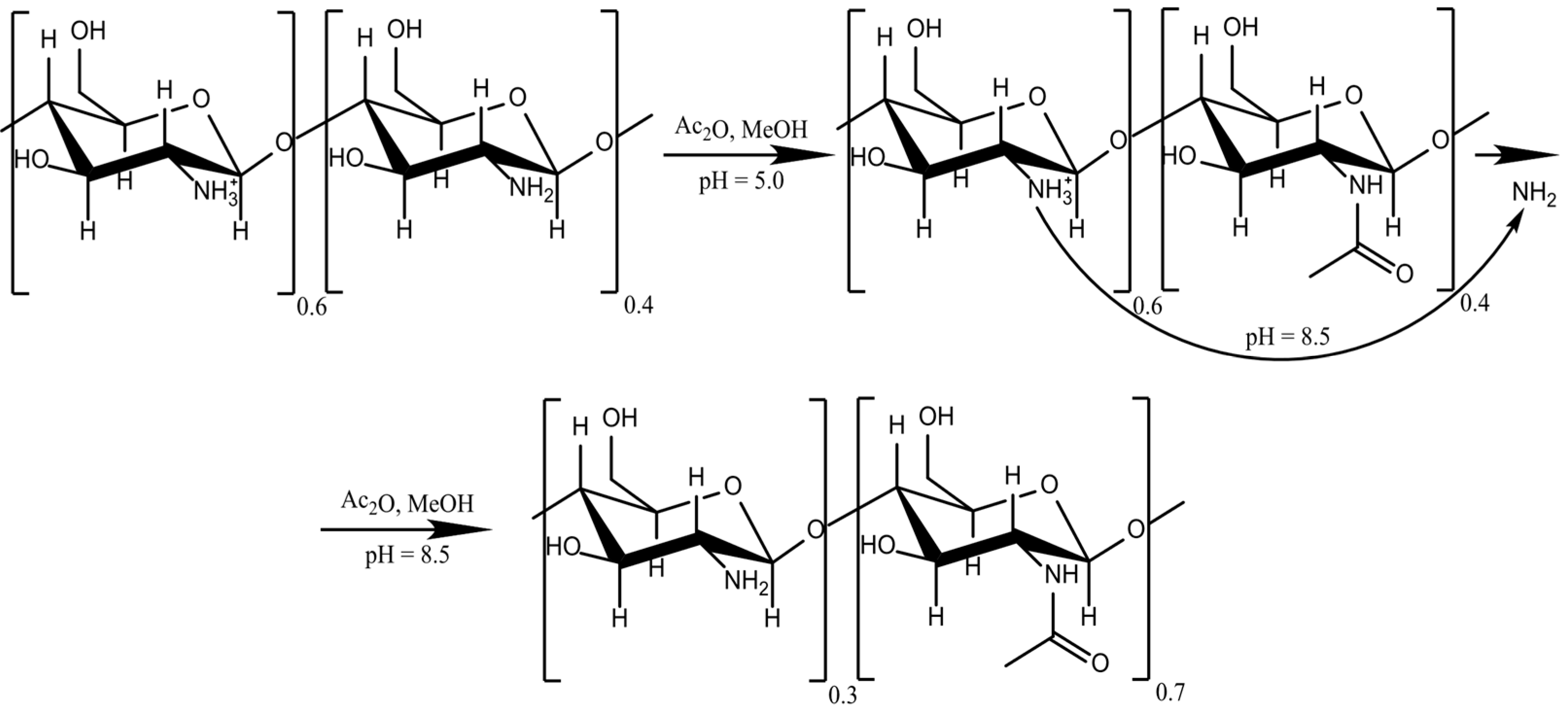
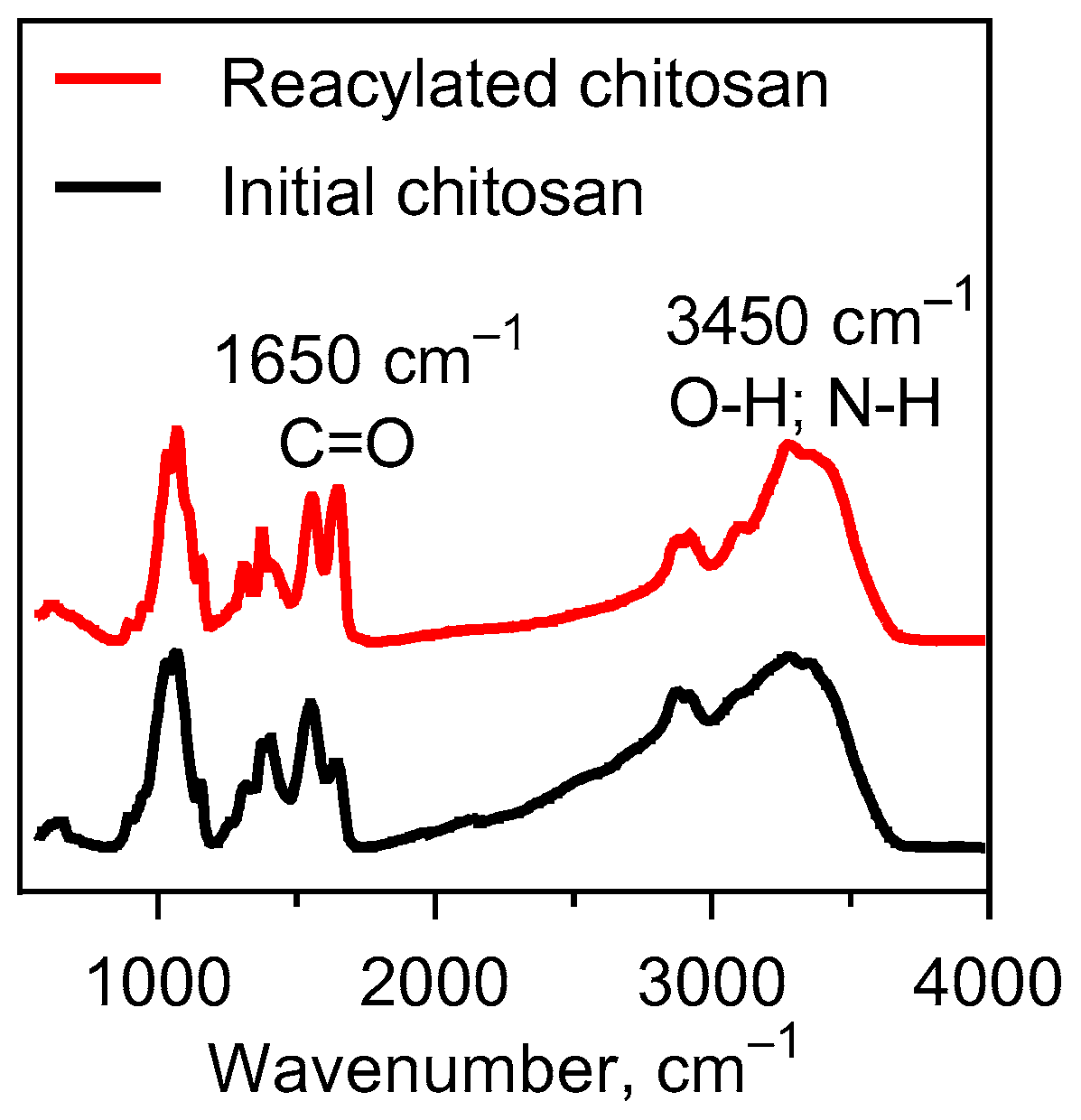
4.2. Chitosan Reacetylation
4.3. Highly Porous Polylactide (PLA) Granules
4.4. BMP-2 Impregnation
4.5. Chitosan Hydrogel Filled with PLA Granules
4.6. Material Mechanical Testing
4.7. In Vitro Studies
4.8. In Vivo Studies
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benzel, E.C.; Francis, T.B. Spine Surgery: Techniques, Complication Avoidance, and Management; Elsevier/Saunders: Philadelphia, PA, USA, 2012; ISBN 978-1-4557-2332-4. [Google Scholar]
- Fonseca, R.J. Oral and Maxillofacial Surgery; Elsevier/Saunders: St. Louis, MO, USA, 2020; ISBN 978-0-323-44442-2. [Google Scholar]
- Kulakov, A.A.; Gvetadze, R.S.; Brailovskayav, T.V.; Kharkova, A.A.; Dzikovitskaya, L.S. Modern approaches to the use of the method of dental implantation for atrophy and defects in the bone tissue of the jaws. Stomatologiya 2017, 96, 43–45. [Google Scholar]
- Dental Bone Graft Substitutes Market by Material (Demineralized Bone Matrix, Autograft, Allograft, Xenograft, and Synthetic Bone Graft Substitute), by Application (Ridge Augmentation, Socket Preservation, Periodontal Defect Regeneration, Implant Bone Regeneration, Sinus Lift, and Others), by Product (Bio-Oss, Osteograf, Grafton, and Others), and by End-User (Hospitals, Ambulatory Surgical Centers, and Dental Clinics): Global Industry Perspective, Comprehensive Analysis, and Forecast, 2018–2025. Zion Market Research (New York). 2019, p. 110. Available online: https://www.zionmarketresearch.com/report/dental-bone-graft-substitutes-market (accessed on 28 June 2019).
- Deb, S.; Chana, S. Biomaterials in relation to dentistry. In Frontiers of Oral Biology; Deb, S., Ed.; Karger: Basel, Switzerland, 2015; Volume 17, pp. 1–12. ISBN 978-3-318-02460-9. [Google Scholar]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.; Korolenkova, M.V.; Zorina, O.A.; Chvalun, S.N.; Goldshtein, D.V.; Kulakov, A.A. Development prospects of curable osteoplastic materials in dentistry and maxillofacial surgery. Heliyon 2020, 6, e04686. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Verné, E.; Bruno, M.; Miola, M.; Maina, G.; Bianco, C.; Cochis, A.; Rimondini, L. Composite bone cements loaded with a bioactive and ferrimagnetic glass-ceramic: Leaching, bioactivity and cytocompatibility. Mater. Sci. Eng. C 2015, 53, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Kuznetsova, V.S.; Vasilyev, A.V.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; Buharova, T.B.; Goldshtein, D.V.; Kulakov, A.A. Safety and efficiency of application of morphogenetic proteins of bone 2 and 7 in dentistry. Stomatologiya 2017, 98, 66–72. [Google Scholar]
- Vasilyev, A.V.; Bukharova, T.B.; Kuznetsova, V.S.; Zagoskin, Y.D.; Minaeva, S.A.; Grigoriev, T.E.; Antonov, E.N.; Osidak, E.O.; Galitsyna, E.V.; Babichenko, I.I.; et al. Comparison of impregnated bone morphogenetic protein-2 release kinetics from biopolymer scaffolds. Inorg. Mater. Appl. Res. 2019, 10, 1093–1100. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Galitsina, E.V.; Fatkhudinova, N.L.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; et al. Osteoinductive potential of highly porous polylactide granules and Bio-Oss impregnated with low doses of BMP-2. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Hsu, S.; Whu, S.W.; Tsai, C.-L.; Wu, Y.-H.; Chen, H.-W.; Hsieh, K.-H. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a biomaterial: Influence of degree of deacetylation on its physiochemical, material and biological properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [PubMed]
- Mathaba, M.; Daramola, M.O. Effect of chitosan’s degree of deacetylation on the performance of PES membrane infused with chitosan during AMD treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.C.; Piazza, R.D.; Marques, R.C.; Gabriela, M.; Campos, N. Successful encapsulation of hydrophilic drug in poly (lactic acid)/chitosan core/ shell nanoparticles. Int. J. Nanotechnol. Nanomed. 2019, 4. [Google Scholar] [CrossRef]
- Hamilton, V.; Yuan, Y.; Rigney, D.A.; Puckett, A.D.; Ong, J.L.; Yang, Y.; Elder, S.H.; Bumgardner, J.D. Characterization of chitosan films and effects on fibroblast cell attachment and proliferation. J. Mater. Sci. Mater. Med. 2006, 17, 1373–1381. [Google Scholar] [CrossRef]
- Chou, C.-M.; Mi, F.-L.; Horng, J.-L.; Lin, L.-Y.; Tsai, M.-L.; Liu, C.-L.; Lu, K.-Y.; Chu, C.-Y.; Chen, Y.-T.; Lee, Y.-L.A.; et al. Characterization and toxicology evaluation of low molecular weight chitosan on zebrafish. Carbohydr. Polym. 2020, 240, 116164. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Grigoriev, T.E.; Zagoskin, Y.D.; Belousov, S.I.; Vasilyev, A.V.; Bukharova, T.B.; Leonov, G.E.; Galitsyna, E.V.; Goldshtein, D.V.; Chvalun, S.N.; Kulakov, A.A.; et al. Influence of molecular characteristics of chitosan on properties of in situ formed scaffolds. BioNanoScience 2017, 7, 492–495. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Zhang, N.; Tan, H.; Gao, C. Preparation and properties of an injectable scaffold of poly(lactic-co-glycolic acid) microparticles/chitosan hydrogel. J. Mech. Behav. Biomed. Mater. 2008, 1, 352–359. [Google Scholar] [CrossRef]
- Hong, Y.; Gong, Y.; Gao, C.; Shen, J. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: Injectable scaffold for cartilage regeneration. J. Biomed. Mater. Res. Part A 2008, 85A, 628–637. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Susin, C.; Lee, J.; Fiorini, T.; Bisch, F.C.; Dixon, D.R.; McPherson, J.C.; Buxton, A.N.; Wikesjö, U.M.E. Effect of rhBMP-2 dose on bone formation/maturation in a rat critical-size calvarial defect model. J. Clin. Periodontol. 2014, 41, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; Kulakov, A.A. Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels. Molecules 2021, 26, 261. https://doi.org/10.3390/molecules26020261
Vasilyev AV, Kuznetsova VS, Bukharova TB, Grigoriev TE, Zagoskin YD, Nedorubova IA, Babichenko II, Chvalun SN, Goldstein DV, Kulakov AA. Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels. Molecules. 2021; 26(2):261. https://doi.org/10.3390/molecules26020261
Chicago/Turabian StyleVasilyev, Andrey Vyacheslavovich, Valeriya Sergeevna Kuznetsova, Tatyana Borisovna Bukharova, Timofei Evgenevich Grigoriev, Yuriy Dmitrievich Zagoskin, Irina Alekseevna Nedorubova, Igor Ivanovich Babichenko, Sergey Nicolaevich Chvalun, Dmitry Vadimovich Goldstein, and Anatoliy Alekseevich Kulakov. 2021. "Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels" Molecules 26, no. 2: 261. https://doi.org/10.3390/molecules26020261
APA StyleVasilyev, A. V., Kuznetsova, V. S., Bukharova, T. B., Grigoriev, T. E., Zagoskin, Y. D., Nedorubova, I. A., Babichenko, I. I., Chvalun, S. N., Goldstein, D. V., & Kulakov, A. A. (2021). Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels. Molecules, 26(2), 261. https://doi.org/10.3390/molecules26020261





