Preparation, Bioactivities and Applications in Food Industry of Chitosan-Based Maillard Products: A Review
Abstract
1. Introduction
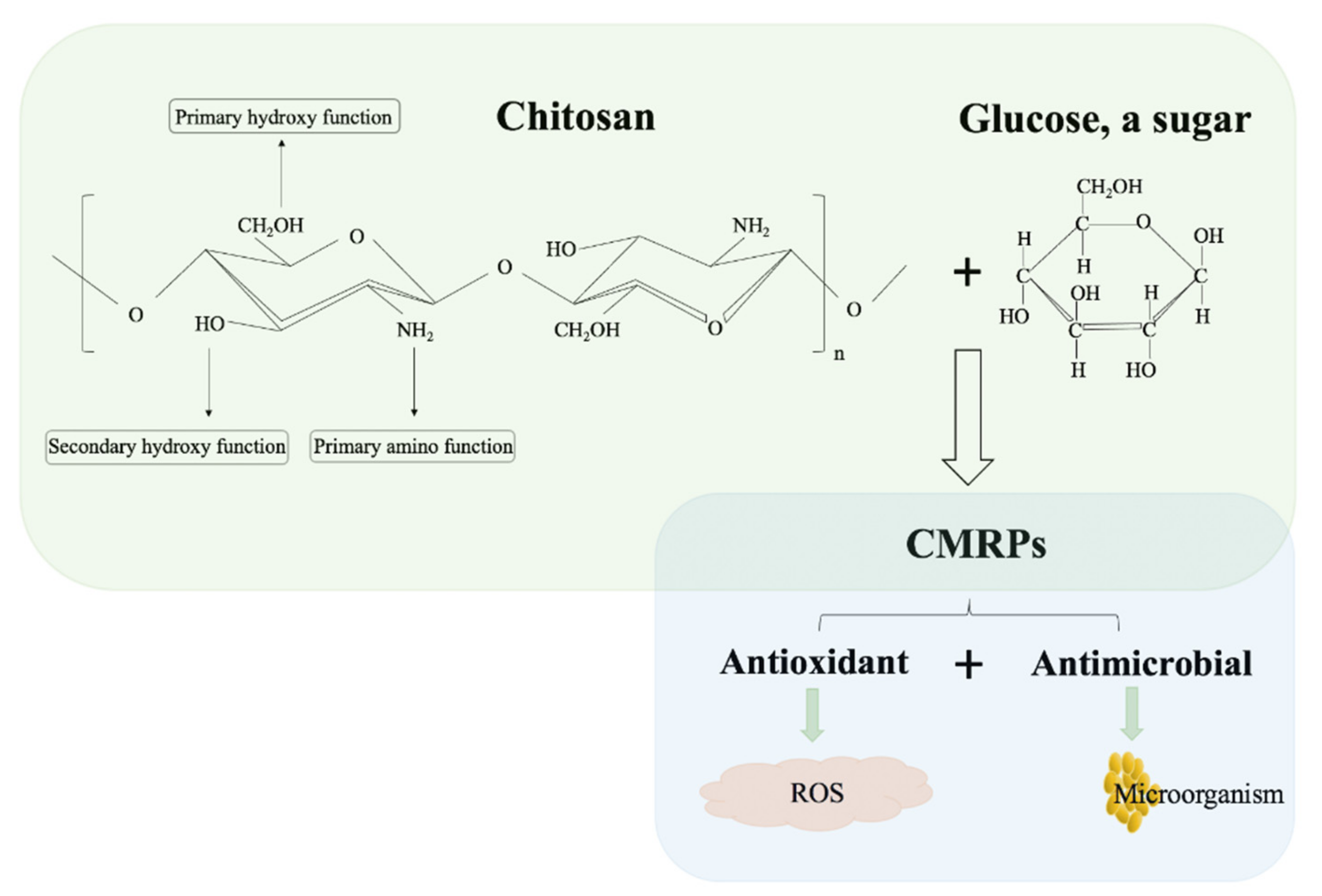
2. Chitosan
2.1. Physicochemical Characteristics
2.2. Biological Activities
2.3. Other Applications of Chitosan
3. Preparation and Identification of Chitosan-Based Maillard Products
3.1. The Preparation of CMRPs
3.2. Methods Used for the Identification of CMRP Formation
3.2.1. UV Absorbance and Fluorescence Intensity
3.2.2. Fourier Transform Infrared (FTIR) Analysis
3.2.3. High Performance Liquid Chromatography-Size Exclusion Chromatography Analysis (HPLC-SEC)
3.2.4. Color Measurements
3.2.5. Colloid Titration Method
3.2.6. Proton Nuclear Magnetic Resonance (1H-NMR)
4. Biological Activities of CMRPs
4.1. Enhanced Antimicrobial Activity
4.2. Enhanced Antioxidant Activity
5. Applications of CMRPs
5.1. Application in Food Storage
5.2. Application in Health Foods or Medicine
6. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, P.K.; Duta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar] [CrossRef]
- García-Bermejo, A.B.; Cardelle-Cobas, A.; Ruiz-Matute, A.I.; Montañés, F.; Olano, A.; Corzo, N. Effect of drying methods on the reactivity of chitosan towards Maillard reaction. Food Hydrocoll. 2012, 29, 27–34. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, F.; Liu, F.; Wang, Y.; Gao, Y. Structure and antimicrobial mechanism of ε-polylysine-chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014, 70, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 7b04528. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; Des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv. Polym. Sci. 2011, 244. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Koc, B.; Mujtaba, M.; Ilk, S.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Yildiz, A. Diatomite as a novel composite ingredient for chitosan film with enhanced physicochemical properties. Int. J. Biol. Macromol. 2017, S014181301731841X. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chou, C.C.; Li, C.F. Preparation, water solubility and rheological property of the N-alkylated mono or disaccharide chitosan derivatives. Food Res. Int. 2002, 35, 713. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Zhao, Y. High intensity ultrasound assisted heating to improve solubility, antioxidant and antibacterial properties of chitosan-fructose Maillard reaction products. LWT Food Sci. Technol. 2015, 60, 253–262. [Google Scholar] [CrossRef]
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Gullón, B.; Montenegro, M.I.; Ruiz-Matute, A.I.; Cardelle-Cobas, A.; Corzo, N.; Pintado, M.E. Synthesis, optimization and structural characterization of a chitosan-glucose derivative obtained by the Maillard reaction. Carbohydr. Polym. 2016, 137, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Acyl modified chitosan derivatives for oral delivery of insulin and curcumin. J. Mater. Sci. Mater. Med. 2010, 21, 2133–2140. [Google Scholar] [CrossRef]
- Kamboj, S.; Singh, K.; Tiwary, A.K.; Rana, V. Optimization of microwave assisted Maillard reaction to fabricate and evaluate corn fiber gum-chitosan IPN films. Food Hydrocoll. 2015, 44, 260–276. [Google Scholar] [CrossRef]
- Terbojevich, M.; Cosani, A.; Muzzarelli, R.A.A. Molecular parameters of chitosans depolymerized with the aid of papain. Carbohydr. Polym. 1996, 29, 63–68. [Google Scholar] [CrossRef]
- Zhang, J.; Mei, Z.; Huang, X.; Ding, Y.; Liang, Y.; Mei, Y. Inhibition of Maillard reaction in production of low-molecular-weight chitosan by enzymatic hydrolysis. Carbohydr. Polym. 2020, 236, 116059. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Nácher, A.; Díez-Sales, O.; Merino-Sanjuán, M.; Fadda, A.M.; Manconi, M. Chitosan-xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J. Microencapsul. 2014, 31, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Falah, F.; Zareie, Z.; Tabatabaei Yazdi, F.; Shahidi, F.; Mortazavi, S.A. Antioxidant potential and antimicrobial activity of chitosan–inulin conjugates obtained through the Maillard reaction. Food Sci. Biotechnol. 2019, 28, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Yeh, J.Y.; Tsai, C.F. Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the Maillard reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef] [PubMed]
- Vanden Braber, N.L.; Díaz Vergara, L.I.; Morán Vieyra, F.E.; Borsarelli, C.D.; Yossen, M.M.; Vega, J.R.; Correa, S.G.; Montenegro, M.A. Physicochemical characterization of water-soluble chitosan derivatives with singlet oxygen quenching and antibacterial capabilities. Int. J. Biol. Macromol. 2017, 102, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Nasri, R.; Ben Azaza, Y.; Li, S.; Nasri, M. Conception of novel blue crab chitosan films crosslinked with different saccharides via the Maillard reaction with improved functional and biological properties. Carbohydr. Polym. 2020, 241, 116303. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 11–27. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Tazdait, D.; Abdi, N.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Assessment of coating tomato fruit with shrimp shell chitosan and N,O-carboxymethyl chitosan on postharvest preservation. J. Food Meas. Charact. 2013, 7, 66–74. [Google Scholar] [CrossRef]
- Rashmi, S.H.; Biradar, B.; Maladkar, K.; Kittur, A.A. Extraction of chitin from prawn shell and preparation of chitosan. Res. J. Chem. Environ. Sci. 2016, 4, 70–73. [Google Scholar]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccines Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef]
- Chawla, S.P.; Kanatt, S.R.; Sharma, A.K. Chitosan. In Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: Berlin, Germany, 2015; ISBN 9783319162980. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Kara, A.; Stevens, R. Characterisation of biscuit fired bone china body microstructure. Part I: XRD and SEM of crystalline phases. J. Eur. Ceram. Soc. 2002, 22, 731–736. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Arca, H.Ç.; Günbeyaz, M.; Şenel, S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 2009, 8, 937–953. [Google Scholar] [CrossRef]
- Ma, J.; Xin, C.; Tan, C. Preparation, physicochemical and pharmaceutical characterization of chitosan from Catharsius molossus residue. Int. J. Biol. Macromol. 2015, 80, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Maji, T.K.; Maji, T.K. Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Rev. Téc. Ing. Univ. Zulia 2014, 2, 4–12. [Google Scholar]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3568–3580. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Sugimoto, M.; Morimoto, M.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr. Polym. 1998, 36, 49–59. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 10–52. [Google Scholar] [CrossRef]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Zamani, A.; Taherzadeh, M.J. Production of low molecular weight chitosan by hot dilute sulfuric acid. BioResources 2010, 5, 1554–1564. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2844. [Google Scholar] [CrossRef]
- Sekiguchi, S.; Miura, Y.; Kaneko, H.; Nishimura, S.I.; Nishi, N.; Iwase, M.; Tokura, S. Molecular Weight Dependency of Antimicrobial Activity by Chitosan Oligomers. Food Hydrocoloidsl. US 1994, 71–76. [Google Scholar] [CrossRef]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of Chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.J.; Zhang, S.L.; Shieh, P.L. Antimicrobial Activity of a Low-Molecular-Weight Chitosan Obtained from Cellulase Digestion of Chitosan. J. Food Prot. 2004, 67, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Castro Marín, A.; Culcasi, M.; Cassien, M.; Stocker, P.; Thétiot-Laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. 2019, 285, 67–76. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Thinesh, T.; Selvin, J.; Selvan, K.M.; Shanmugam, V.; Shanmugam, A. Characterization of bioactive chitosan and sulfated chitosan from Doryteuthis singhalensis (Ortmann, 1891). Int. J. Biol. Macromol. 2017, 99, 682–691. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Negm, N.A.; Kana, M.T.H.A.; Abubshait, S.A.; Betiha, M.A. Effectuality of chitosan biopolymer and its derivatives during antioxidant applications. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan Membrane as a Wound-Healing Dressing: Characterization and Clinical Application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Torchilin, V.P. Nanocarriers. Pharm. Res. 2007, 24, 2333–2334. [Google Scholar] [CrossRef]
- Nomanbhay, S.M.; Palanisamy, K. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005, 8, 43–53. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Sobeh, M.; Majdoub, H.; Yasri, A. Antioxidant activity improvement of apples juice supplemented with chitosan-galactose Maillard reaction products. Molecules 2019, 24, 4557. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan-glucose conjugates: Influence of extent of maillard reaction on antioxidant properties. J. Agric. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Bao, E.; Zhao, Y. Preparation, characterization and toxicology properties of α- and β-chitosan Maillard reaction products nanoparticles. Int. J. Biol. Macromol. 2016, 89, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Lin, J.; Chen, X.M. Effect of chitosan molecular weight on the functional properties of chitosan-maltose Maillard reaction products and their application to fresh-cut Typha latifolia L. Carbohydr. Polym. 2014, 102, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-oxidant and anti-diabetes potential of water-soluble chitosan-glucose derivatives produced by maillard reaction. Polymers 2019, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.X.; Li, J.; Li, M.; Guo, X.N.; Peng, W.; Zhou, H.M. Functional properties of chitosan-xylose Maillard reaction products and their application to semi-dried noodle. Carbohydr. Polym. 2013, 92, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Kawai, S. Modification of chitosan by the Maillard reaction using cellulose model compounds. Carbohydr. Polym. 2007, 68, 242–248. [Google Scholar] [CrossRef]
- Arata Badano, J.; Vanden Braber, N.; Rossi, Y.; Díaz Vergara, L.; Bohl, L.; Porporatto, C.; Falcone, R.D.; Montenegro, M. Physicochemical, in vitro antioxidant and cytotoxic properties of water-soluble chitosan-lactose derivatives. Carbohydr. Polym. 2019, 224, 115258. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Jiang, W.; Du, Q.; Yu, C.; Sun, Q.; Zhang, S. An Enhanced Stability Nanoparticle Preparation by Corn Protein Hydrolysate-Carboxymethyl Chitosan Maillard Conjugates Loaded with Rutin. J. Food Sci. 2019. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Li, J.; Ma, F.K.; Dang, Q.F.; Liang, X.G.; Chen, X.G. Glucose-conjugated chitosan nanoparticles for targeted drug delivery and their specific interaction with tumor cells. Front. Mater. Sci. 2014, 8, 363–372. [Google Scholar] [CrossRef]
- Morales, F.J.; Van Boekel, M.A.J.S. A study on advanced maillard reaction in heated casein/sugar solutions: Fluorescence accumulation. Int. Dairy J. 1997, 7, 683. [Google Scholar] [CrossRef]
- Jing, H.; Kitts, D.D. Chemical and biochemical properties of casein-sugar Maillard reaction products. Food Chem. Toxicol. 2002, 40, 1015. [Google Scholar] [CrossRef]
- Rao, M.S.; Chawla, S.P.; Chander, R.; Sharma, A. Antioxidant potential of Maillard reaction products formed by irradiation of chitosan-glucose solution. Carbohydr. Polym. 2011, 83, 714–719. [Google Scholar] [CrossRef]
- Zaja̧c, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocoll. 2019. [Google Scholar] [CrossRef]
- Umemura, K.; Kawai, S. Preparation and characterization of maillard reacted chitosan films with hemicellulose model compounds. J. Appl. Polym. Sci. 2008, 108, 2481–2487. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tsai, C.F.; Li, C.F. Preparation and characterization of water-soluble chitosan produced by Maillard reaction. Fish. Sci. 2006, 72, 1096–1103. [Google Scholar] [CrossRef]
- Ying, G.Q.; Xiong, W.Y.; Wang, H.; Sun, Y.; Liu, H.Z. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr. Polym. 2011, 83, 1787–1796. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; Fang, J.; Deng, Y.; Wang, D.; Zhao, Y. Optimization of the fermentation conditions of Rhizopus japonicus M193 for the production of chitin deacetylase and chitosan. Carbohydr. Polym. 2014, 101, 57–67. [Google Scholar] [CrossRef]
- Zhang, L.; Dudhani, A.; Lundin, L.; Kosaraju, S.L. Macromolecular conjugate based particulates: Preparation, characterisation and evaluation of controlled release properties. Eur. Polym. J. 2009, 45, 1960–1969. [Google Scholar] [CrossRef]
- Jiang, Z.; Brodkorb, A. Structure and antioxidant activity of Maillard reaction products from α-lactalbumin and β-lactoglobulin with ribose in an aqueous model system. Food Chem. 2012, 133, 960–968. [Google Scholar] [CrossRef]
- Jiang, Z.; Rai, D.K.; O’Connor, P.M.; Brodkorb, A. Heat-induced Maillard reaction of the tripeptide IPP and ribose: Structural characterization and implication on bioactivity. Food Res. Int. 2013, 50, 266–274. [Google Scholar] [CrossRef][Green Version]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard reaction products as antimicrobial components for packaging films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, X.; Huang, Z.; Jia, S.; Zhang, Y.; Shi, J.; Hong, H.; Feng, L.; Luo, Y. Modification of gelatin hydrolysates from grass carp (Ctenopharyngodon idellus) scales by Maillard reaction: Antioxidant activity and volatile compounds. Food Chem. 2019. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Zhang, Y.; Ma, L.; Xu, X. Comparative studies on chemical parameters and antioxidant properties of stipes and caps of shiitake mushroom as affected by different drying methods. J. Sci. Food Agric. 2013, 93, 3107–3113. [Google Scholar] [CrossRef]
- Perez-Locas, C.; Yaylayan, V.A. The Maillard reaction and food quality deterioration. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9781845694951. [Google Scholar] [CrossRef]
- Zheng, X.; He, Y.; Zhou, H.; Xiong, C. Effects of Chitosan Oligosaccharide–Nisin Conjugates Formed by Maillard reaction on the preservation of Collichthys niveatus. J. Food Process. Preserv. 2019. [Google Scholar] [CrossRef]
- Huang, J.R.; Huang, C.Y.; Huang, Y.W.; Chen, R.H. Shelf-life of fresh noodles as affected by chitosan and its Maillard reaction products. LWT Food Sci. Technol. 2007, 40, 1291. [Google Scholar] [CrossRef]
- Do Amaral, D.S.; Cardelle-Cobas, A.; de Castro Querino Dias, C.; Lima, D.A.S.; de Ferreira Pereira, S.; de Oliveira Arcanjo, N.M.; Dalmás, P.S.; Madruga, M.S.; Pintado, M.M.E. Low fat goat meat sausage with chitosan-glucose maillard reaction product: Impact on quality and shelf life. Food Sci. Technol. 2020, 40. [Google Scholar] [CrossRef]
- Chang, H.L.; Chen, Y.C.; Tan, F.J. Antioxidative properties of a chitosan-glucose Maillard reaction product and its effect on pork qualities during refrigerated storage. Food Chem. 2011, 124, 589–595. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jing, Y.; Yan, F. Chitooligosaccharide–Lysine Maillard Reaction Products: Preparation and Potential Application on Fresh-Cut Kiwifruit. Food Bioprocess Technol. 2019, 12, 1133–1143. [Google Scholar] [CrossRef]
- Yan, F.; Yu, X.; Jing, Y. Optimized preparation, characterization, and antioxidant activity of chitooligosaccharide–glycine Maillard reaction products. J. Food Sci. Technol. 2018, 55, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.K.; Dwivedi, S. Diabetes and cardiovascular diseases. J. Int. Med. Sci. Acad. 2015. [Google Scholar] [CrossRef]
- Zhou, F.; Guo, J.; Han, X.; Gao, Y.; Chen, Q.; Huang, W.; Zhan, J.; Huang, D.; You, Y. Cranberry polyphenolic extract exhibits an antiobesity effect on high-fat diet-fed mice through increased thermogenesis. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Faber, M.A.; Pascal, M.; El Kharbouchi, O.; Sabato, V.; Hagendorens, M.M.; Decuyper, I.I.; Bridts, C.H.; Ebo, D.G. Shellfish allergens: Tropomyosin and beyond. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 842–848. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Wang, J.; Ni, S.; Wang, Y. Maillard reaction with ribose, galacto-oligosaccharide or chitosan-oligosaccharide reduced the allergenicity of shrimp tropomyosin by inducing conformational changes. Food Chem. 2019, 274, 789–795. [Google Scholar] [CrossRef]
| Materials Reacted with Chitosan | Stirring Methods | Heating Methods | Performance | References |
|---|---|---|---|---|
| Polylysine | Not specified | Dry-heated with different periods (0, 3, 6, 9 and 12 h) in a desiccator (80 °C, relative humidity: 79%) containing saturated potassium bromide solution at the bottom | Extension in the dry-heating time reduced the antibacterial activity of conjugates | [3] |
| Fructose | Not specified | Put in a test tube subjected for ultrasound-assisted water bath at 80 °C for 8 h | DPPH free radical scavenging capacity from UA heating were higher than those from water-bath heating alone | [13] |
| Glucose | Under shaking at 40 °C, 60 °C or 80 °C 100 rpm for different reaction time | The best conditions were 60 °C and 32 h of reaction. | [16] | |
| Inulin | Stirred for 30 min | Autoclaved for 15 min | The reaction time was reduced by using autoclave | [23] |
| Glucosamine hydrochloride | Under shaking at 65 °C for 48 h in an oven | After 48 h of heating, the complete CMRPs were obtained from the original chitosan | [25] | |
| Glucose, fructose, arabinose and xylose | Gentle stirred for 24 h at 25 °C | Heated for 24 h in an oven at approximatively 90 °C and an 30% relative humidity | Heat treatment did not affect their mechanical parameters, while decrease their hydrophobicity | [26] |
| Galactose | Stirring (other were not specified) | Put inside a test tubes and for heated for 3 h at 100 °C in water bath | Heated chitosan with 2% (w/v) of galactose for 3 h was the most efficiency concentration | [70] |
| Glucose | Not specified | Sealed in metal cans and heated at 98 °C up to 2 h | Increased all of these indices of the extent of Maillard reaction | [71] |
| Maltose | Not specified | Heated for 6 h at 100 °C in water bath | Prolonged heating time increased the DPPH free radical scavenging activity of CMRPs | [73] |
| Glucose | Not specified | Heated at 100 °C (using a water bath) or 121 °C (using an autoclave) for 1–4 h | Heating temperature at 121 °C had a higher efficiency than 100 °C in the formation of intermediates | [74] |
| Xylose | Not specified | Heated at 80 °C in water bath for up to 8 h | Higher chelating power was observed as the increase of heating time | [75] |
| Glucose and cellobiose | Stirred for 30 min at 25 °C | Dried in an oven at 50 °C for about 20 h | Not specified | [76] |
| Lactose | Under shaking at 70 °C, 120 rpm for 96 h | The reduction in the molecular weight | [77] | |
| Corn protein hydrolysate | Not specified | Incubated for 48 h in a desiccator (60 °C, relative humidity: 79%) containing saturated potassium bromide solution at the bottom | Under dry heating conditions, corn protein hydrolysate could be easier to undergo the Maillard reaction | [78] |
| Materials Reacted with Chitosan | Application Fields | Effect | References |
|---|---|---|---|
| Galactose | Food storage | Used as additives that improved the antioxidant quality of apple juice | [70] |
| Maltose | Food storage | Inhibited the discoloration and ascorbic acid content of fresh-cut Typha latifolia L. | [73] |
| Glucose | Health food or medicine | Exhibited higher anti-α-amylase and anti-α-glucosidase activities which could be used to treat Type 2 diabetes | [74] |
| Xylose | Food storage | Exhibited an extended shelf-life of semi-dried noodle for more than 7 days and limited the discoloration and darkening | [75] |
| Nisin | Health food or medicine | Exhibited anti-obesity effect by the alleviated microphage accumulation in mice liver and gut dysbiosis | [99] |
| Glucose | Food storage | Inhibited microbial growth and made the fresh goat sausages firmer and more stable | [100] |
| Glucose | Food storage | Inhibited lipid oxidation of fresh pork samples | [101] |
| Tropomyosin | Health food or medicine | Reduced the allergenicity of tropomyosin which is correlated with the reduction of α-helix and increase of β-sheet | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, Y.; Zhou, F.; Guo, J.; Tang, J.; Han, Y.; Li, Z.; Fu, C. Preparation, Bioactivities and Applications in Food Industry of Chitosan-Based Maillard Products: A Review. Molecules 2021, 26, 166. https://doi.org/10.3390/molecules26010166
Yang H, Zhang Y, Zhou F, Guo J, Tang J, Han Y, Li Z, Fu C. Preparation, Bioactivities and Applications in Food Industry of Chitosan-Based Maillard Products: A Review. Molecules. 2021; 26(1):166. https://doi.org/10.3390/molecules26010166
Chicago/Turabian StyleYang, Huijuan, Yuyu Zhang, Fang Zhou, Juanjuan Guo, Jiajie Tang, Yanqing Han, Zhanming Li, and Caili Fu. 2021. "Preparation, Bioactivities and Applications in Food Industry of Chitosan-Based Maillard Products: A Review" Molecules 26, no. 1: 166. https://doi.org/10.3390/molecules26010166
APA StyleYang, H., Zhang, Y., Zhou, F., Guo, J., Tang, J., Han, Y., Li, Z., & Fu, C. (2021). Preparation, Bioactivities and Applications in Food Industry of Chitosan-Based Maillard Products: A Review. Molecules, 26(1), 166. https://doi.org/10.3390/molecules26010166