Gallato Zirconium (IV) Phtalocyanine Complex Conjugated with SiO2 Nanocarrier as a Photoactive Drug for Photodynamic Therapy of Atheromatic Plaque
Abstract
1. Introduction
2. Results and Discussion
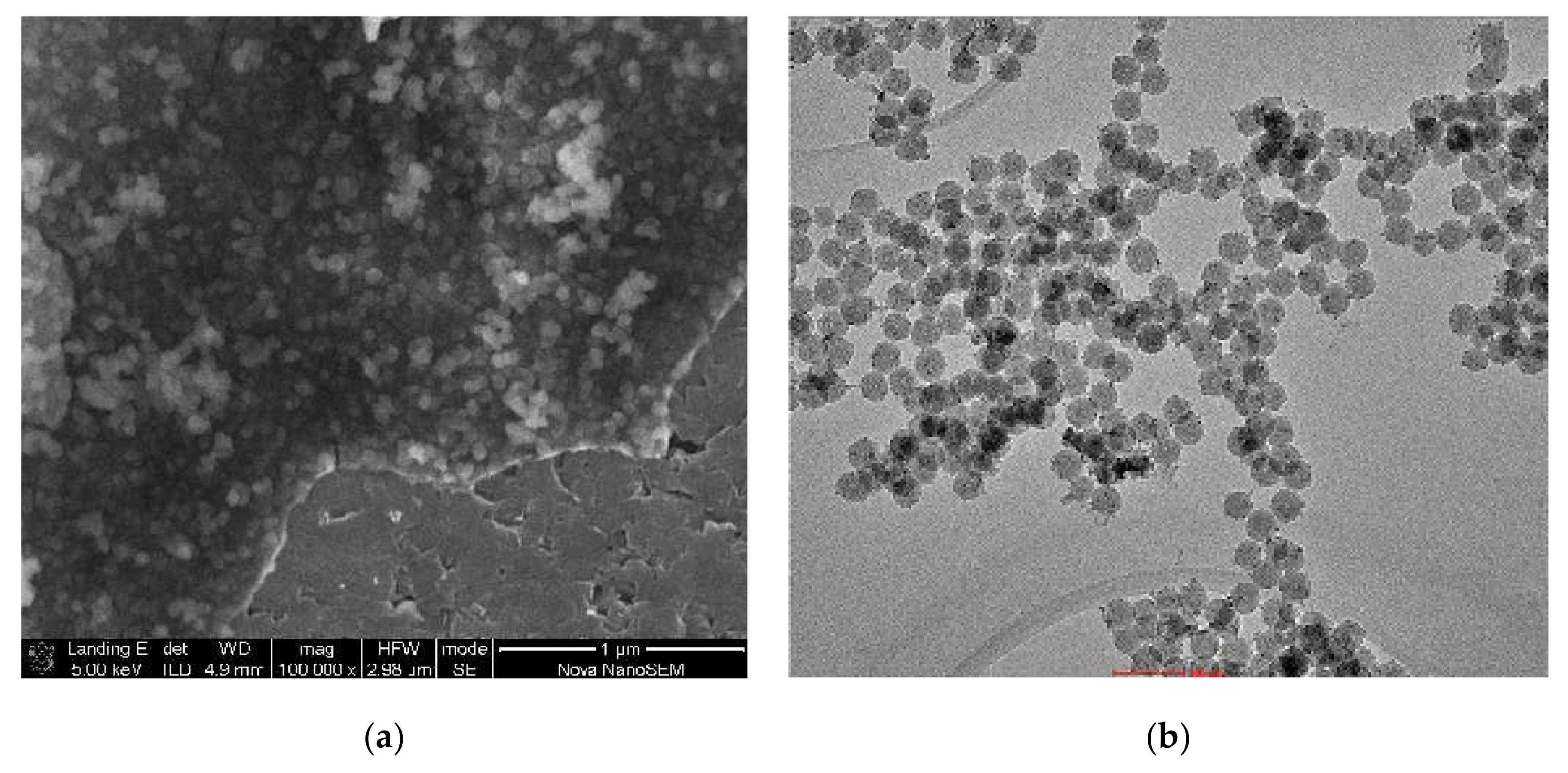
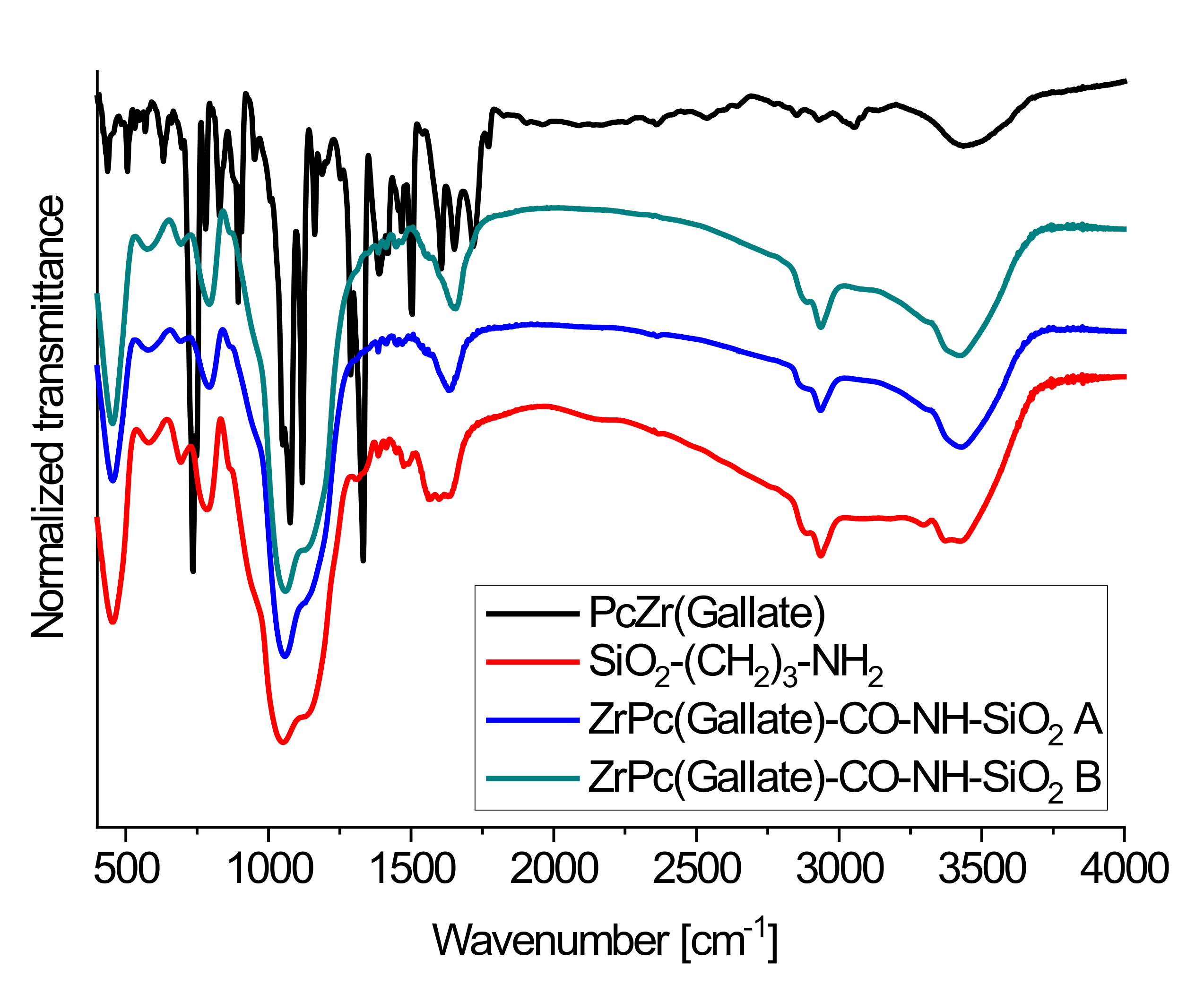
2.1. The Structure and Morphology of Obtained Conjugates
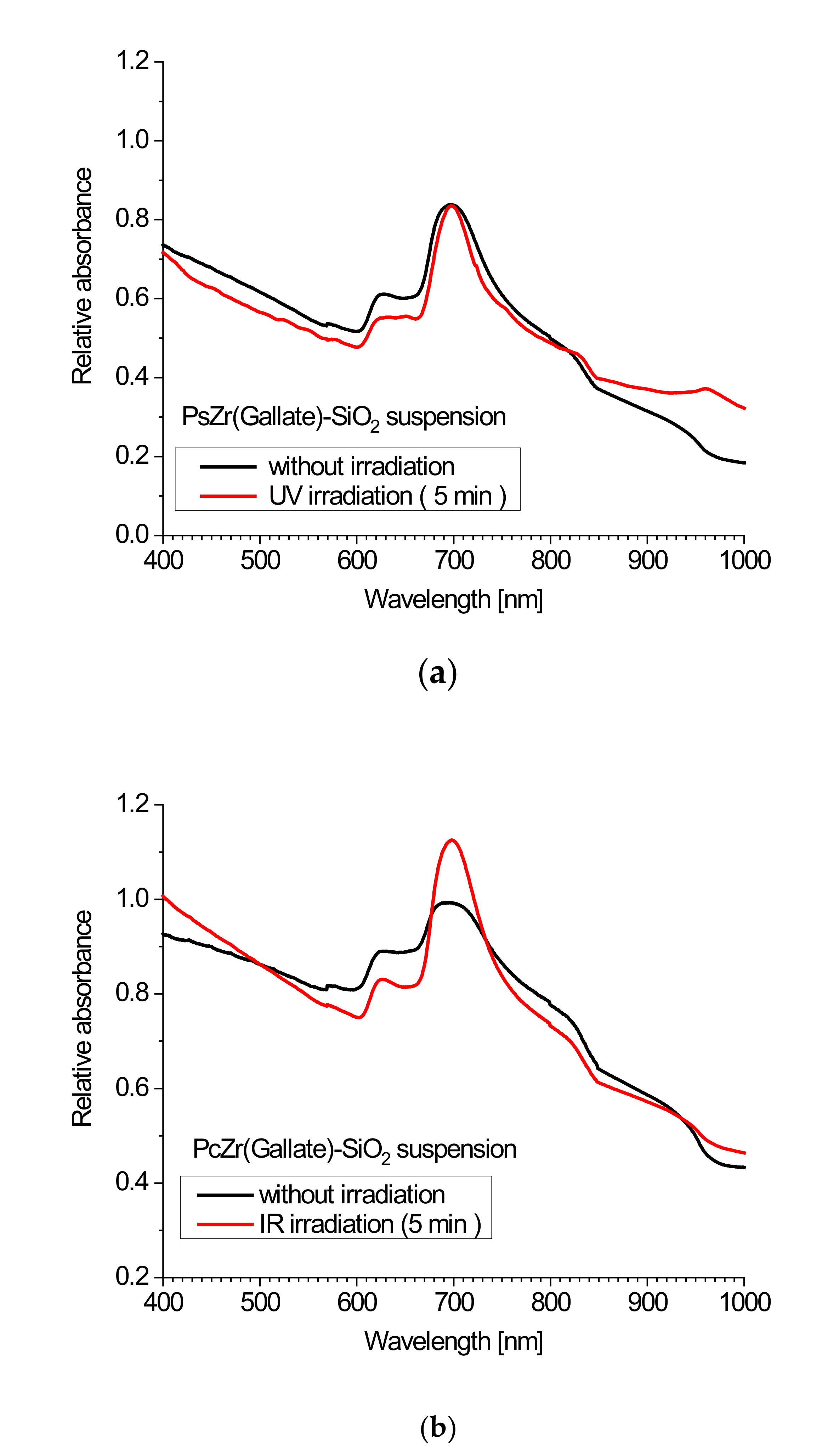
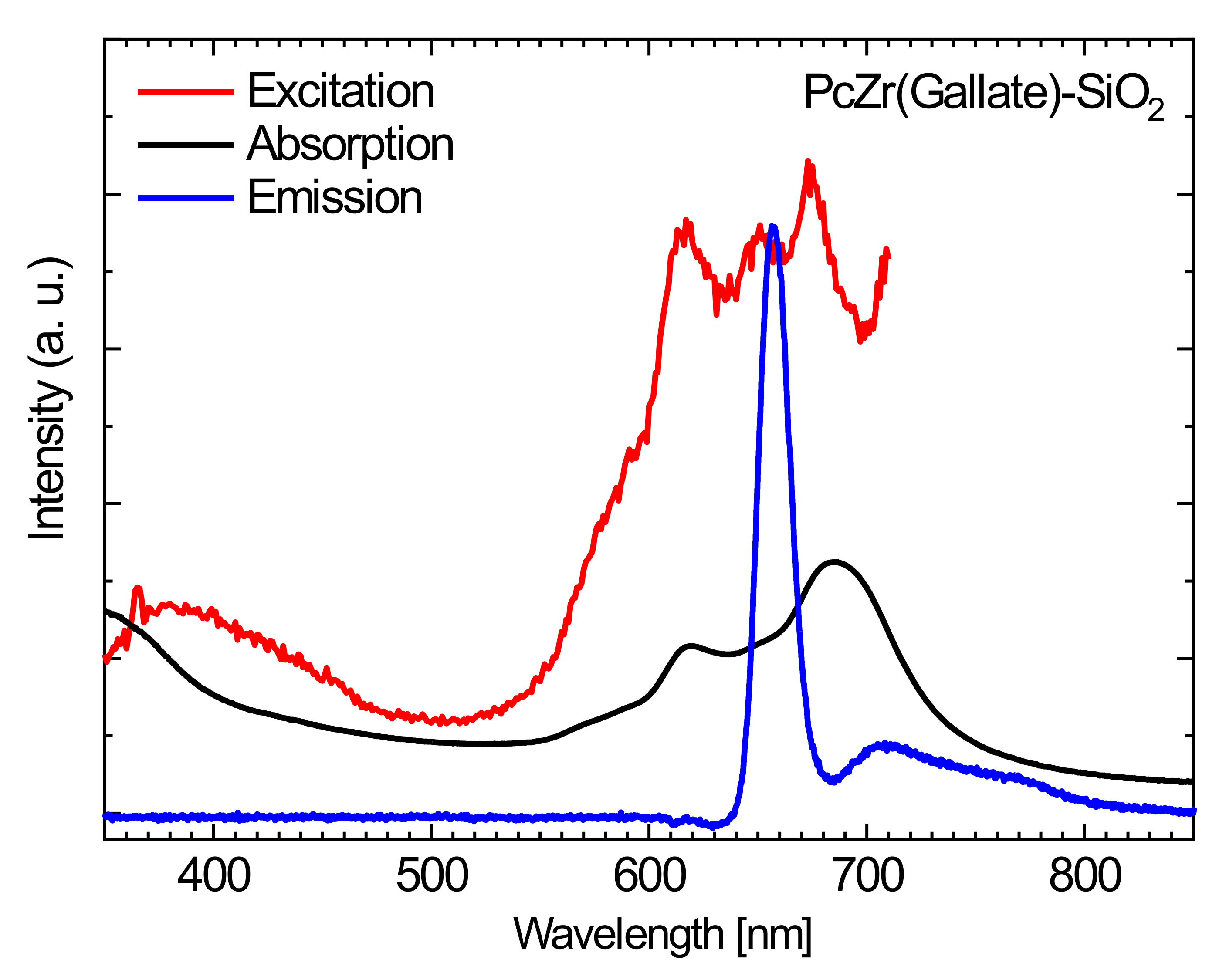
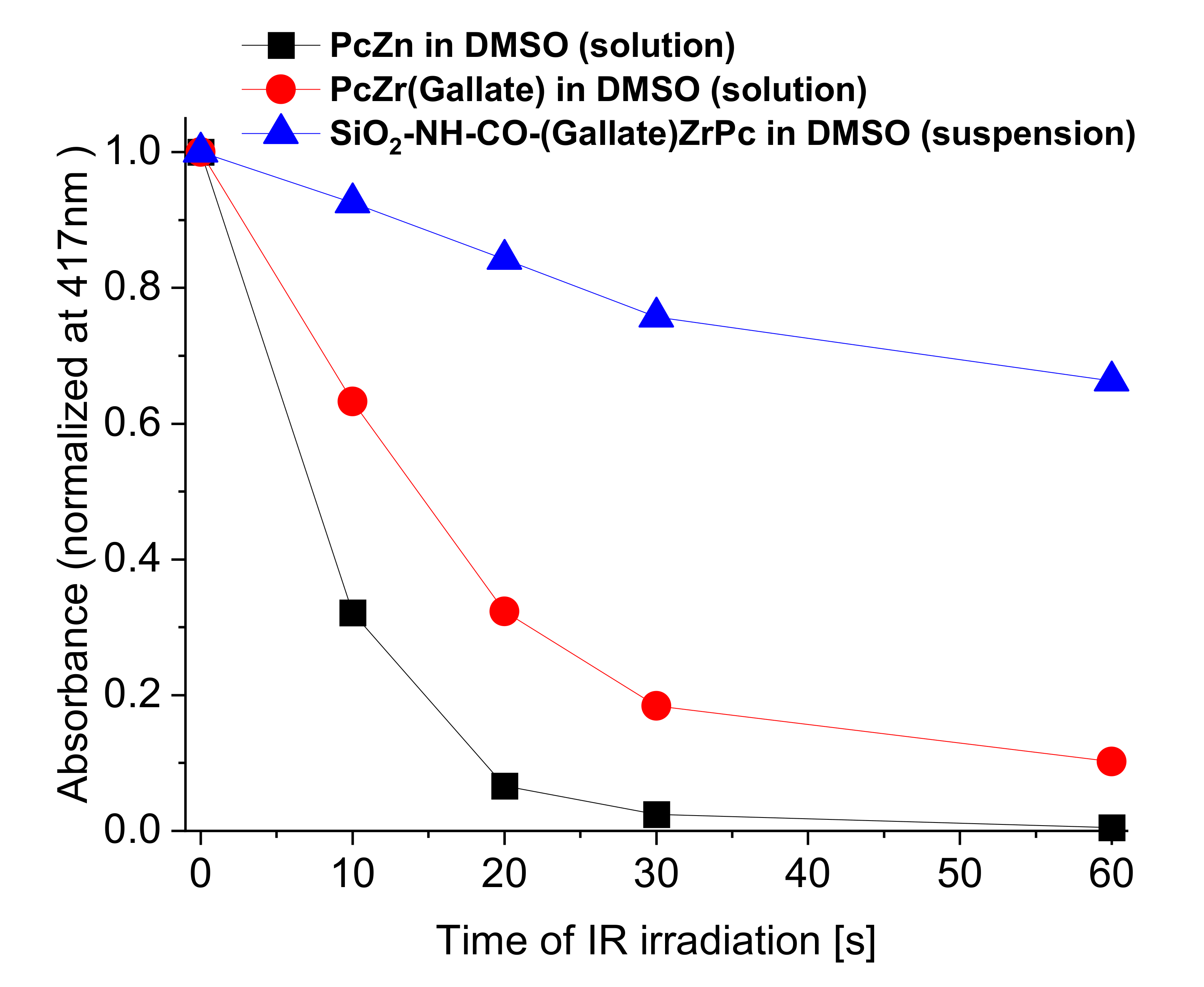
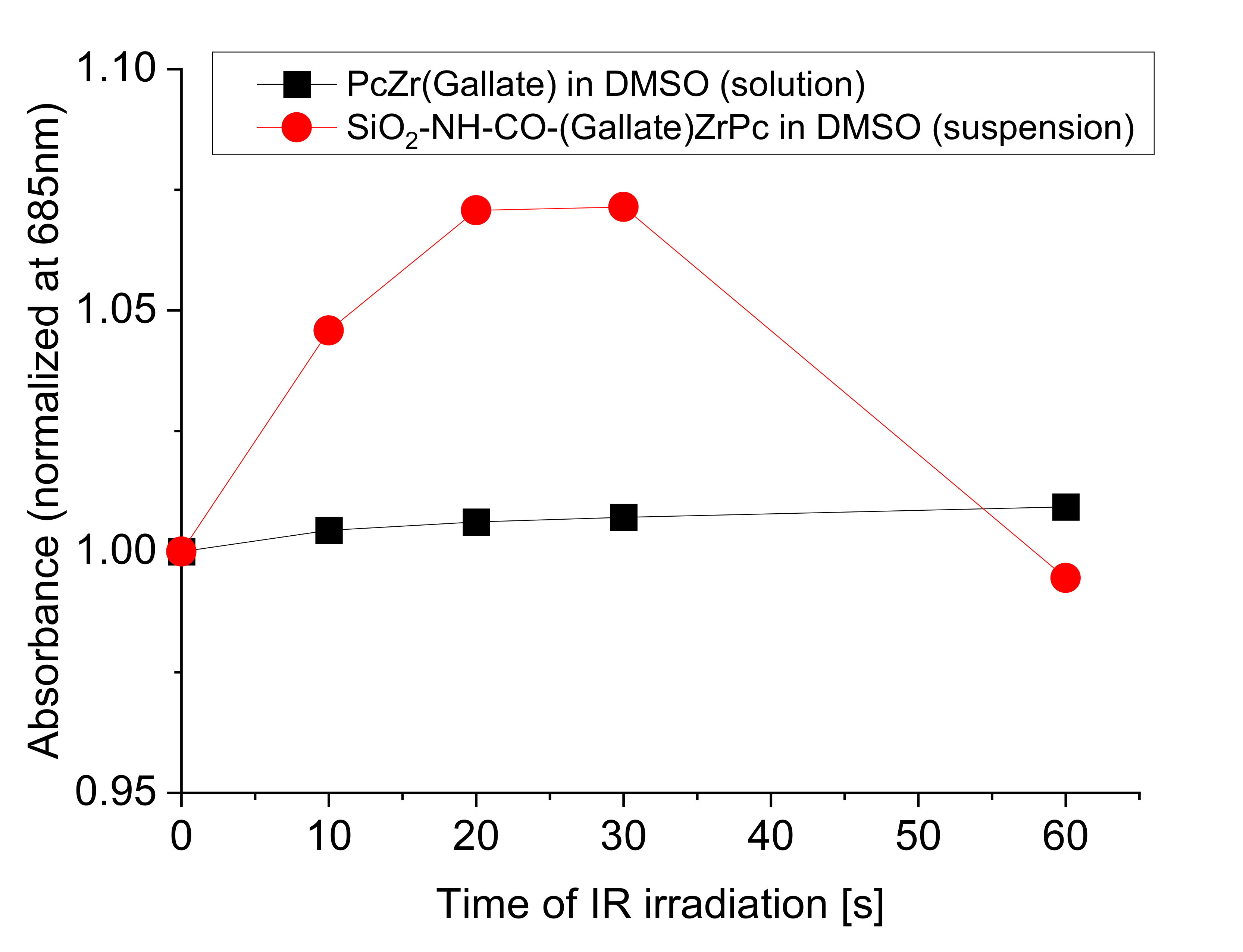
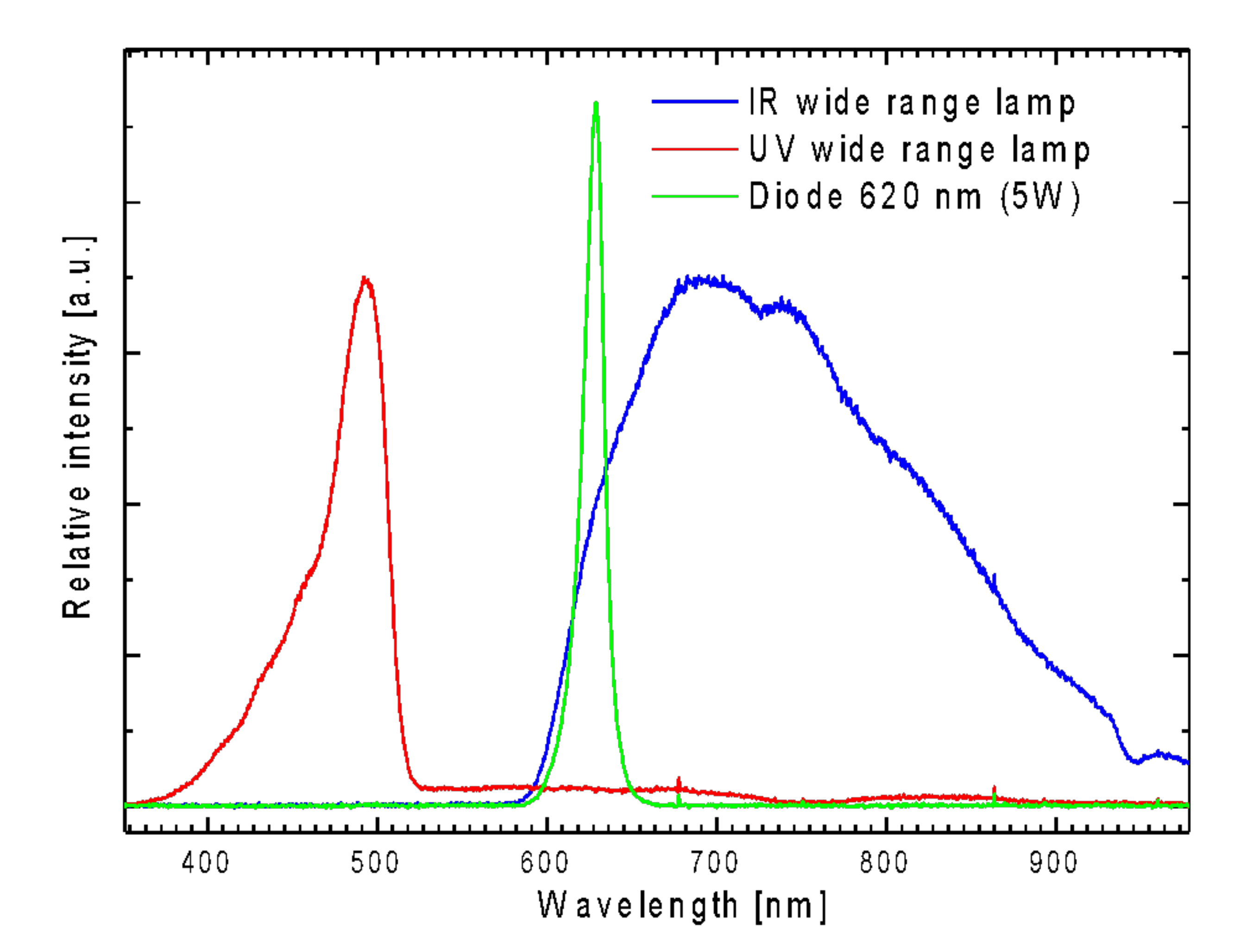
2.2. Spectroscopic Studies of Free Phthalocyanine and Its Conjugate with SiO2 Nanocarrier in Saline
Reactive Oxygen Species (ROS) Generation in DMSO
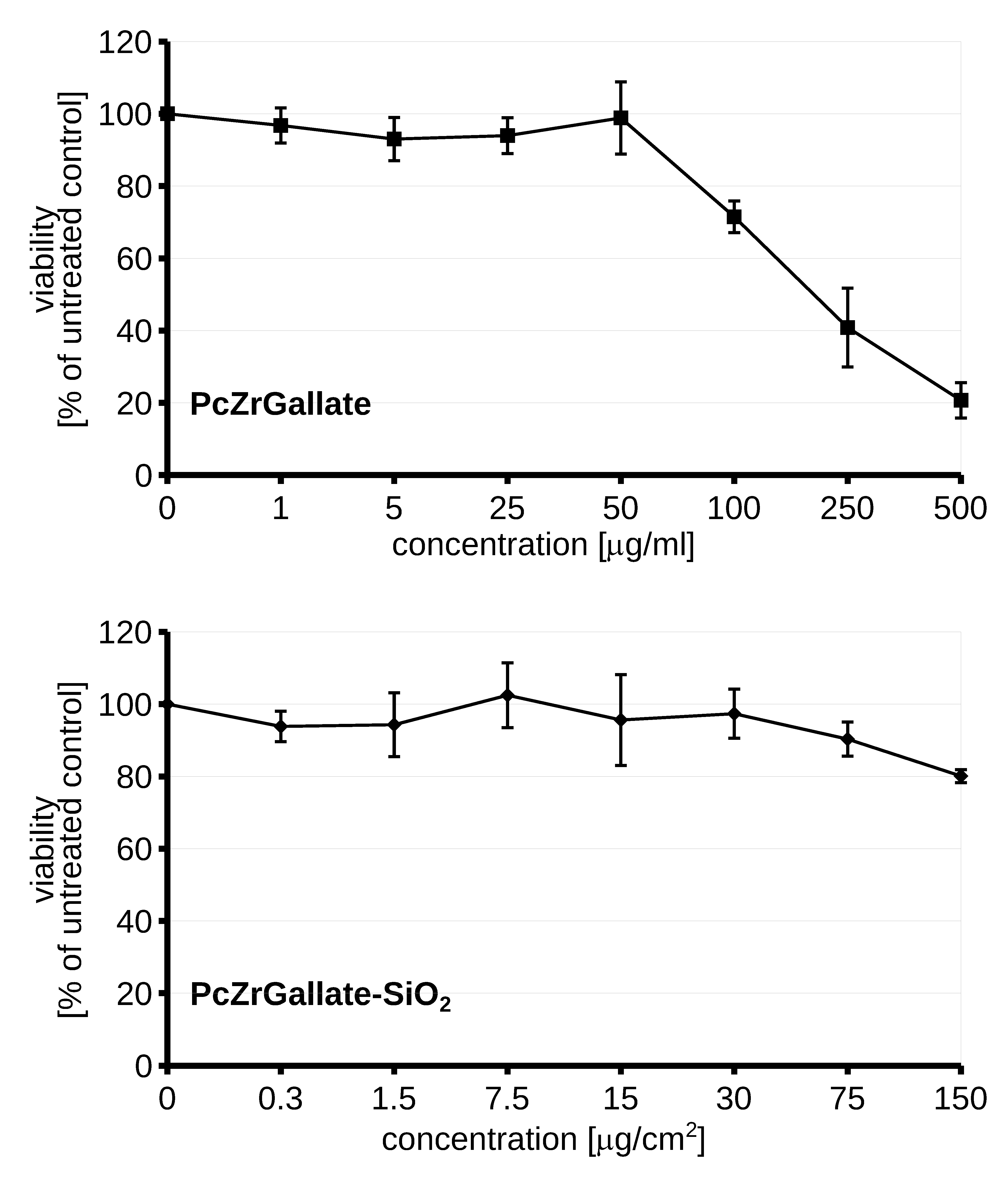
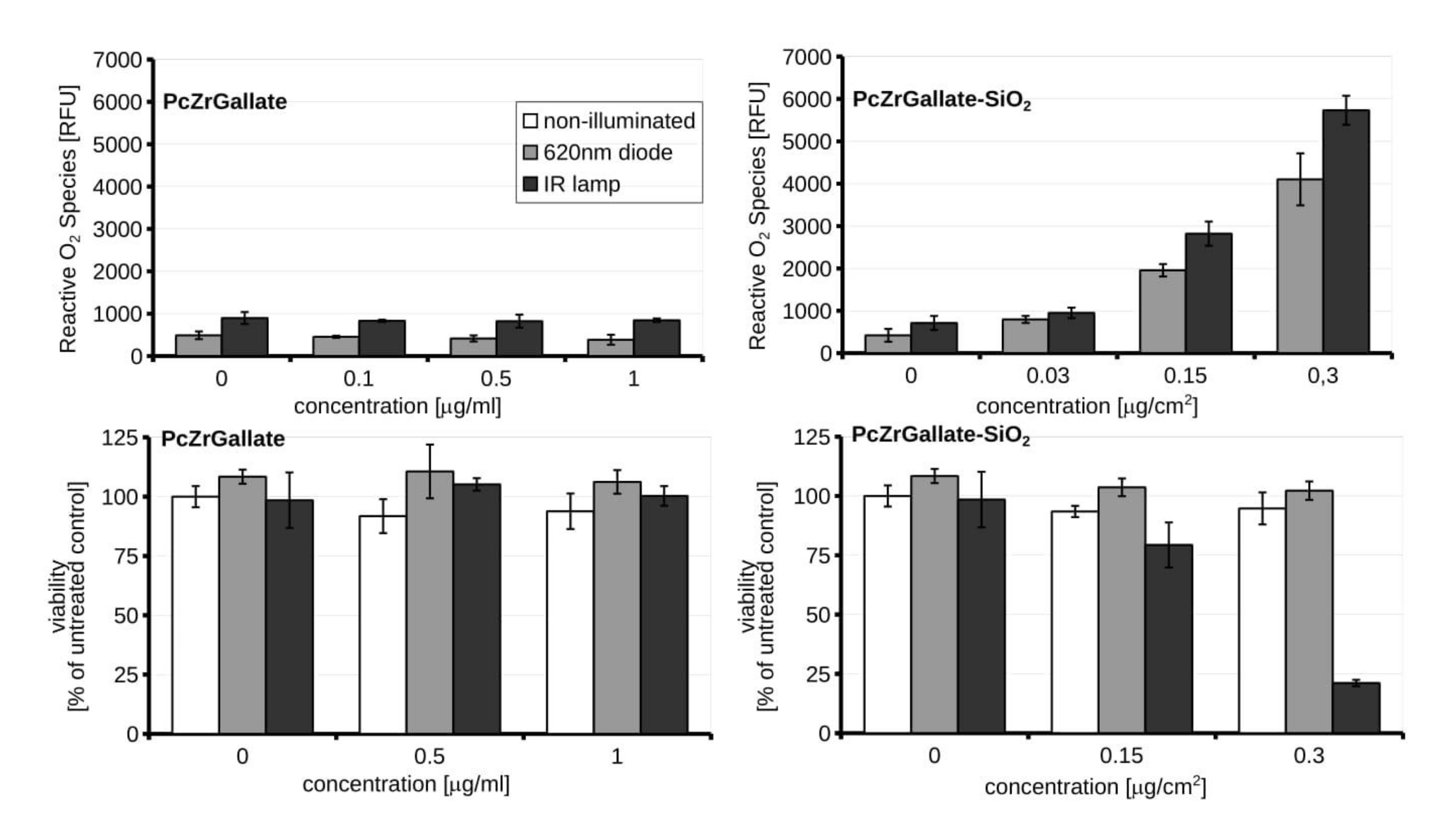
2.3. Biocompatibility and Phototoxicity of PcZrGallate Preparations
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. PcZrGallate-APTES and PcZrGallate-SiO2 Conjugates (Sample A)
3.1.2. PcZrGallate Modified SiO2 Nanoparticles (B-Sample)
3.2. Structure and Morphology Measurements
3.3. Optical Spectroscopy Measurements
Reactive Oxygen Species (ROS) Generation Measurements
3.4. Cell Culture
3.5. Cell Treatment Protocols
3.5.1. Detection of Reactive Oxygen Species
3.5.2. Cell Cytotoxic Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Patel, S.K.; Janjic, J.M. Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics 2015, 5, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Zellweger, M.; Wagnières, G.; Van den Bergh, H.; Cook, S.; Giraud, M.N. Photodynamic therapy for the treatment of atherosclerotic plaque: Lost in translation? Cardiovasc. Ther. 2017, 35, 1755–5922. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Wu, Y.; Nguyen, T.; Wang, X.; Peter, K.; Ta, H.T. The choice of targets and ligands for site-specific delivery of nanomedicine to atherosclerosis. Cardiovasc. Res. 2020, 116, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef]
- Wawrzyńska, M.; Kałas, W.; Biały, D.; Zioło, E.; Arkowski, J.; Mazurek, W.; Strzadała, L. In vitro photodynamic therapy with chlorin e6 leads to apoptosis of human vascular smooth muscle cells. Arch. Immunol. Ther. Exp. 2010, 58, 67–75. [Google Scholar] [CrossRef]
- Kopaczyńska, M.; Sobieszczańska, B.; Ulatowska-Jarża, A.; Hołowacz, I.; Buzalewicz, I.; Wasyluk, Ł.; Tofail, S.; Biały, D.; Wawrzyńska, M.; Podbielska, H. Photoactivated titania-based nanomaterials for potential application as cardiovascular stent coatings. Biocybern. Biomed. Eng. 2014, 34, 189–197. [Google Scholar] [CrossRef]
- Goldman, M.P. Photodynamic Therapy; Elsevier Ltd.: Oxford, UK, 2007. [Google Scholar]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Gelfond, M.L. Photodynamic Therapy in Oncology. Pract. Oncol. 2007, 8, 204–210. [Google Scholar]
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; Van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar]
- McKeown, N.B. Phthalocyanine Materials: Synthesis, Structure, and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Gerasymchuk, Y.S.; Volkov, S.V.; Chernii, V.; Tomachynski, L.A.; Radzki, S. Synthesis and spectral properties of axially substituted zirconium(IV) and hafnium(IV) water soluble phthalocyanines in solutions. J. Alloys Compd. 2004, 380, 186–190. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.S.; Chernii, V.; Tomachynski, L.A.; Legendziewicz, J.; Radzki, S. Spectroscopic characterization of zirconium(IV) and hafniumf(IV) gallate phthalocyanines in monolithic silica gels obtained by sol-gel method. Opt. Mater. 2005, 27, 1484–1494. [Google Scholar] [CrossRef]
- Cheng, Y.; Meyers, J.D.; Broome, A.-M.; Kenney, M.E.; Basilion, J.P.; Burda, C. Deep Penetration of a PDT Drug into Tumors by Noncovalent Drug-Gold Nanoparticle Conjugates. J. Am. Chem. Soc. 2011, 133, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Burda, C. 2.01—Nanoparticles for Photodynamic Therapy Comprehensive. Nanosci. Technol. 2011, 2, 1–28. [Google Scholar]
- Kałas, W.; Wysokińska, E.; Przybyło, M.; Langner, M.; Ulatowska-Jarża, A.; Biały, D.; Wawrzyńska, M.; Zioło, E.; Gil, W.; Trzeciak, A.M.; et al. Photoactive liposomal formulation of PVP-conjugated chlorin e6 for photodynamic reduction of atherosclerotic plaque. Int. J. Mol. Sci. 2019, 20, 15. [Google Scholar] [CrossRef]
- Borak, B.; Arkowski, J.; Skrzypiec, M.; Ziółkowsk, P.; Krajewska, B.; Wawrzyńska, M.; Grotthus, B.; Gliniak, H.; Szelag, A.; Mazurek, W.; et al. Behavior of silica particles introduced into an isolated rat heart as potential drug carriers. Biomed. Mater. 2007, 2, 220–223. [Google Scholar] [CrossRef]
- Wawrzyńska, M.; Duda, M.; Hołowacz, I.; Kaczorowska, A.; Ulatowska-Jarża, A.; Buzalewicz, I.; Kałas, W.; Wysokińska, E.; Biały, D.; Podbielska, H.; et al. Photoactive Pore Matrix for In Situ Delivery of a Photosensitizer in Vascular Smooth Muscle Cells Selective PDT. Materials 2019, 9, 4110. [Google Scholar] [CrossRef]
- Dube, E.; Oluwole, D.O.; Nyokong, T. Photophysicochemical behaviour ofanionic indium phthalocyanine when grafted onto AgxAuy and porous silicananoparticles. J. Lumin. 2017, 190, 353–363. [Google Scholar] [CrossRef]
- Zou, J.-L.; Chen, X.-L. Using silica nanoparticles as a catalyst carrier to the highly sensitive determination of thiamine. Microchem. J. 2007, 86, 42–47. [Google Scholar] [CrossRef]
- Tomachynski, L.A.; Chernii, V.Y.; Gorbenko, H.N.; Filonenko, V.V.; Volkov, S.V. Synthesis, Spectral Properties, and Anti-tumor Activity of a New Axially Substituted Phthalocyanine Complex of Zirconium(IV)with Citric Acid. Chem. Biodivers. 2004, 1, 862–867. [Google Scholar] [CrossRef]
- Collinsom, M.M. Recent trends in analytical applications of organically modified silicate materials. Trends Anal. Chem. 2002, 21, 30–38. [Google Scholar]
- Gerasymchuk, Y.; Tomachynski, L.; Tretyakova, I.; Hanuza, J.; Legendziewicz, J. Axially substituted ytterbium(III) monoph-thalocyanine—Synthesis and their spectral properties in solid state, solution and in monolithic silica blocks. J. Photochem. Photobiol. A Chem. 2010, 214, 128–134. [Google Scholar] [CrossRef]
- Pişkin, M.; Durmuş, M.; Bulut, M. Highly soluble 7-oxy-3-(4-methoxyphenyl)coumarin bearing zinc phthalocyanines: Synthesis and investigation of photophysical and photochemical properties. J. Photochem. Photobiol. A Chem. 2011, 223, 37–49. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.S.; Chernii, V.; Tomachynskii, L.A.; Kowalska, M.; Legendziewicz, J.; Radzki, S. Correlation between computer models of structure of 5-sulfosalicylato Zr(IV) phthalocyanine with results obtained by NMR, ESI-MS and UV–Vis spectra. Opt. Mater. 2010, 32, 1193–1201. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Böhmert, L.; König, L.; Sieg, H.; Lichtenstein, D.; Paul, N.; Braeuning, A.; Voigt, A.; Lampen, A. In vitro nanoparticle dosimetry for adherent growing cell monolayers covering bottom and lateral walls. Part. Fibre Toxicol. 2018, 15, 42. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.N.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Delcassian, D.; Patel, A.K.; Cortinas, A.B.; Langer, R. Drug delivery across length scales. J. Drug Target. 2019, 27, 229–243. [Google Scholar] [CrossRef]
- Park, E.-J.; Yi, J.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Carney, S.S.; Brunetti, R.; Malvindi, V.; Al-Juffali, M.A.; Vecchio, N.; Janes, G.; Bakr, S.; Cingolani, O.; Stellacci, R.; Pompa, F. A General Mechanism for Intracellular Toxicity of Metal-Containing Nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar]
- Avşar, G.; Sari, F.A.; Yuzer, A.C.; Soylu, H.M.; Erd, O.; Ince, M.; Yurt, L.F. Intracellular uptake and fluorescence imaging potential in tumor cell of zinc phthalocyanine. Int. J. Pharm. 2016, 505, 369–375. [Google Scholar] [CrossRef]
- Nyokong, T.; Antunes, E. Influence of nanoparticle materials on the photophysical behavior of phthalocyanines. Coord. Chem. Rev. 2013, 257, 2401–2418. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasymchuk, Y.; Kałas, W.; Arkowski, J.; Marciniak, Ł.; Hreniak, D.; Wysokińska, E.; Strządała, L.; Obremska, M.; Tomachynski, L.; Chernii, V.; et al. Gallato Zirconium (IV) Phtalocyanine Complex Conjugated with SiO2 Nanocarrier as a Photoactive Drug for Photodynamic Therapy of Atheromatic Plaque. Molecules 2021, 26, 260. https://doi.org/10.3390/molecules26020260
Gerasymchuk Y, Kałas W, Arkowski J, Marciniak Ł, Hreniak D, Wysokińska E, Strządała L, Obremska M, Tomachynski L, Chernii V, et al. Gallato Zirconium (IV) Phtalocyanine Complex Conjugated with SiO2 Nanocarrier as a Photoactive Drug for Photodynamic Therapy of Atheromatic Plaque. Molecules. 2021; 26(2):260. https://doi.org/10.3390/molecules26020260
Chicago/Turabian StyleGerasymchuk, Yuriy, Wojciech Kałas, Jacek Arkowski, Łukasz Marciniak, Dariusz Hreniak, Edyta Wysokińska, Leon Strządała, Marta Obremska, Larysa Tomachynski, Viktor Chernii, and et al. 2021. "Gallato Zirconium (IV) Phtalocyanine Complex Conjugated with SiO2 Nanocarrier as a Photoactive Drug for Photodynamic Therapy of Atheromatic Plaque" Molecules 26, no. 2: 260. https://doi.org/10.3390/molecules26020260
APA StyleGerasymchuk, Y., Kałas, W., Arkowski, J., Marciniak, Ł., Hreniak, D., Wysokińska, E., Strządała, L., Obremska, M., Tomachynski, L., Chernii, V., & Stręk, W. (2021). Gallato Zirconium (IV) Phtalocyanine Complex Conjugated with SiO2 Nanocarrier as a Photoactive Drug for Photodynamic Therapy of Atheromatic Plaque. Molecules, 26(2), 260. https://doi.org/10.3390/molecules26020260





