Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubility of Dextrins
2.2. Dextrins Dextrose Equivalents (DE)
2.3. Color Parameters (L* a* b*)
2.4. Total Dietary Fiber Content
2.5. SEM
2.6. X-ray Diffraction (XRD)
2.7. Thermal Properties of Dextrins (DSC)
2.8. Glycosidic-Linkage Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Dextrins (Continuous and Discountinuous Process) Using Microwave-Assisted Heating
3.3. Solubility of Dextrins
3.4. Dextrose Equivalent (DE) of Dextrins
3.5. Color Parameters (L* a* b*)
3.6. Total Dietary Fiber Content According to AOAC 2009.01 Method
3.7. SEM
3.8. X-ray Diffraction (XRD)
3.9. Thermal Properties of Dextrins (DSC)
3.10. Methylation Analysis
3.11. GC-MS Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Granato, D.; Nunes, D.S.; Barba, F. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Fang, H.; Xu, W.; Yan, Y.; Xu, H.; Liu, Y.; Mo, M.; Zhang, H.; Zhao, Y. Dietary fiber intake and risk of type 2 diabetes: A dose-response analysis of prospective studies. Eur. J. Epidemiol. 2014, 29, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, M.; Zhou, L.; Lingh, S.; Li, Y.; Kong, B.; Huang, P. Dietary fiber intake and risks of proximal and distal colon cancers: A meta-analysis. Medicine 2018, 97, e11678. [Google Scholar] [CrossRef]
- Prasadi, N.V.P.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Wang, Z.; Cheng, H.; Zhang, Y.; Lin, B.; Qin, L.; Bai, Y. Effects of reaction condition on glycosidic linkage structure, physical–chemical properties and in vitro digestibility of pyrodextrins prepared from native waxy maize starch. Food Chem. 2020, 320, 126491. [Google Scholar] [CrossRef]
- Ohkuma, K.; Hanno, Y.; Inada, K.; Matsuda, I.; Katta, Y. Process for Preparing Dextrin Containing Food Fiber. U.S. Patent 5620873, 15 April 1997. [Google Scholar]
- Wang, Y.J.; Kozłowski, R.; Delgado, G.A. Enzyme resistant dextrins from high amylose corn mutant starches. Starke 2001, 53, 21–26. [Google Scholar] [CrossRef]
- Jochym, K.; Kapusniak, J.; Barczynska, R.; Slizewska, K. New starch preparations resistant to enzymatic digestion. J. Sci. Food Agric. 2012, 92, 886–891. [Google Scholar] [CrossRef]
- Lovera, M.; de Castro, G.M.C.; da Rocha Pires, N.; do Socorro Rocha Bastos, M.; Holanda-Araujo, M.L.; Laurentin, A.; de Azevedo Moreira, R.; de Oliveira, H.D. Pyrodextrinization of yam (Dioscorea sp.) starch isolated from tubers grown in Brazil and physicochemical characterization of yellow pyrodextrins. Carbohydr. Polym. 2020, 242, 116382. [Google Scholar] [CrossRef] [PubMed]
- Trithavisup, K.; Krusong, K.; Tananuwonga, K. In-depth study of the changes in properties and molecular structure of cassava starch during resistant dextrin preparation. Food Chem. 2019, 297, 124996. [Google Scholar] [CrossRef]
- Guerin-Deremaux, L.; Li, S.; Pochat, M.; Wils, D.; Mubasher, M.; Reifer, C.; Miller, L.E. Effects of NUTRIOSE® dietary fiber supplementation on body weight, body composition, energy intake, and hunger in overweight men. Int. J. Food Sci. Nutr. 2011, 62, 628–635. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.J.; Chen, Y.; Wei, N.; Hou, Y.; Bai, W.; Hu, S.Q. Effect of water-soluble dietary fiber resistant dextrin on flour and bread qualities. Food Chem. 2020, 317, 126452. [Google Scholar] [CrossRef] [PubMed]
- Kapusniak, K.; Wojcik, M.; Wrobel, K.; Rosicka-Kaczmarek, J.; Kapusniak, J. Assessment of physicochemical and thermal properties of soluble dextrin fiber from potato starch for use in fruit mousses. J. Sci. Food Agric. 2021, 101, 4125–4133. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.S.; Kumar, K.; Redhu, S.; Bhardwaj, S. Microwave assisted synthesis: A green chemistry approach. Int. J. Appl. Pharm. Sci. Res. 2013, 3, 278–285. [Google Scholar]
- Anwar, J.; Shafique, U.; Zaman, W.; Rehman, R.; Salman, M.; Dar, A.; Anzano, J.M.; Ashraf, U.; Ashraf, S. Microwave chemistry: Effect of ions on dieletric heating in microwave ovens. Arab. J. Chem. 2015, 8, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Gaba, M.; Passi, N.D. Microwave Chemistry: General Features and Applications. Indian J. Pharm. Educ. Res. 2010, 45, 175–183. [Google Scholar]
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of microwave treatment on the physicochemical properties of potato starch granules. Chem. Cent. J. 2013, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, D.; Wang, L.; Zhang, N.; Xiong, L.; Huang, L.; Zhao, J.; Wang, M.; Zhang, H. Full-time response of starch subjected to microwave heating. Sci. Rep. 2017, 7, 3967. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Fan, D.; Huang, L.; Gao, Y.; Lian, H.; Zhao, J.; Zhang, H. Effects of microwaves on molecular arrangements in potato starch. RSC Adv. 2017, 7, 14348. [Google Scholar] [CrossRef] [Green Version]
- Braşoveanu, M.; Nemţanu, M.R. Behaviour of starch exposed to microwave radiation treatment. Starke 2014, 66, 3–14. [Google Scholar] [CrossRef]
- Kapusniak, K.; Nebesny, E. Enzyme-resistant dextrins from potato starch for potential application in the beverage industry. Carbohydr. Polym. 2017, 172, 152–158. [Google Scholar]
- Stevenson, D.G.; Biswas, A.; Inglett, G.E. Thermal and Pasting Properties of Microwaved Corn Starch. Starke 2005, 57, 347–353. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Fan, J.; Budarin, V.L.; Bouxin, F.P.; Clark, J.H.; Tsang, D.C.W. Evidences of starch-microwave interactions under hydrolytic and pyrolytic conditions. Green Chem. 2020, 22, 7109–7118. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S. Low power microwave-assisted enzymatic esterification of starch. Starke 2012, 64, 188–197. [Google Scholar] [CrossRef]
- Horchani, H.; Chaâbouni, M.; Gargouri, Y.; Sayari, A. Solvent-free lipase-catalyzed synthesis of long-chain starch esters using microwave heating: Optimization by response surface methodology. Carbohydr. Polym. 2010, 79, 466–474. [Google Scholar] [CrossRef]
- Kaur, M.; Oberoi, D.P.S.; Sogi, D.S.; Gill, B.S. Physicochemical, morphological and pasting properties of acid treated starches from different botanical sources. J. Food Sci. Technol. 2011, 48, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ji, J.; Yang, L.; Lei, N.; Wang, J.; Sun, B. Structrural and physicochemical property changes during pyroconversion of native maize starch. Carbohydr. Polym. 2020, 245, 116560. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes 2020, 8, 759. [Google Scholar] [CrossRef]
- Toraya-Aviles, R.; Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Effects of pyroconversion and enzymatic hydrolysis on indigestible starch content and physicochemical properties of cassava (Manihot esculenta) starch. Starke 2016, 69, 1600267. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of microwave treatment (low power and varying time) on potato starch: Microstructure, thermo-functional, pasting and rheological properties. Int. J. Biol. Macromol. 2020, 155, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Lin, J.-H.; Zeng, H.-M.; Wu, Y.-H.; Chang, Y.-H. Indigestible pyrodextrins prepared from corn starch in the presence of glacial acetic acid. Carbohydr. Polym. 2018, 188, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Weil, W.; Weil, R.C.; Keawsompong, S.; Sriroth, K.; Seib, P.A.; Shi, Y.-C. Pyrodextrins from waxy and normal tapioca starches: Molecular structure and in vitro digestibility. Carbohydr. Polym. 2021, 252, 117140. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Sun, Y.; Shi, J.; Xu, X.; Shi, Y.-C. Hypoglycemic Effects of Pyrodextrins with Different Molecular Weights and Digestibilities in Mice with Diet Induced Obesity. J. Agric. Food Chem. 2018, 66, 2988–2995. [Google Scholar] [CrossRef]
- Glaring, M.A.; Koch, C.B.; Blennow, A. Genotype-Specific Spatial Distribution of Starch Molecules in the Starch Granule: A Combined CLSM and SEM Approach. Biomacromolecules 2006, 7, 2310–2320. [Google Scholar] [CrossRef]
- Svihus, B.; Uhlen, A.H.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Lei, N.; Chai, S.; Xu, M.; Ji, J.; Mao, H.; Yan, S.; Gao, Y.; Li, H.; Wang, J.; Sun, B. Effect of dry heating treatment on multi-levels of structure and physicochemical properties of maize starch: A thermodynamic study. Int. J. Biol. Macromol. 2020, 147, 109–116. [Google Scholar] [CrossRef]
- Singh, V.; Ali, S.Z.; Somashekar, R.; Mukherjee, P.S. Nature of crystallinity in native and acid modified starches. Int. J. Food Prop. 2006, 9, 845–854. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castano, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Zhang, Y.; Chen, Y.; Wu, Y.; Ouyang, J. Effect of microwave irradiation-retrogradation treatment on the digestive and physicochemical properties of starches with different crystallinity. Food Chem. 2019, 298, 125015. [Google Scholar] [CrossRef]
- Remya, R.; Jyothia, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.C.; Gutkokski, L.C.; Martin, V.G. Impact of acid hydrolysis and esterification process in rice and potato starch properties. Int. J. Biol. Macromol. 2018, 120, 959–965. [Google Scholar] [CrossRef]
- Ba, K.; Blecker, C.; Danthine, S.; Tine, E.; Destain, J.; Thonart, P. Physicochemical characterization of dextrins prepared with amylases from sorghum malt. Starke 2013, 65, 962–968. [Google Scholar] [CrossRef]
- Olvera-Hernández, V.; Ble-Castillo, J.-L.; Betancur-Ancona, D.; Acevedo-Fernández, J.-J.; Castellanos-Ruelas, A.; Chel-Guerrero, L. Effects of modified banana (Musa cavendish) starch on glycemic control and blood pressure in rats with high sucrose diet. Nutr. Hosp. 2018, 35, 588–595. [Google Scholar] [CrossRef]
- Chen, P.; Liu, X.; Zhang, X.; Sangwan, P.; Yu, L. Phase transition of waxy and normal wheat starch granules during gelatinization. Int. J. Polym. Sci. 2015, 2015, 397128. [Google Scholar] [CrossRef]
- Ubwa, S.T.; Abah, J.; Asemave, K.; Shambe, T. Studies on the gelatinization temperature of some cereal starches. Int. J. Chem. 2012, 4, 22–28. [Google Scholar] [CrossRef]
- Hana, X.; Kangb, J.; Baib, Y.; Xueb, M.; Shib, Y. Structure of pyrodextrin in relation to its retrogradation properties. Food Chem. 2018, 242, 169–173. [Google Scholar] [CrossRef]
- Nunes, F.M.; Lopes, E.S.; Moreira, A.S.P.; Simões, J.; Coimbra, M.A.; Domingues, R.M. Formation of type 4 resistant starch and maltodextrins from amylose and amylopectin upon dry heating: A model study. Carbohydr. Polym. 2016, 141, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Shi, Y.-C. Chemical structures in pyrodextrin determined by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2016, 151, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Augustat, S.; Schierbaum, F. Ausgewahlte Methoden der Stärkechemie; VEB Fachbuchverlag: Leipzig, Germany, 1968. [Google Scholar]
- PN-A-A74701:1978, Hydrolizaty Skrobiowe (Krochmalowe)—Metody Badan. Available online: https://sklep.pkn.pl/pn-a-74701-1978p.html (accessed on 16 June 2021). (In Polish).
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K. Determination of Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2010, 93, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Hulleman, S.H.D.; Kalisvaart, M.G.; Janssen, F.H.P.; Feil, H.; Vliegenthart, J.F.G. Origins of B-type crystallinity in glycerol-plasticised, compression-moulded potato starches. Carbohydr. Polym. 1999, 39, 351–360. [Google Scholar] [CrossRef]
- Rosicka-Kaczmarek, J.; Tkaczyk, M.; Makowski, B.; Komisarczyk, A.; Nebesny, E. The influence of non-starch polysaccharide on thermodynamic properties of starches from facultative wheat varieties. Eur. Food Res. Technol. 2017, 243, 2243–2253. [Google Scholar] [CrossRef] [Green Version]


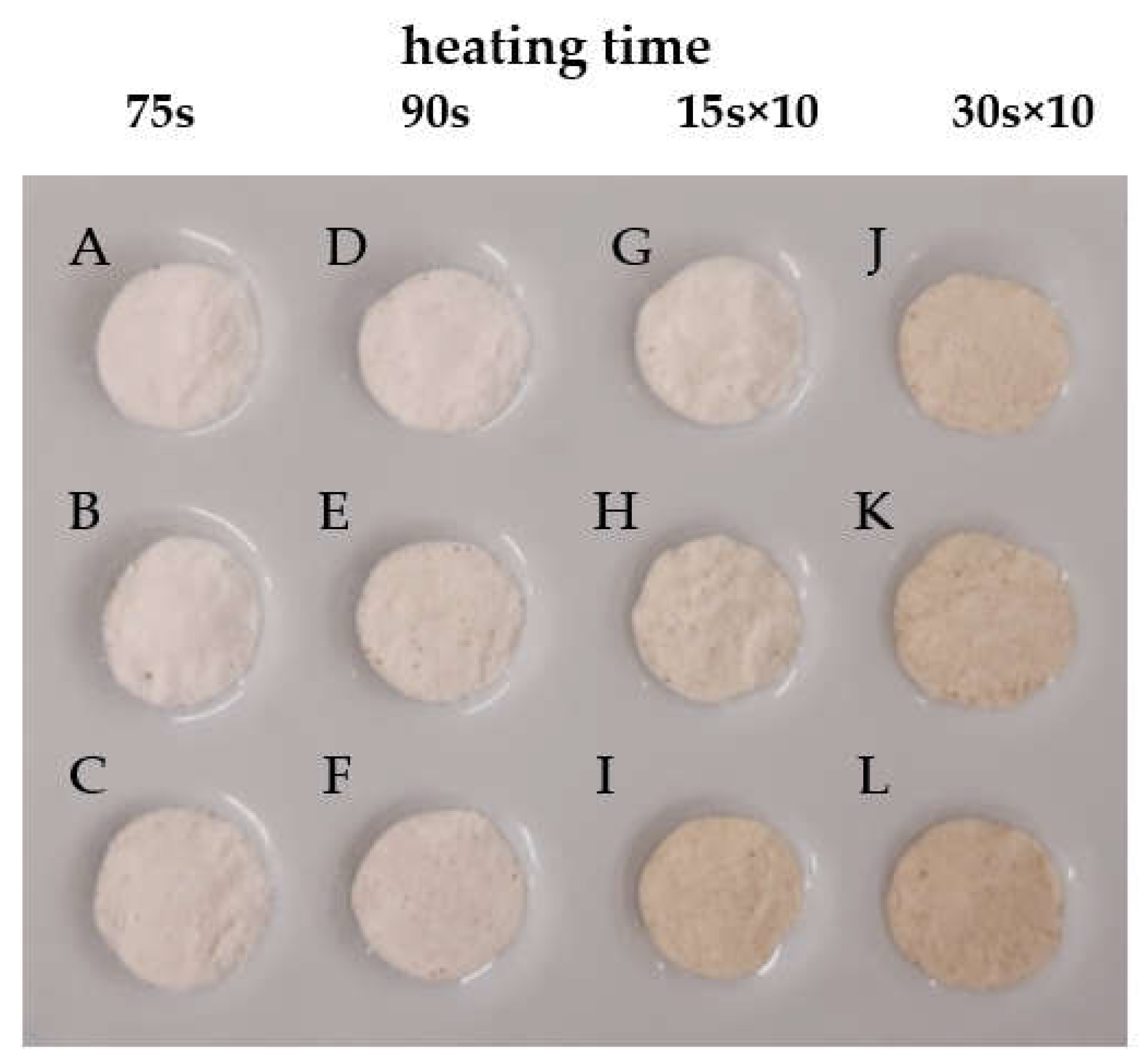

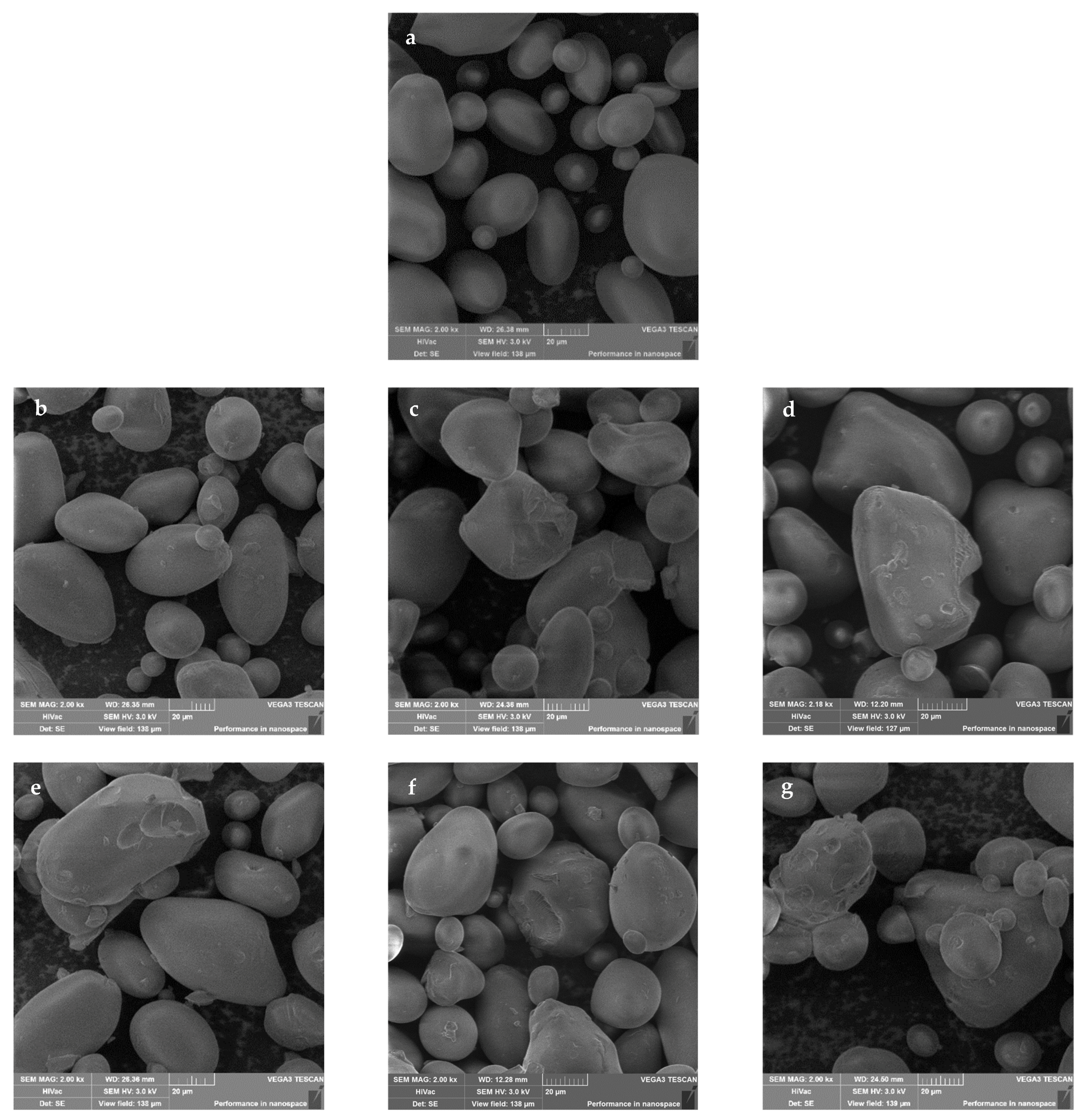

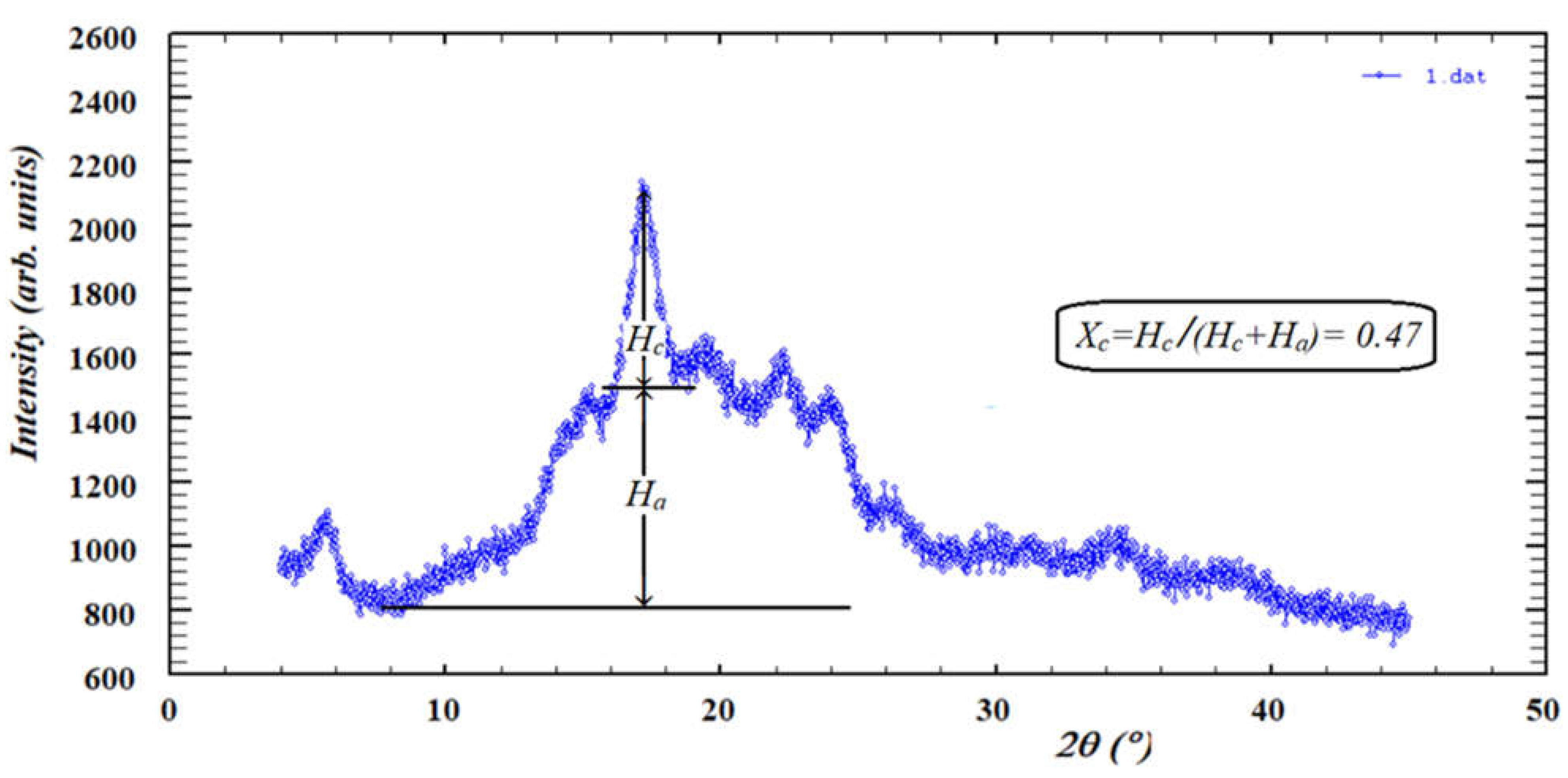
| Dextrin | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Native potato starch | 94.06 | 0.07 | 1.82 | |
| 40 W 75 s | 88.89 ± 0.14 a | 0.56 ± 0.03 j | 9.89 ± 0.11 h | 9.59 ± 0.10 i |
| 45 W 75 s | 87.31 ± 0.13 c | 1.00 ± 0.04 g | 10.28 ± 0.12 g | 10.86 ± 0.07 h |
| 50 W 75 s | 86.69 ± 0.12 d | 1.37 ± 0.04 f | 11.14 ± 0.11 d | 11.95 ± 0.10 f |
| 40 W 90 s | 88.00 ± 0.12 b | 0.99 ± 0.04 g | 10.91 ± 0.08 e | 10.96 ± 0.08 h |
| 45 W 90 s | 86.33 ± 0.09 d | 1.49 ± 0.03 e | 10.91 ± 0.07 e | 12.02 ± 0.07 f |
| 50 W 90 s | 85.61 ± 0.14 e | 1.52 ± 0.02 e | 10.67 ± 0.12 f | 12.32 ± 0.06 e |
| 100 W 15 s × 10 | 88.21 ± 0.09 b | 0.67 ± 0.07 h | 8.56 ± 0.11 i | 8.69 ± 0.08 j |
| 110 W 15 s × 10 | 86.95 ± 0.23 d | 0.6 ± 0.05 i | 10.94 ± 0.26 def | 11.41 ± 0.23 g |
| 120 W 15 s × 10 | 80.44 ± 0.20 g | 2.13 ± 0.05 d | 12.87 ± 0.15 c | 17.26 ± 0.12 d |
| 100 W 30 s × 10 | 81.12 ± 0.18 f | 2.32 ± 0.08 c | 14.64 ± 0.16 b | 18.03 ± 0.19 c |
| 110 W 30 s × 10 | 79.78 ± 0.11 h | 2.71 ± 0.06 b | 15.29 ± 0.22 a | 19.47 ± 0.10 b |
| 120 W 30 s × 10 | 79.38 ± 0.25 i | 3.07 ± 0.16 a | 15.16 ± 0.17 a | 19.71 ± 0.12 a |
| Sample | Xc | |
|---|---|---|
| Native potato starch | 0.47 | |
| 40 W 75 s | 0.28 | Continuous heating |
| 45 W 75 s | 0.23 | |
| 50 W 75 s | 0.21 | |
| 40 W 90 s | 0.24 | |
| 45 W 90 s | 0.18 | |
| 50 W 90 s | 0.17 | |
| 100 W 15 s × 10 | 0.24 | |
| 110 W 15 s × 10 | 0.22 | Discontinuous heating |
| 120 W 15 s × 10 | 0.14 | |
| 100 W 30 s × 10 | 0.12 | |
| 110 W 30 s × 10 | 0.10 | |
| 120 W 30 s × 10 | 0.07 |
| Dextrin | To | Tp | Tc | ΔTr | ΔH |
|---|---|---|---|---|---|
| (°C) | (J g−1 d.w.) | ||||
| Native starch | 58.76 ± 0.14 a | 63.23 ± 0.11 a | 72.33 ± 0.15 a | 13.57 ± 0.11 a | 14.30 ± 0.25 a |
| 40 W 75 s | 60.19 ± 0.22 bAA* | 64.95 ± 0.16 bAA* | 73.01 ± 0.10 bAA* | 12.82 ± 0.17 bAA* | 8.37 ± 0.17 bAA* |
| 45 W 75 s | 62.59 ± 0.21 cBA* | 66.80 ± 0.20 cBA* | 74.50 ± 0.14 cBA* | 11.91 ± 0.10 cBA* | 7.90 ± 0.19 cBA* |
| 50 W 75 s | 63.34 ± 0.17 dCA* | 67.21 ± 0.19 dCA* | 74.96 ± 0.21 dCA* | 11.62 ± 0.18 dCA* | 4.48 ± 0.15 dCA* |
| 40 W 90 s | 62.35 ± 0.20 eAB* | 70.10 ± 0.12 eAB* | 75.03 ± 0.11 eAB* | 11.22 ± 0.14 eAB* | 3.09 ± 0.11 eAB* |
| 45 W 90 s | 65.87 ± 0.15 fBB* | 71.88 ± 0.21 fBB* | 75.97 ± 0.27 fBB* | 10.10 ± 0.17 fBB* | 1.50 ± 0.21 fBB* |
| 50 W 90 s | 68.96 ± 0.14 gCB* | 73.90 ± 0.27 gCB* | 76.91 ± 0.21 gCB* | 7.95 ± 0.20 gCB* | 0.50 ± 0.19 gCB* |
| 100 W 15 s × 10 | 59.17 ± 0.21 hAA* | 73.85 ± 0.54 hAA* | 76,74 ± 0.23 hAA* | 17.57 ± 0.21 hAA* | 1.30 ± 0.17 hAA* |
| 110 W 15 s × 10 | 60.75 ± 0.33 iBA* | 66.96 ± 0.16 iBA* | 69.55 ± 0.21 iBA* | 8.80 ± 0.17 iBA* | 0.33 ± 0.15 iBA* |
| 120 W 15 s × 10 | No endothermic peak | ||||
| 100 W 30 s × 10 | 68.23 ± 0.12 jAB* | 70.37 ± 0.24 jAB* | 72.97 ± 0.21 jAB* | 4.74 ± 0.24 jAB* | 0.43 ± 0.10 jAB* |
| 110 W 30 s × 10 | No endothermic peak | ||||
| 120 W 30 s × 10 | No endothermic peak | ||||
| Sample | t-Glcp | 2-Glcp | 3-Glcp | 4-Glcp | 6-Glcp | 2,3-Glcp | 2,4-Glcp | 2,6-Glcp | 3,4-Glcp | 4,6-Glcp | 2,3,6-Glcp | 2,4,6-Glcp | 3,4,6-Glcp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| potato starch | 3.9 | 93.0 | 3.1 | ||||||||||
| 40 W 75 s | 9.2 | 85.6 | 0.6 | 0.3 | 0.3 | 4.1 | |||||||
| 45 W 75 s | 10.0 | 84.9 | 0.5 | 0.3 | 0.2 | 4.2 | |||||||
| 50 W 75 s | 11.0 | 0.4 | 0.4 | 79.0 | 2.3 | 0.6 | 0.4 | 5.9 | |||||
| 40 W 90 s | 12.9 | 0.6 | 0.4 | 77.4 | 1.6 | 0.5 | 0.2 | 0.5 | 5.6 | 0.1 | 0.2 | ||
| 45 W 90 s | 10.6 | 0.6 | 0.3 | 79.7 | 1.5 | 0.6 | 0.1 | 0.5 | 5.9 | 0.1 | 0.1 | ||
| 50 W 90 s | 8.2 | 0.6 | 0.2 | 81.5 | 1.2 | 0.4 | 3.3 | 0.6 | 3.5 | 0.1 | 0.2 | 0.2 | |
| 100 W 15 s × 10 | 12.4 | 2.4 | 1.4 | 75.8 | 1.2 | 0.5 | 0.1 | 0.4 | 5.8 | ||||
| 110 W 15 s × 10 | 15.3 | 0.7 | 0.7 | 68.8 | 4.4 | 0.8 | 0.6 | 8.7 | |||||
| 120 W 15 s × 10 | 17.4 | 0.8 | 0.8 | 65.0 | 5.3 | 0.9 | 0.6 | 9.1 | |||||
| 100 W 30 s × 10 | 16.2 | 1.0 | 0.8 | 65.0 | 5.3 | 0.9 | 0.6 | 9.8 | 0.2 | 0.2 | |||
| 110 W 30 s × 10 | 17.9 | 1.2 | 1.1 | 59.6 | 7.1 | 1.2 | 0.8 | 11.0 | 0.3 | ||||
| 120 W 30 s × 10 | 16.4 | 1.8 | 1.1 | 62.4 | 3.1 | 0.1 | 1.4 | 0.3 | 1.0 | 11.3 | 0.1 | 0.5 | 0.5 |
| Continuous Heating | Discontinuous Heating | |
|---|---|---|
| Processing conditions | 40 W, 45 W, or 50 W for 75 or 90 s (1 time processed) | 100 W, 110 W or 120 W for 15 s or 30 s (10 times processed) |
| Advantages | simple, fast, one-step process, better repeatability | high degree of possible modification, more homogeneous samples |
| Disadvantages | more heterogeneous samples, low degree of possible modification | longer, 10-step process, worse repeatability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusniak, K.; Lubas, K.; Wojcik, M.; Rosicka-Kaczmarek, J.; Pavlyuk, V.; Kluziak, K.; Gonçalves, I.; Lopes, J.; Coimbra, M.A.; Kapusniak, J. Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules 2021, 26, 5619. https://doi.org/10.3390/molecules26185619
Kapusniak K, Lubas K, Wojcik M, Rosicka-Kaczmarek J, Pavlyuk V, Kluziak K, Gonçalves I, Lopes J, Coimbra MA, Kapusniak J. Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules. 2021; 26(18):5619. https://doi.org/10.3390/molecules26185619
Chicago/Turabian StyleKapusniak, Kamila, Karolina Lubas, Malwina Wojcik, Justyna Rosicka-Kaczmarek, Volodymyr Pavlyuk, Karolina Kluziak, Idalina Gonçalves, Joana Lopes, Manuel A. Coimbra, and Janusz Kapusniak. 2021. "Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production" Molecules 26, no. 18: 5619. https://doi.org/10.3390/molecules26185619
APA StyleKapusniak, K., Lubas, K., Wojcik, M., Rosicka-Kaczmarek, J., Pavlyuk, V., Kluziak, K., Gonçalves, I., Lopes, J., Coimbra, M. A., & Kapusniak, J. (2021). Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules, 26(18), 5619. https://doi.org/10.3390/molecules26185619









