Cerium-Promoted Ginsenosides Accumulation by Regulating Endogenous Methyl Jasmonate Biosynthesis in Hairy Roots of Panax ginseng
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ginseng Hairy Roots Formation
2.2. Growth Index of Ginseng Hairy Roots
2.3. Ce3+ Stimulates Ginsenoside Accumulation in Ginseng Hairy Roots
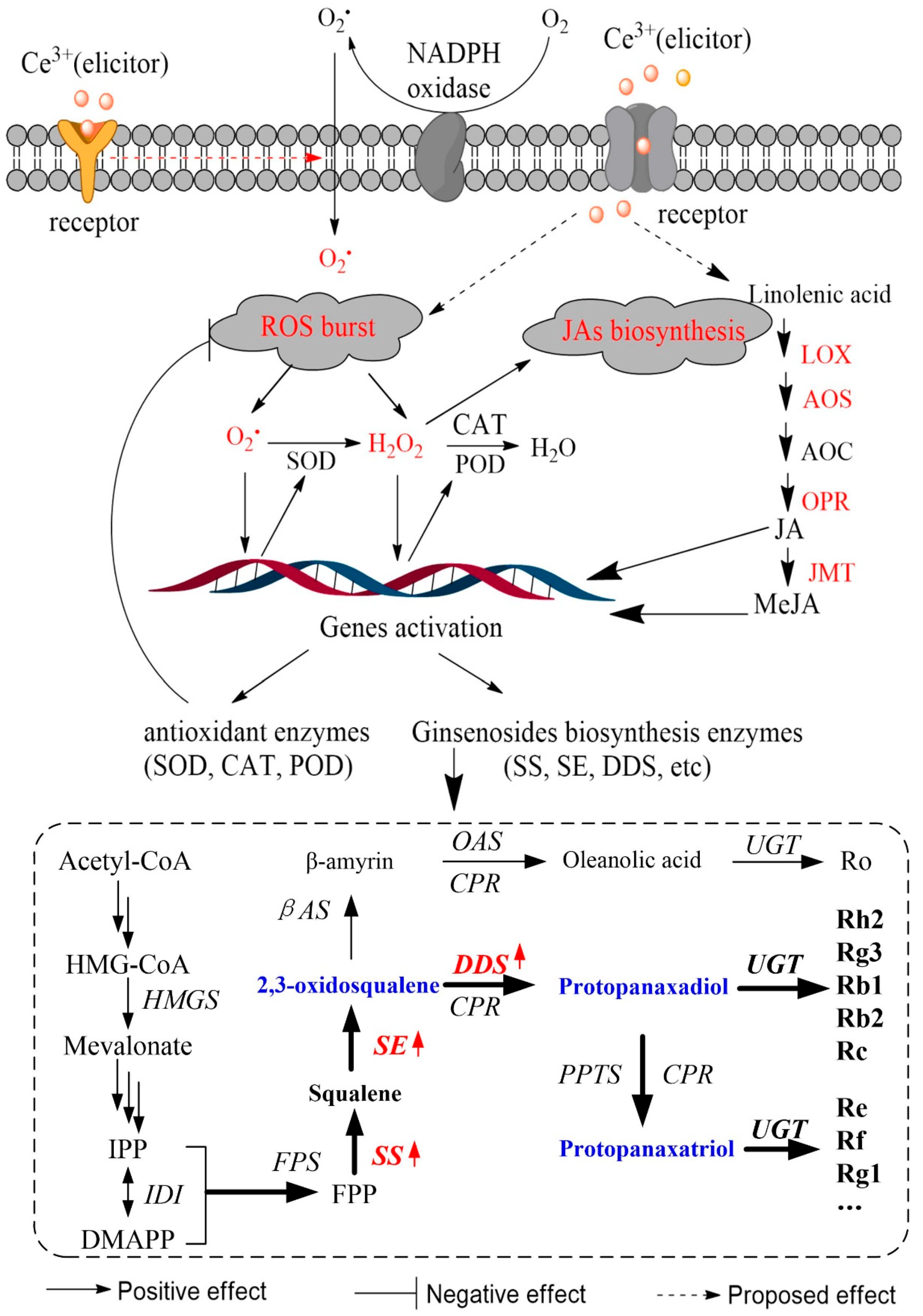
2.4. Ce3+-Induced ROS Production and Antioxidant Enzyme Activities
2.5. Ce3+-Induced MeJA Accumulation and Its Biosynthesis Key Enzyme Genes Expression
2.6. Ce3+-Induced PgSS, PgSS, PgDDS Expression and Ginsenosides Biosynthesis
3. Materials and Methods
3.1. Chemicals, Materials and Treatment
3.2. Determination of Hairy Roots Growth Parameters
3.3. Determination of Ginsenosides Content
3.4. Determination of O2•−, H2O2 and MDA
3.5. Extraction and Assay of Enzyme
3.6. Measurement of JAs Level
3.7. Genes Expression Analysis of Antioxidant Enzymes, Key Enzymes of JAs and Ginsenosides Biosynthesis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jung, J.H.; Kim, H.Y.; Kim, H.S.; Jung, S.H. Transcriptome analysis of Panax ginseng response to high light stress. J. Ginseng Res. 2020, 44, 312–320. [Google Scholar] [CrossRef]
- Kim, H.M.; Song, Y.; Hyun, G.H.; Long, N.P.; Park, J.H.; Hsieh, Y.S.; Kwon, S.W. Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax ginseng C. A. Meyer. Molecules 2020, 25, 791. [Google Scholar] [CrossRef] [Green Version]
- Nakhjavani, M.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Anti-angiogenic properties of ginsenoside Rg3. Molecules 2020, 25, 4905. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Deng, J.; Wang, J.; Weng, W.; Chu, H.; Meng, Q. Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J. Ginseng Res. 2020, 44, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lv, Q.; Li, Y.; Jin, Y.-H. The Anti-Tumor Effect and Underlying Apoptotic Mechanism of Ginsenoside Rk1 and Rg5 in Human Liver Cancer Cells. Molecules 2021, 26, 3926. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Smith, S.A.; Nwangwa, E.E.; Arivett, B.A.; Bryant, D.L.; Fuller, M.L.; Hayes, D.; Bowling, J.L.; Nelson, D.E.; DuBois, J.D.; et al. Panax quinquefolius (North American ginseng) cell suspension culture as a source of bioactive polysaccharides: Immunostimulatory activity and characterization of a neutral polysaccharide AGC1. Int. J. Biol. Macromol. 2019, 139, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Qiang, B.; Miao, J.; Phillips, N.; Wei, K.; Gao, Y. Recent advances in the tissue culture of american ginseng (Panax Quinquefoli-us). Chem. Biodivers. 2020, 17, e2000366. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Lin, Y.; Chen, Z.; Xu, H.; Wang, L. The effects of cerium on the growth and some antioxidant metabolisms in rice seedlings. Environ. Sci. Pollut. Res. 2012, 19, 3282–3291. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Zhang, M.; Zhang, N.; Li, M. Effects of rare earth elements on growth and metabolism of medicinal plants. Acta Pharm. Sin. B 2013, 3, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Shan, C. Lanthanum improves the antioxidant capacity in chloroplast of tomato seedlings through ascorbate-glutathione cycle under salt stress. Sci. Hortic. 2018, 232, 264–268. [Google Scholar] [CrossRef]
- Lu, C.; Ma, Y.; Wang, J. Lanthanum elicitation on hypocrellin A production in mycelium cultures of Shiraia bambusicola is mediated by ROS generation. J. Rare Earths 2019, 37, 895–902. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Zhou, Q. Protective Effect of Rare Earth against Oxidative Stress Under Ultraviolet-B Radiation. Biol. Trace Elem. Res. 2008, 128, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhou, Q. Antioxidant Capacity of Flavonoid in Soybean Seedlings under the Joint Actions of Rare Earth Element La(III) and Ultraviolet-B Stress. Biol. Trace Elem. Res. 2008, 127, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; García-Morales, S.; Pérez-Sato, J.A.; Gómez-Merino, F.C. Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef]
- Xin, P.; Shuang-Lin, Z.; Jun-Yao, H.; Li, D. Influence of Rare Earth Elements on Metabolism and Related Enzyme Activity and Isozyme Expression in Tetrastigma hemsleyanum Cell Suspension Cultures. Biol. Trace Elem. Res. 2013, 152, 82–90. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Wang, Y.; Guo, G. Effect of La(NO3)3 and Ce(NO3)3 on shoot induction and seedling growth of in vitro cul-tured Anoectochilus roxburghii. J. Plant Biol. 2016, 59, 105–113. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, F.; Xiao, R. Effect of Lanthanum Ions (La3+) on the Reactive Oxygen Species Scavenging Enzymes in Wheat Leaves. Biol. Trace Elem. Res. 2003, 91, 243–252. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Liu, X.; Wang, H.; Deng, R.; Yu, H.; Cheng, Z. Identification and Allelopathy of Green Garlic (Allium sativum L.) Volatiles on Scavenging of Cucumber (Cucumis sativus L.) Reactive Oxygen Species. Molecules 2019, 24, 3263. [Google Scholar] [CrossRef] [Green Version]
- Sathiyaraj, G.; Lee, O.R.; Parvin, S.; Khorolragchaa, A.; Kim, Y.-J.; Yang, D.C. Transcript profiling of antioxidant genes during biotic and abiotic stresses in Panax ginseng C. A. Meyer. Mol. Biol. Rep. 2010, 38, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Neill, S.; Cai, W.; Tang, Z. Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol. Plant. 2003, 118, 414–421. [Google Scholar] [CrossRef]
- Ali, M.B.; Yu, K.-W.; Hahn, E.-J.; Paek, K.-Y. Differential responses of anti-oxidants enzymes, lipoxygenase activity, ascorbate content and the production of saponins in tissue cultured root of mountain Panax ginseng C.A. Mayer and Panax quinquefolium L. in bioreactor subjected to methyl jasmonate stress. Plant Sci. 2005, 169, 83–92. [Google Scholar] [CrossRef]
- Ali, M.B.; Yu, K.-W.; Hahn, E.-J.; Paek, K.-Y. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006, 25, 613–620. [Google Scholar] [CrossRef]
- Wu, C.H.; Popova, E.V.; Hahn, E.J.; Paek, K.Y. Linoleic and α-linolenic fatty acids affect biomass and secondary metabolite production and nutritive properties of Panax ginseng adventitious roots cultured in bioreactors. Biochem. Eng. J. 2009, 47, 109–115. [Google Scholar] [CrossRef]
- Tewari, R.K.; Hahn, E.-J.; Paek, K.-Y. Function of nitric oxide and superoxide anion in the adventitious root development and antioxidant defence in Panax ginseng. Plant Cell Rep. 2007, 27, 563–573. [Google Scholar] [CrossRef]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-A.; Zhao, B.; Wang, X.; Yuan, X.; Wang, Y. Promotion of the growth of Crocus sativus cells and the production of crocin by rare earth elements. Biotechnol. Lett. 2004, 26, 27–30. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, X.; Zhao, B.; Yuan, X.; Wang, Y. Effects of rare earth elements on the growth of Cistanche deserticola cells and the production of phenylethanoid glycosides. J. Biotechnol. 2003, 102, 129–134. [Google Scholar] [CrossRef]
- Zicari, M.A.; D’Aquino, L.; Paradiso, A.; Mastrolitti, S.; Tommasi, F. Effect of cerium on growth and antioxidant metabolism of Lemna minor L. Ecotoxicol. Environ. Saf. 2018, 163, 536–543. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, L.; He, X.; Bai, W.; Ding, Y.; Zhang, Z.; Zhao, Y.; Chai, Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.Á.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the Differential Biotransformation and Genotoxicity of ZnO and CeO2 Nanoparticles on Soybean (Glycine max) Plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Ze, Y.; Liu, C.; Li, N.; Zhou, M.; Duan, Y.; Hong, F. Cerium Relieves the Inhibition of Nitrogen Metabolism of Spinach Caused by Magnesium Deficiency. Biol. Trace Elem. Res. 2009, 132, 247–258. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jeon, J.-N.; Jang, M.-G.; Oh, J.Y.; Kwon, W.-S.; Jung, S.-K.; Yang, D.-C. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J. Ginseng Res. 2014, 38, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Wang, Q.; Zhao, B.; Wang, Y. Improved cell growth and total flavonoids of Saussurea medusa on solid culture medi-um supplemented with rare earth elements. Biotechnol. Lett. 2002, 24, 1889–1892. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Hu, G.W.; Wang, C.G.; Jing, Y.; Zhou, Y.Q.; Shen, P.W. Effect of La, Ce on Taxus cuspidata cell growth, biosynthesis and release of taxol. J. Rare Earths 1998, 16, 300–306. [Google Scholar]
- Yuan, Y.-J.; Li, J.-C.; Ge, Z.-Q.; Wu, J.-C. Superoxide anion burst and taxol production induced by Ce4+ in suspension cultures of Taxus cuspidata. J. Mol. Catal. B Enzym. 2002, 18, 251–260. [Google Scholar] [CrossRef]
- Yang, S.; Lu, S.-H.; Yuan, Y.-J. Cerium elicitor-induced phosphatidic acid triggers apoptotic signaling development in Taxus cuspidata cell suspension cultures. Chem. Phys. Lipids 2009, 159, 13–20. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.; Hu, Q. Promotion of indole alkaloid production in Catharanthus roseus cell cultures by rare earth elements. Biotechnol. Lett. 2000, 22, 825–828. [Google Scholar] [CrossRef]
- Bian, L.H.; Zou, L.; Zhou, B.Q.; Liu, W.; Zhou, J.; Wang, X. Effect of lanthanum on accumulation of activeconstituent and key enzymes expression of Salvia miltiorrhiza hairy root. China J. Chin. Mater. Med. 2016, 41, 4344–4349. [Google Scholar]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, J.P.; Scott, E.R.; Liu, J.W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.Y. Exogenous melatonin alleviates cold stress by pro-moting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.-M.; Chen, H. Antioxidant responses of rice seedling to Ce4+ under hydroponic cultures. Ecotoxicol. Environ. Saf. 2011, 74, 1693–1699. [Google Scholar] [CrossRef]
- Huang, S.-F.; Li, Z.-Y.; Wang, X.-Q.; Wang, Q.-X.; Hu, F.-F. Cerium caused life span shortening and oxidative stress resistance in Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2010, 73, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Morales, M.I.; McCreary, R.; Castillo-Michel, H.; Barrios, A.C.; Hong, J.; Tafoya, A.; Lee, W.-Y.; Varela-Ramirez, A.; Peralta-Videa, J.R.; et al. Cerium Oxide Nanoparticles Modify the Antioxidative Stress Enzyme Activities and Macromolecule Composition in Rice Seedlings. Environ. Sci. Technol. 2013, 47, 14110–14118. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Qiu, N.; Hu, F.; Sheng, L.; Wang, R.; Cao, F. Ce3+ induces flavonoids accumulation by regulation of pigments, ions, chlorophyll fluorescence and antioxidant enzymes in suspension cells of Ginkgo biloba L. Plant Cell Tissue Organ Cult. 2015, 123, 283–296. [Google Scholar] [CrossRef]
- Dias, M.C.; Mariz-Ponte, N.; Santos, C. Lead induces oxidative stress in Pisum sativum plants and changes the levels of phyto-hormones with antioxidant role. Plant Physiol. Biochem. 2019, 137, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced reactive oxygen species scavenging by overproduction of super-oxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Wang, P.-Y.; Sun, L.-G.; Zhang, J.-J.; Yu, J.; Wang, Y.-W.; Chen, G.-X. Alleviation of cadmium toxicity by cerium in rice seedlings is related to improved photosynthesis, elevated antioxidant enzymes and decreased oxidative stress. Plant Growth Regul. 2014, 74, 251–260. [Google Scholar] [CrossRef]
- Cao, F.; Wang, N.; Zhang, M.; Dai, H.; Dawood, M.; Zhang, G.; Wu, F. Comparative study of alleviating effects of GSH, Se and Zn under combined contamination of cadmium and chromium in rice (Oryza sativa). BioMetals 2013, 26, 297–308. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; Sun, N.; Liu, H.; Zhao, Y.; Liang, Y.; Zhang, L.; Han, Y. Functional diversity of jasmonates in rice. Rice 2015, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, S.; Kim, Y.-J.; Sukweenadhi, J.; Zhang, D.; Yang, D.-C. PgLOX6encoding a lipoxygenase contributes to jasmonic acid biosynthesis and ginsenoside production in Panax ginseng. J. Exp. Bot. 2016, 67, 6007–6019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creelman, R.A.; Tierney, M.L.; Mullet, J.E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc. Natl. Acad. Sci. USA 1992, 89, 4938–4941. [Google Scholar] [CrossRef] [Green Version]
- Gundlach, H.; Müller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cul-tures. Proc. Natl. Acad. Sci. USA 1992, 89, 2389–2393. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yuan, G.; Yuan, S.; Duan, W.; Wang, P.; Bai, J.; Zhang, F.; Gao, S.; Zhang, L.; Zhao, C. TaOPR2 encodes a 12-oxo-phytodienoic acid reductase involved in the biosynthesis of jasmonic acid in wheat (Triticum aestivum L.). Biochem. Biophys. Res. Commun. 2016, 470, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Niu, J.; Li, B.; Huang, Y.; Han, L.; Liu, Y.; Zhou, W.; Hu, S.; Li, L.; Wang, D.; et al. Molecular Characterization and Overexpression of SmJMT Increases the Production of Phenolic Acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 3788. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-Y.; Ge, X. Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol. Bioeng. 2004, 85, 714–721. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Rahimi, S.; Balusamy, S.D.; Khorolragchaa, A.; Kim, Y.J.; Kim, J.H.; Jung, S.; Yang, D. Effect of salicylic acid and yeast ex-tract on the accumulation of jasmonic acid and sesquiterpenoids in Panax ginseng adventitious roots. Russ. J. Plant Phys. 2014, 61, 811–817. [Google Scholar] [CrossRef]
- Liu, T.; Luo, T.; Guo, X.; Zou, X.; Zhou, D.; Afrin, S.; Li, G.; Zhang, Y.; Zhang, R.; Luo, Z. PgMYB2, a MeJA-Responsive Transcription Factor, Positively Regulates the Dammarenediol Synthase Gene Expression in Panax Ginseng. Int. J. Mol. Sci. 2019, 20, 2219. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.-W.; Jung, J.; Ha, Y.I.; Park, H.-W.; In, D.S.; Chung, H.-J.; Liu, J.R. Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep. 2004, 23, 557–566. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing aglycons of ginsenosides in bakers’ yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar] [CrossRef]
- Balusamy, S.R.D.; Rahimi, S.; Sukweenadhi, J.; Kim, Y.-J.; Yang, D.-C. Exogenous methyl jasmonate prevents necrosis caused by mechanical wounding and increases terpenoid biosynthesis in Panax ginseng. Plant Cell Tissue Organ Cult. 2015, 123, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, J.; Ji, J.; Li, P.; Yu, L.; Allah, E.A.; Luo, Y.; Hu, L.; Hu, X. High Temperature Induces Expression of Tobacco Transcription Factor NtMYC2a to Regulate Nicotine and JA Biosynthesis. Front. Physiol. 2016, 7, 465. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Zhang, D.; Zhuang, J.; Huang, Y.; Mu, Y.; Lv, S. Study on the correlation between gene expression and enzyme activity of seven key enzymes and ginsenoside content in ginseng in over time in Ji’an, China. Int. J. Mol. Sci. 2017, 18, 2682. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhong, J.-J. Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process. Biochem. 2013, 48, 1227–1234. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, B.-L.; Li, G.-C.; Xie, T.; Hu, T.; Luo, Z.-Y. Enhancement of ginsenoside Rg1 in Panax ginseng hairy root by overexpressing the α-l-rhamnosidase gene from Bifidobacterium breve. Biotechnol. Lett. 2015, 37, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid–a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Lefebvre-De Vos, D.; Le Disquet, I.; Leprince, A.S.; Bordenave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savoure, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baque, A.; Hahn, E.-J.; Paek, K.-Y. Growth, secondary metabolite production and antioxidant enzyme response of Morinda citrifolia adventitious root as affected by auxin and cytokinin. Plant Biotechnol. Rep. 2010, 4, 109–116. [Google Scholar] [CrossRef]
- Zhang, R.; Tan, S.Q.; Zhang, B.L.; Guo, Z.Y.; Tian, L.Y.; Weng, P.; Luo, Z.Y. Two key amino acids variant of alpha-L-arabinofuranosidase from Bacillus subtilis Str. 168 with altered activity for selective conversion ginsenoside Rc to Rd. Molecules 2021, 26, 1733. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, S.; Yao, L.; Wang, J.; Gao, W. Quality evaluation of Panax ginseng adventitious roots based on ginsenoside constituents, functional genes, and ferric-reducing antioxidant power. J. Food Biochem. 2019, 43, e12901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, J.; Cao, H.-Z.; An, Y.-R.; Huang, J.-J.; Chen, X.-H.; Mohammed, N.; Afrin, S.; Luo, Z.-Y. Molecular cloning and expression analysis of PDR1-like gene in ginseng subjected to salt and cold stresses or hormonal treatment. Plant Physiol. Biochem. 2013, 71, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Xiao, Y.; Cao, L.-L.; Yan, X.; Li, C.; Shi, H.-Y.; Wang, J.-W.; Ye, Y.-H. Cerebroside C Increases Tolerance to Chilling Injury and Alters Lipid Composition in Wheat Roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef] [Green Version]
- Fournier, J.; Pouénat, M.-L.; Rickauer, M.; Rabinovltch-Chable, H.; Rigaud, M.; Marie-Thérèse, E.-T. Purification and charac-terization of elicitor-induced lipoxygenase in tobacco cells. Plant J. 1993, 3, 63–70. [Google Scholar] [CrossRef]
- You, C.; Zhu, H.; Xu, B.; Huang, W.; Wang, S.; Ding, Y.; Liu, Z.; Li, G.; Chen, L.; Ding, C.; et al. Effect of Removing Superior Spikelets on Grain Filling of Inferior Spikelets in Rice. Front. Plant Sci. 2016, 7, 1161. [Google Scholar] [CrossRef] [Green Version]
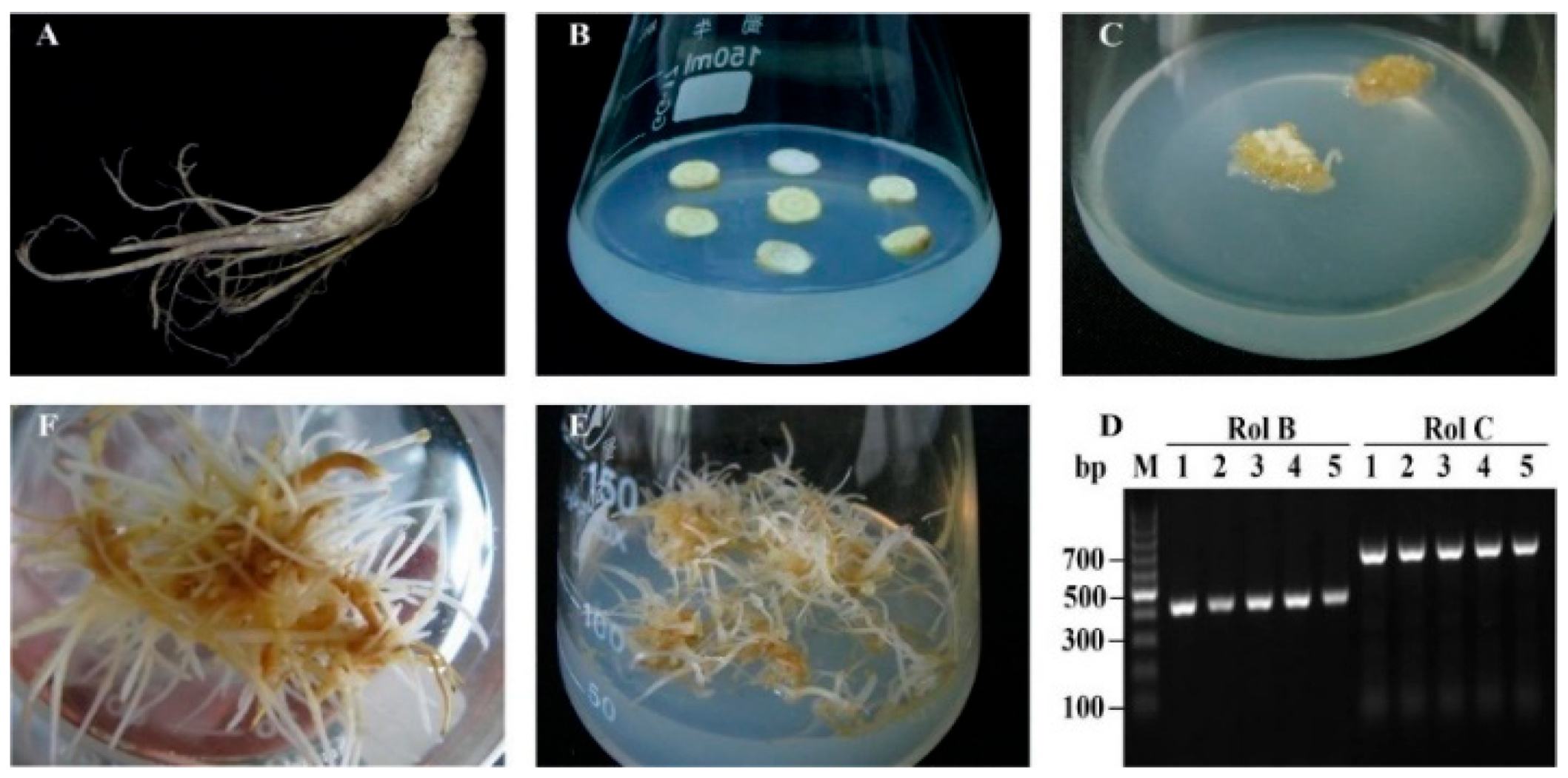

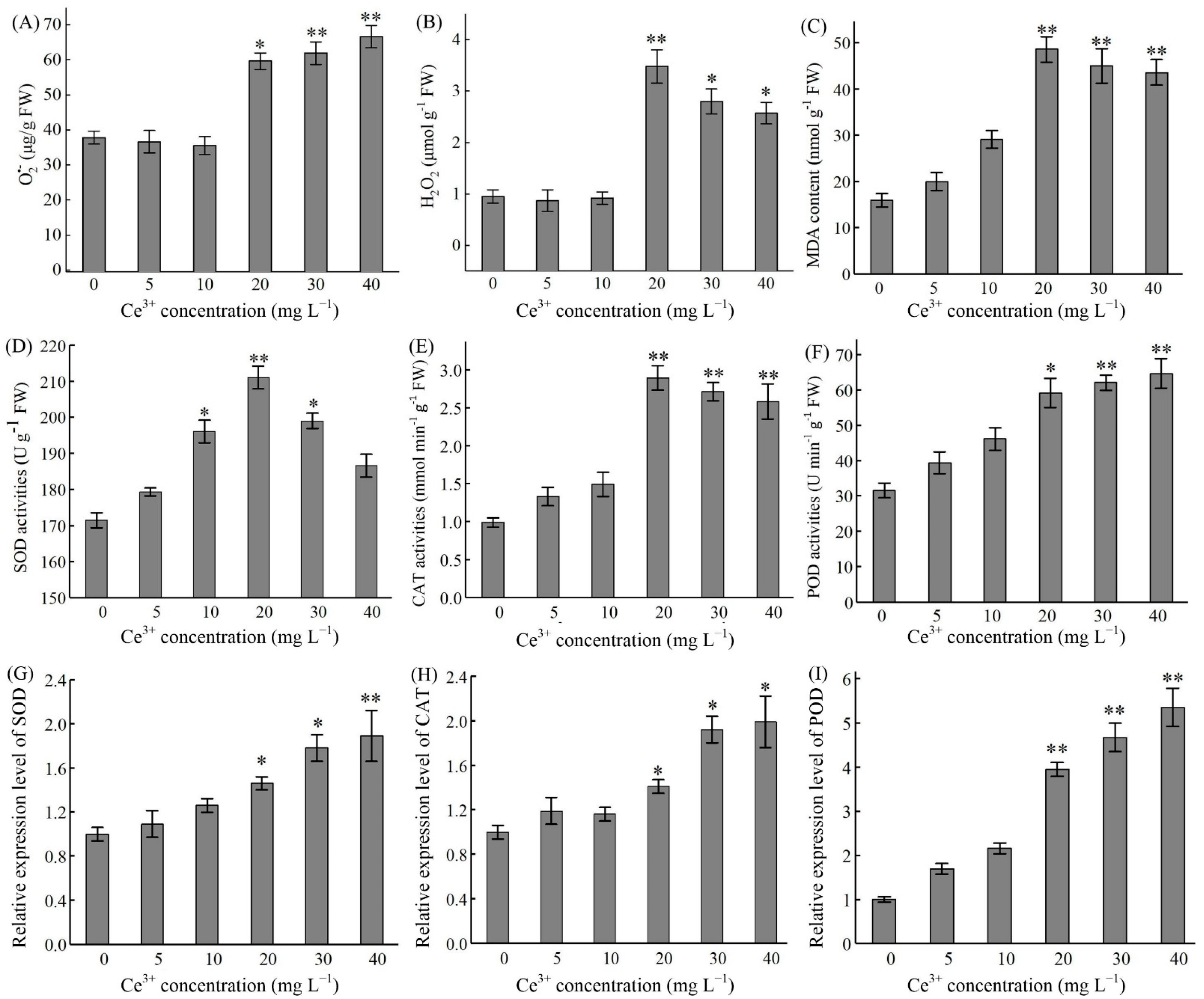
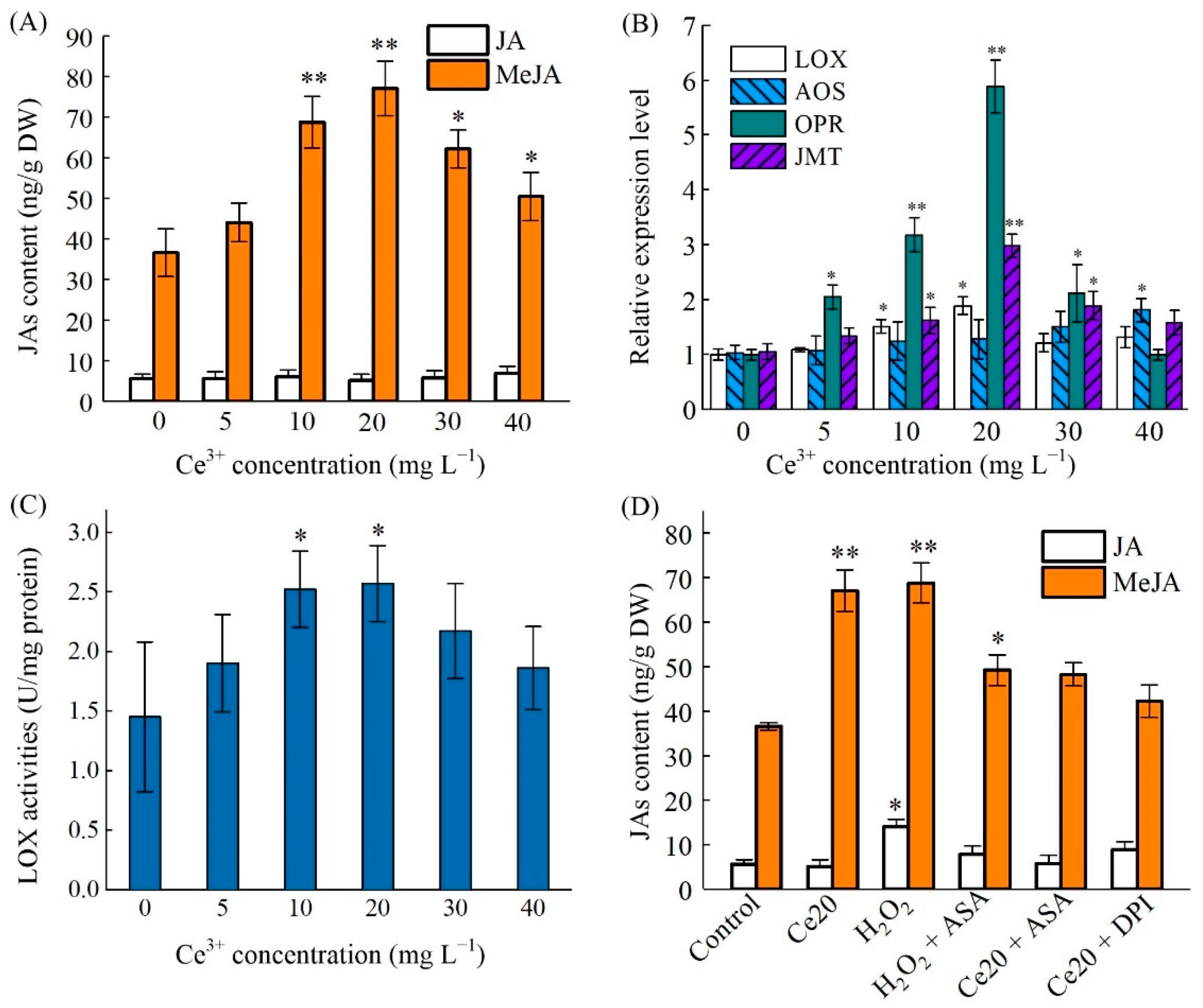
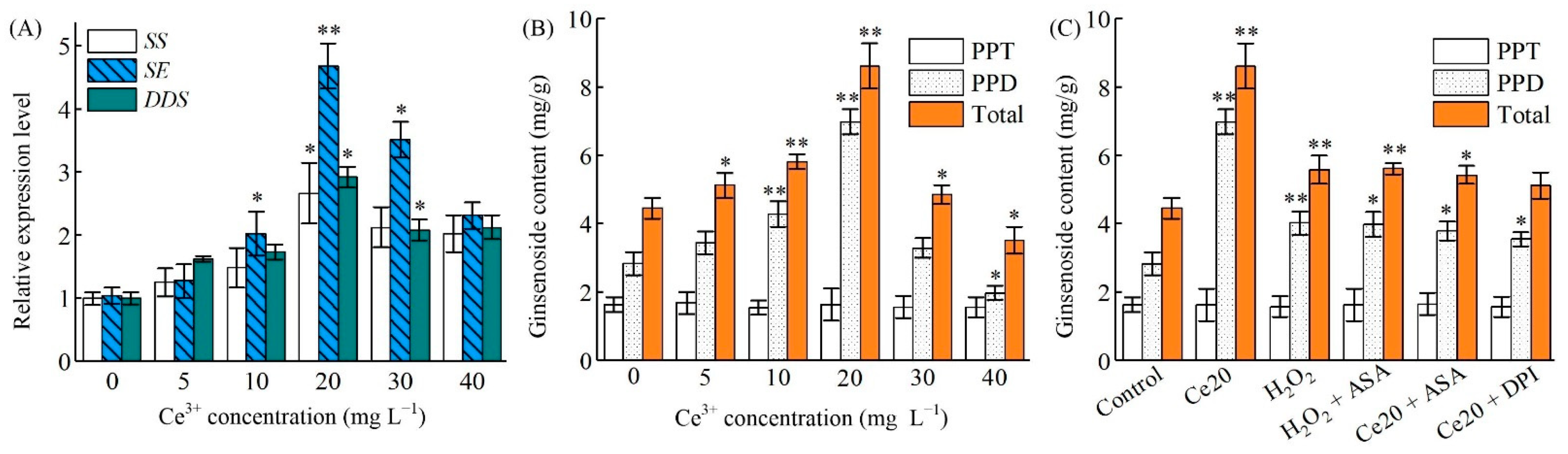
| Ce Concentration mg L−1 | Hairy Roots Growth Parameters | ||||
|---|---|---|---|---|---|
| Average Root Length (mm) | Fresh Weight (g) | Dry Weight (g) | Dry Matter Content (%) | Growth Ratio | |
| 0 | 3.6 ± 0.1 a | 9.2 ± 0.2 a | 0.8 ± 0.2 a | 8.2 ± 0.3 a | 8.0 ± 0.5 a |
| 5 | 4.0 ± 0.2 b | 12.7 ± 0.3 b | 1.1 ± 0.1 b | 8.4 ± 0.2 a | 11.2 ± 0.7 b |
| 10 | 5.1 ± 0.2 c | 16.0 ± 0.5 c | 1.7 ± 0.1 c | 10.8 ± 0.8 b | 13.9 ± 0.7 c |
| 20 | 5.0 ± 0.1 c | 14.8 ± 0.6 d | 1.6 ± 0.2 c | 11.1 ± 0.9 b | 13.3 ± 0.8 c |
| 30 | 3.6 ± 0.2 a | 10.7 ± 0.4 e | 1.0 ± 0.2 b | 9.6 ± 0.5 c | 9.2 ± 0.6 d |
| 40 | 3.4 ± 0.3 a | 8.1 ± 0.3 f | 0.7 ± 0.1 a | 8.5 ± 0.3 a | 6.7 ± 0.5 e |
| Ce3+ (mg L−1) | Ginsenoside (mg/g FW) | Total Content(mg/g) | Total Yield (mg) | |||||
|---|---|---|---|---|---|---|---|---|
| Rb1 | Rb2 | Rc | Rd | Rg1 | Re | |||
| 0 | 2.2 ± 0.08 a | 0.3 ± 0.01 a | 0.4 ± 0.06 a | 1.9 ± 0.1 a | 2.0 ± 0.1 a | 2.9 ± 0.1 a | 9.6 ± 0.13 a | 88.7 ± 2.1 a |
| 5 | 2.5 ± 0.07 a | 0.4 ± 0.02 b | 0.6 ± 0.08 b | 2.2 ± 0.1 a | 2.1 ± 0.1 a | 3.1 ± 0.3 a | 10.9 ± 0.4 a | 137.8 ± 5.2 b |
| 10 | 3.6 ± 0.2 b | 0.5 ± 0.06 b | 1.3 ± 0.1 c | 3.0 ± 0.3 b | 2.6 ± 0.2 b | 3.2 ± 0.4 b | 14.2 ± 0.5 b | 227.6 ± 8.3 c |
| 20 | 3.9 ± 0.3 b | 0.9 ± 0.08 c | 1.4 ± 0.1 c | 3.9 ± 0.2 c | 2.8 ± 0.1 b | 3.6 ± 0.3 b | 16.4 ± 0.5 c | 242.2 ± 5.6 d |
| 30 | 3.8 ± 0.2 b | 0.8 ± 0.04 c | 1.4 ± 0.1 c | 3.6 ± 0.2 b | 2.8 ± 0.3 b | 3.4 ± 0.4 b | 15.8 ± 0.3 c | 168.8 ± 6.4 e |
| 40 | 2.0 ± 0.1 a | 0.3 ± 0.05 a | 0.3 ± 0.06 a | 1.9 ± 0.1 a | 2.0 ± 0.1 a | 2.9 ± 0.3 a | 10.1 ± 0.2 a | 81.7 ± 4.2 a |
| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| PgSOD | Forward | CTAAACCCCTCACCGTCGTC |
| Reverse | TTCACTGTAGTTGGGCCGTC | |
| PgCAT | Forward | AGATACCGGACTTTTGCGCC |
| Reverse | GACACCCATATGCTGCGGAT | |
| PgPOD | Forward | GGATTCTTCCACCGCTCCAA |
| Reverse | CCTTTGTCGGCGAACGTTTT | |
| PgLOX | Forward | CGTGGTGGACAGAAATCCGA |
| Reverse | TTGAGGGGTTTTGAGGACGG | |
| PgAOS | Forward | CTGCAGGTGGAATTAGCGGA |
| Reverse | CATCCGGAGCGATTCGTACA | |
| PgOPR | Forward | GATGGCTCTAGCGTTGCAGA |
| Reverse | TGACTGTTAACACCTACCGGC | |
| PgJMT | Forward | ACAGGAGGCCGAATGGTTTT |
| Reverse | CCTTAAGGGCGACGGCTAAT | |
| PgSS | Forward | TAGGCATGCGGAAAAGCAGA |
| Reverse | TGTTGAATGACGAGGCCGAA | |
| PgSE | Forward | TCGCGATCTTCTTAGGCCAC |
| Reverse | CCACTGGCTTGCGTAATGTG | |
| PgDDS | Forward | CCCTGCAGTGCCTACTGTTA |
| Reverse | CTCCCAAACTGCGAAACCAC | |
| RolB | Forward | GCTCTTGCAGTGCTAGATTT |
| Reverse | GAAGGTGCAAGCTACCTCTC | |
| RolC | Forward | CTCCTGACATCAAACTCGTC |
| Reverse | TGCTTCGAGTTATGGGTACA | |
| β-actin | Forward | TGCCCCAGAAGAGCACCCTGT |
| Reverse | AGCATACAGGGAAAGATCGGCTTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Tan, S.; Zhang, B.; Hu, P.; Li, L. Cerium-Promoted Ginsenosides Accumulation by Regulating Endogenous Methyl Jasmonate Biosynthesis in Hairy Roots of Panax ginseng. Molecules 2021, 26, 5623. https://doi.org/10.3390/molecules26185623
Zhang R, Tan S, Zhang B, Hu P, Li L. Cerium-Promoted Ginsenosides Accumulation by Regulating Endogenous Methyl Jasmonate Biosynthesis in Hairy Roots of Panax ginseng. Molecules. 2021; 26(18):5623. https://doi.org/10.3390/molecules26185623
Chicago/Turabian StyleZhang, Ru, Shiquan Tan, Bianling Zhang, Pengcheng Hu, and Ling Li. 2021. "Cerium-Promoted Ginsenosides Accumulation by Regulating Endogenous Methyl Jasmonate Biosynthesis in Hairy Roots of Panax ginseng" Molecules 26, no. 18: 5623. https://doi.org/10.3390/molecules26185623
APA StyleZhang, R., Tan, S., Zhang, B., Hu, P., & Li, L. (2021). Cerium-Promoted Ginsenosides Accumulation by Regulating Endogenous Methyl Jasmonate Biosynthesis in Hairy Roots of Panax ginseng. Molecules, 26(18), 5623. https://doi.org/10.3390/molecules26185623





