In Vitro Study of the Bioavailability and Bioaccessibility of the Main Compounds Present in Ayahuasca Beverages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Main Compounds in Initial Samples and after Digestive Process
2.2. Cell Culture
2.2.1. Evaluation of Cell Viability
2.2.2. Evaluation of the Electrical Resistance of the Cell Transendothelial Membrane
2.2.3. Evaluation of Cell Monolayer Permeability
2.2.4. Characterization of the Main Compounds after Cell Incubation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sample and Working Solutions Preparation
3.3. In Vitro Simulation of Human Digestion Process
3.4. Cell Culture
3.4.1. MTT Cell Viability Assay
3.4.2. Transepithelial Electrical Resistance Assay
3.4.3. Lucifer Yellow Permeability Assay
3.5. Instrumental and Chromatographic Conditions
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Estrella-Parra, E.A.; Almanza-Pérez, J.C.; Alarcón-Aguilar, F.J. Ayahuasca: Uses, Phytochemical and Biological Activities. Nat. Prod. Bioprospect. 2019, 9, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Hamill, J.; Hallak, J.; Dursun, S.M.; Baker, G. Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness. Curr. Neuropharmacol. 2018, 17, 108–128. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Luís, Â.; Gallardo, E.; Duarte, A.P. Psychoactive substances of natural origin: Toxicological aspects, therapeutic properties and analysis in biological samples. Molecules 2021, 26, 1397. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.S.; de Oliveira, R.; da Silva, M.L.; Von Zuben, M.V.; Grisolia, C.K.; Domingues, I.; Caldas, E.D.; Pic-Taylor, A. Exposure to ayahuasca induces developmental and behavioral alterations on early life stages of zebrafish. Chem. Biol. Interact. 2018, 293, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gable, R.S. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007, 102, 24–34. [Google Scholar] [CrossRef]
- Gonçalves, J.; Luís, Â.; Gradillas, A.; García, A.; Restolho, J.; Fernández, N.; Domingues, F.; Gallardo, E.; Duarte, A.P. Ayahuasca beverages: Phytochemical analysis and biological properties. Antibiotics 2020, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.Y.; Gonçalves, J.; Duarte, A.P.; Barroso, M.; Cristóvão, A.C.; Gallardo, E. Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review. Medicines 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Riba, J.; Valle, M.; Urbano, G.; Yritia, M.; Morte, A.; Barbanoj, M.J. Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J. Pharmacol. Exp. Ther. 2003, 306, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Callaway, J.C.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Raymon, L.P.; Poland, R.E.; Andrade, E.N.; Andrade, E.O.; Mash, D.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Bouso, J.C.; Hallak, J.E.C. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Ther. Adv. Psychopharmacol. 2017, 7, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Gaujac, A.; Dempster, N.; Navickiene, S.; Brandt, S.D.; De Andrade, J.B. Determination of N,N-dimethyltryptaminein beverages consumed in religious practices by headspace solid-phase microextraction followed by gas chromatography ion trap mass spectrometry. Talanta 2013, 106, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Padoan, E.; Ajmone-Marsan, F. Soil particle size fraction and potentially toxic elements bioaccessibility: A review. Ecotoxicol. Environ. Saf. 2021, 209, 111806. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Ramos, R.; Luís, Â.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef] [Green Version]
- Versantvoort, C.H.M.; Oomen, A.G.; Van De Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Barthe, L.; Woodley, J.; Houin, G. Gastrointestinal absorption of drugs: Methods and studies. Fundam. Clin. Pharmacol. 1999, 13, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar] [CrossRef]
- Devkar, S.; Kandhare, A.; Sloley, B.; Jagtap, S.; Lin, J.; Tam, Y.; Katyare, S.; Bodhankar, S.; Hegde, M. Evaluation of the bioavailability of major withanolides of Withania somnifera using an in vitro absorption model system. J. Adv. Pharm. Technol. Res. 2015, 6, 159–164. [Google Scholar]
- Cameron, L.P.; Olson, D.E. Dark Classics in Chemical Neuroscience: N, N-Dimethyltryptamine (DMT). ACS Chem. Neurosci. 2018, 9, 2344–2357. [Google Scholar] [CrossRef]
- Riba, J.; Romero, S.; Grasa, E.; Mena, E.; Carrió, I.; Barbanoj, M.J. Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology 2006, 186, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Araujo, D.B.; Ribeiro, S.; Cecchi, G.A.; Carvalho, F.M.; Sanchez, T.A.; Pinto, J.P.; de Martinis, B.S.; Crippa, J.A.; Hallak, J.E.C.; Santos, A.C. Seeing with the eyes shut: Neural basis of enhanced imagery following ayahuasca ingestion. Hum. Brain Mapp. 2012, 33, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed on 9 June 2021).
- Bensalem, S.; Soubhye, J.; Aldib, I.; Bournine, L.; Nguyen, A.T.; Vanhaeverbeek, M.; Rousseau, A.; Boudjeltia, K.Z.; Sarakbi, A.; Kauffmann, J.M.; et al. Inhibition of myeloperoxidase activity by the alkaloids of Peganum harmala L. (Zygophyllaceae). J. Ethnopharmacol. 2014, 154, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Wang, Y.H.; Rumalla, C.S.; Smillie, T.J.; Khan, I.A. Simultaneous determination of alkaloids and flavonoids from aerial parts of Passiflora species and dietary supplements using UPLC-UV-MS and HPTLC. Nat. Prod. Commun. 2012, 7, 1177–1180. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.P.S.; De Oliveira, C.D.R.; Moura, S.; Dörr, F.A.; Silva, W.A.E.; Yonamine, M. Gas chromatographic analysis of dimethyltryptamine and β-carboline alkaloids in Ayahuasca, an amazonian psychoactive plant beverage. Phytochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef]
- Souza, R.C.Z.; Zandonadi, F.S.; Freitas, D.P.; Tófoli, L.F.F.; Sussulini, A. Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1124, 197–203. [Google Scholar] [CrossRef]
- Chambers, M.I.; Appley, M.G.; Longo, C.M.; Musah, R.A. Detection and Quantification of Psychoactive N, N-Dimethyltryptamine in Ayahuasca Brews by Ambient Ionization High-Resolution Mass Spectrometry. ACS Omega 2020, 5, 28547–28554. [Google Scholar] [CrossRef]
- Kaasik, H.; Souza, R.C.Z.; Zandonadi, F.S.; Tófoli, L.F.; Sussulini, A. Chemical Composition of Traditional and Analog Ayahuasca. J. Psychoact. Drugs 2021, 53, 65–75. [Google Scholar] [CrossRef]
- Taylor, W.I. Simple Derivatives Of Tryptophan. In Indole Alkaloids; Taylor, W.I., Ed.; Pergamon Press, Elsevier: Amsterdam, The Netherlands, 1966; Volume 1, pp. 29–51. ISBN 9781483196718. [Google Scholar]
- Katchborian-Neto, A.; Santos, W.T.; Nicácio, K.J.; Corrêa, J.O.A.; Murgu, M.; Martins, T.M.M.; Gomes, D.A.; Goes, A.M.; Soares, M.G.; Dias, D.F.; et al. Neuroprotective potential of Ayahuasca and untargeted metabolomics analyses: Applicability to Parkinson’s disease. J. Ethnopharmacol. 2020, 255, 112743. [Google Scholar] [CrossRef]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbrecht, D.H.; Long, C.J.; Hickman, J.J. Transepithelial/endothelial Electrical Resistance (TEER) theory and ap-plications for microfluidic body-on-a-chip devices Keywords TEER Body-on-a-chip Barrier tissue Blood-brain barrier Organ Endothelial cells Epithelial cells Human-on-a-chip. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar]
- Hellinger, É.; Veszelka, S.; Tóth, A.E.; Walter, F.; Kittel, Á.; Bakk, M.L.; Tihanyi, K.; Háda, V.; Nakagawa, S.; Dinh Ha Duy, T.; et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012, 82, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Perego, S.; Ardoino, I.; Marasco, E.; Lombardi, G.; Fiorilli, A.; Biganzoli, E.; Tettamanti, G.; Ferraretto, A. Evaluation of a possible direct effect by casein phosphopeptides on paracellular and vitamin D controlled transcellular calcium transport mechanisms in intestinal human HT-29 and Caco2 cell lines. Food Funct. 2013, 4, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Yokoyama, T.; Ogawa, N.; Fujiwara-Hatano, Y.; Shimizu, M. The changes in the neuronal PC12 and the intestinal epithelial Caco-2 cells during the coculture. The functional analysis using an in vitro coculture system. Cytotechnology 2001, 35, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Piccolino, M.; Neyton, J.; Gerschenfeld, H.M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′:5′ -monophosphate in horizontal cells of turtle retina. J. Neurosci. 1984, 4, 2477–2488. [Google Scholar] [CrossRef]
- Fazzari, M.; Fukumoto, L.; Mazza, G.; Livrea, M.A.; Tesoriere, L.; Di Marco, L. In vitro bioavailability of phenolic compounds from five cultivars of frozen sweet cherries (Prunus avium L.). J. Agric. Food Chem. 2008, 56, 3561–3568. [Google Scholar] [CrossRef]
- Toydemir, G.; Boyacioglu, D.; Capanoglu, E.; Van Der Meer, I.M.; Tomassen, M.M.M.; Hall, R.D.; Mes, J.J.; Beekwilder, J. Investigating the transport dynamics of anthocyanins from unprocessed fruit and processed fruit juice from sour cherry (Prunus cerasus L.) across intestinal epithelial cells. J. Agric. Food Chem. 2013, 61, 11434–11441. [Google Scholar] [CrossRef]
- ATCC Caco-2 [Caco2]|ATCC. Available online: https://www.atcc.org/products/htb-37 (accessed on 2 September 2021).
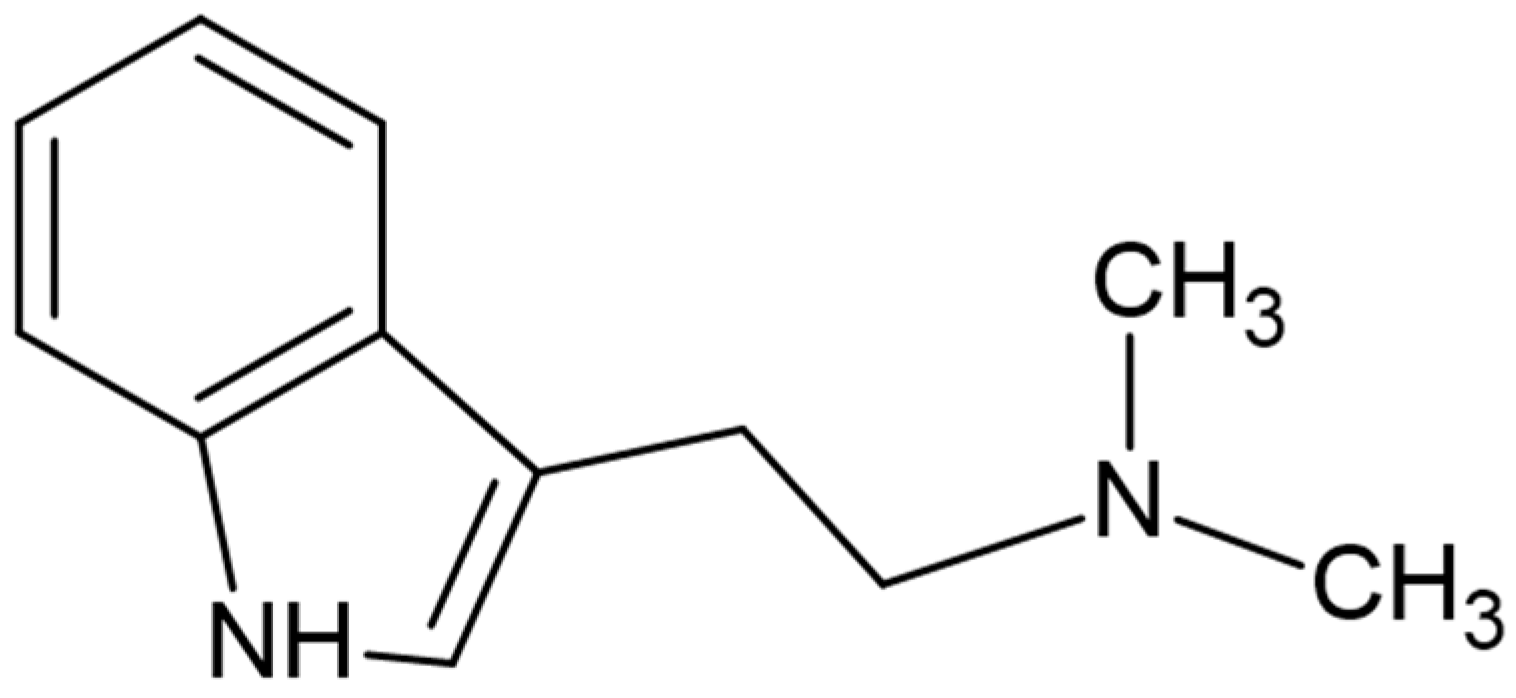

| Samples | Compounds | Initial Concentrations |
|---|---|---|
| P. viridis | DMT | 6.50 ± 0.01 |
| B. caapi | THH | 5.00 ± 0.10 |
| Harmol | 0.14 ± 0.00 | |
| Harmine | 10.00 ± 0.28 | |
| Harmalol | 0.05 ± 0.00 | |
| Harmaline | 4.68 ± 0.14 | |
| P. harmala | THH | 3.05 ± 0.04 |
| Harmol | 0.02 ± 0.00 | |
| Harmine | 12.00 ± 0.00 | |
| Harmalol | 0.66 ± 0.01 | |
| Harmaline | 17.00 ± 0.00 | |
| M. hostilis | DMT | 10.50 ± 0.02 |
| Commercial mixture | DMT | 10.40 ± 0.01 |
| THH | 2.09 ± 0.07 | |
| Harmol | 0.01 ± 0.00 | |
| Harmine | 0.02 ± 0.00 | |
| Harmalol | ND | |
| Harmaline | 0.37 ± 0.02 | |
| P. viridis + B. caapi | DMT | 4.50 ± 0.01 |
| THH | 2.50 ± 0.07 | |
| Harmol | 0.01 ± 0.00 | |
| Harmine | 0.48 ± 0.00 | |
| Harmalol | ND | |
| Harmaline | 0.07 ± 0.00 | |
| P. viridis + P. harmala | DMT | 6.50 ± 0.09 |
| THH | 0.63 ± 0.05 | |
| Harmol | 0.02 ± 0.00 | |
| Harmine | 0.30 ± 0.01 | |
| Harmalol | 0.08 ± 0.00 | |
| Harmaline | 0.48 ± 0.01 | |
| M. hostilis + B. caapi | DMT | 8.00 ± 0.02 |
| THH | 1.90 ± 0.06 | |
| Harmol | 0.03 ± 0.00 | |
| Harmine | 0.82 ± 0.02 | |
| Harmalol | 0.04 ± 0.00 | |
| Harmaline | 0.12 ± 0.00 | |
| M. hostilis + P. harmala | DMT | 8.50 ± 0.01 |
| THH | 3.44 ± 0.05 | |
| Harmol | 0.06 ± 0.00 | |
| Harmine | 9.00 ± 0.00 | |
| Harmalol | 0.36 ± 0.00 | |
| Harmaline | 13.5 ± 0.06 |
| Samples | Compounds | Salivary | Gastric | Duodenal |
|---|---|---|---|---|
| P. viridis | DMT | 0.84 ± 0.60 | 7.77 ± 0.08 | 7.49 ± 0.19 |
| B. caapi | THH | 0.83 ± 0.00 | 0.78 ± 0.13 | 1.05 ± 0.09 |
| Harmol | ND | ND | ND | |
| Harmine | 1.56 ± 0.00 | 4.13 ± 0.03 | 1.98 ± 0.03 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 0.19 ± 0.00 | 0.33 ± 0.00 | 0.21 ± 0.00 | |
| P. harmala | THH | 1.52 ± 0.09 | 1.66 ± 0.12 | 1.32 ± 0.01 |
| Harmol | ND | ND | ND | |
| Harmine | 18.38 ± 0.18 | 19.52 ± 0.05 | 10.02 ± 0.01 | |
| Harmalol | 1.47 ± 0.04 | 1.54 ± 0.02 | 1.03 ± 0.07 | |
| Harmaline | 29.66 ± 0.10 | 26.18 ± 0.14 | 22.88 ± 0.26 | |
| M. hostilis | DMT | 9.55 ± 0.03 | 8.96 ± 0.17 | 8.33 ± 0.00 |
| Commercial mixture | DMT | 4.28 ± 0.05 | 4.09 ± 0.02 | 3.38 ± 0.08 |
| THH | 0.95 ± 0.05 | 1.42 ± 0.00 | 0.50 ± 0.03 | |
| Harmol | ND | ND | ND | |
| Harmine | ND | ND | ND | |
| Harmalol | 1.21 ± 0.00 | 1.02 ± 0.01 | 0.81 ± 0.01 | |
| Harmaline | 1.29 ± 0.03 | 1.12 ± 0.01 | 0.85 ± 0.00 | |
| P. viridis + B. caapi | DMT | 2.37 ± 0.01 | 1.61 ± 0.05 | 2.00 ± 0.02 |
| THH | 3.33 ± 0.05 | 4.05 ± 0.11 | 2.82 ± 0.02 | |
| Harmol | 0.29 ± 0.00 | 0.34 ± 0.00 | 0.26 ± 0.01 | |
| Harmine | 1.23 ± 0.03 | 3.09 ± 0.22 | 1.80 ± 0.05 | |
| Harmalol | 0.26 ± 0.00 | 0.27 ± 0.02 | 0.25 ± 0.00 | |
| Harmaline | 0.19 ± 0.01 | 0.40 ± 0.00 | 0.26 ± 0.01 | |
| P. viridis + P. harmala | DMT | 4.30 ± 0.08 | 3.94 ± 1.33 | 4.56 ± 0.15 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | 1.64 ± 0.01 | 6.70 ± 0.12 | 3.05 ± 0.10 | |
| Harmalol | 0.37 ± 0.01 | 0.38 ± 0.01 | 0.34 ± 0.04 | |
| Harmaline | 4.62 ± 0.02 | 8.93 ± 0.04 | 4.05 ± 0.05 | |
| M. hostilis + B. caapi | DMT | 9.07 ± 0.04 | 5.52 ± 0.09 | 7.36 ± 0.05 |
| THH | 2.89 ± 0.21 | 2.37 ± 0.11 | 2.45 ± 0.04 | |
| Harmol | 0.28 ± 0.02 | 0.24 ± 0.01 | 0.22 ± 0.00 | |
| Harmine | 4.02 ± 0.04 | 10.34 ± 0.07 | 7.69 ± 0.27 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 0.28 ± 0.01 | 0.42 ± 0.01 | 0.29 ± 0.01 | |
| M. hostilis + P. harmala | DMT | 9.65 ± 0.12 | 4.76 ± 0.07 | 6.68 ± 0.16 |
| THH | 0.89 ± 0.01 | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | 4.41 ± 0.07 | 10.92 ± 0.23 | 9.38 ± 0.05 | |
| Harmalol | 0.60 ± 0.03 | 0.87 ± 0.01 | 0.63 ± 0.04 | |
| Harmaline | 11.45 ± 0.20 | 16.96 ± 0.11 | 12.08 ± 0.02 |
| Samples | Cell Viability (%) |
|---|---|
| P. viridis Crude | 156.01 ± 27.31 |
| P. viridis Digested | 128.85 ± 9.03 |
| B. caapi Crude | 95.92 ± 1.83 |
| B. caapi Digested | 113.66 ± 11.59 |
| P. harmala Crude | 171.46 ± 28.88 |
| P. harmala Digested | 117.62 ± 3.59 |
| M. hostilis Crude | 148.28 ± 14.18 |
| M. hostilis Digested | 96.04 ± 12.23 |
| Commercial mixture Crude | 101.50 ± 13.25 |
| Commercial mixture Digested | 79.52 ± 0.93 |
| P. viridis + B. caapi Crude | 148.07 ± 26.83 |
| P. viridis + B. caapi Digested | 103.74 ± 3.43 |
| P. viridis + P. harmala Crude | 162.55 ± 15.63 |
| P. viridis + P. harmala Digested | 127.75 ± 9.97 |
| M. hostilis + B. caapi Crude | 126.61 ± 16.39 |
| M. hostilis + B. caapi Digested | 118.50 ± 1.59 |
| M. hostilis + P. harmala Crude | 138.41 ± 17.63 |
| M. hostilis + P. harmala Digested | 120.70 ± 3.12 |
| Samples | TEER (Ω cm2) | ||
|---|---|---|---|
| Before | After | p-Value | |
| Control | 990 ± 31.11 | 1034 ± 31.11 | 0.293 |
| P. viridis Crude | 1298 ± 155.56 | 1628 ± 207.94 | 0.239 |
| P. viridis Digested | 1518 ± 93.34 | 2046 ± 155.56 | 0.054 |
| B. caapi Crude | 1166 ± 155.56 | 1408 ± 110.73 | 0.146 |
| B. caapi Digested | 1232 ± 134.42 | 1276 ± 116.41 | 0.317 |
| P. harmala Crude | 1386 ± 217.79 | 1408 ± 124.45 | 0.913 |
| P. harmala Digested | 1188 ± 186.68 | 1496 ± 110.73 | 0.107 |
| M. hostilis Crude | 1254 ± 155.56 | 1298 ± 31.11 | 0.733 |
| M. hostilis Digested | 1584 ± 177.82 | 1496 ± 116.41 | 0.112 |
| Commercial mixture Crude | 1694 ± 155.56 | 1716 ± 232.83 | 0.754 |
| Commercial mixture Digested | 1232 ± 44.00 | 1232 ± 25.40 | 0.643 |
| P. viridis + B. caapi Crude | 1364 ± 186.68 | 1496 ± 0.00 | 0.423 |
| P. viridis + B. caapi Digested | 1166 ± 93.34 | 1386 ± 31.11 | 0.087 |
| P. viridis + P. harmala Crude | 1415 ± 93.34 | 1408 ± 91.59 | 0.936 |
| P. viridis + P. harmala Digested | 1100 ± 141.44 | 1144 ± 116.41 | 0.795 |
| M. hostilis + B. caapi Crude | 1232 ± 76.21 | 1276 ± 25.40 | 0.189 |
| M. hostilis + B. caapi Digested | 1188 ± 248.90 | 1408 ± 127.02 | 0.619 |
| M. hostilis + P. harmala Crude | 1254 ± 93.34 | 1430 ± 31.11 | 0.127 |
| M. hostilis + P. harmala Digested | 1232 ± 177.82 | 1452 ± 0.00 | 0.246 |
| Samples | Permeability (%) | p-Value |
|---|---|---|
| Control | 16.94 ± 2.35 | - |
| P. viridis Crude | 19.59 ± 3.00 | 0.281 |
| P. viridis Digested | 13.49 ± 1.03 | 0.165 |
| B. caapi Crude | 16.79 ± 0.14 | 0.879 |
| B. caapi Digested | 16.11 ± 0.49 | 0.823 |
| P. harmala Crude | 15.01 ± 0.46 | 0.462 |
| P. harmala Digested | 17.97 ± 1.37 | 0.523 |
| M. hostilis Crude | 14.38 ± 0.72 | 0.322 |
| M. hostilis Digested | 14.10 ± 0.41 | 0.267 |
| Commercial mixture Crude | 19.88 ± 2.84 | 0.383 |
| Commercial mixture Digested | 16.13 ± 1.83 | 0.865 |
| P. viridis + B. caapi Crude | 16.42 ± 0.40 | 0.959 |
| P. viridis + B. caapi Digested | 16.03 ± 1.50 | 0.463 |
| P. viridis + P. harmala Crude | 13.81 ± 0.49 | 0.225 |
| P. viridis + P. harmala Digested | 13.47 ± 1.85 | 0.283 |
| M. hostilis + B. caapi Crude | 13.07 ± 1.89 | 0.139 |
| M. hostilis + B. caapi Digested | 13.18 ± 0.16 | 0.074 |
| M. hostilis + P. harmala Crude | 12.40 ± 1.64 | 0.069 |
| M. hostilis + P. harmala Digested | 11.65 ± 1.79 | 0.058 |
| Samples | Compounds | Time | ||
|---|---|---|---|---|
| 1 h | 2 h | 4 h | ||
| P. viridis | DMT | 0.50 ± 0.01 | 0.89 ± 0.21 | 1.22 ± 0.05 |
| B. caapi | THH | ND | 0.66 ± 0.02 | 0.59 ± 0.06 |
| Harmol | ND | ND | ND | |
| Harmine | 0.33 ± 0.03 | 1.00 ± 0.01 | 1.35 ± 0.03 | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| P. harmala | THH | ND | ND | 0.72 ± 0.12 |
| Harmol | ND | 0.19 ± 0.02 | ND | |
| Harmine | 2.09 ± 0.02 | 4.41 ± 0.23 | 5.90 ± 0.04 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 3.65 ± 0.06 | 5.71 ± 0.60 | 8.55 ± 0.17 | |
| M. hostilis | DMT | ND | 1.18 ± 0.10 | 1.42 ± 0.06 |
| Commercial mixture | DMT | ND | 0.55 ± 0.04 | 0.73 ± 0.04 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | ND | ND | ND | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| P. viridis + B. caapi | DMT | 0.16 ± 0.01 | 0.47 ± 0.06 | 0.63 ± 0.07 |
| THH | 0.65 ± 0.02 | 0.78 ± 0.06 | 0.93 ± 0.04 | |
| Harmol | ND | ND | ND | |
| Harmine | ND | 0.42 ± 0.06 | 0.76 ± 0.04 | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| P. viridis + P. harmala | DMT | 0.33 ± 0.03 | 1.01 ± 0.16 | 1.10 ± 0.07 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | 0.35 ± 0.02 | 0.39 ± 0.01 | 0.78 ± 0.04 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 0.31 ± 0.00 | 0.67 ± 0.09 | 0.76 ± 0.03 | |
| M. hostilis + B. caapi | DMT | 0.69 ± 0.07 | 1.67 ± 0.11 | 1.87 ± 0.04 |
| THH | 0.74 ± 0.08 | 0.53 ± 0.03 | 0.65 ± 0.07 | |
| Harmol | ND | ND | ND | |
| Harmine | 0.98 ± 0.11 | 1.64 ± 0.11 | 2.42 ± 0.08 | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| M. hostilis + P. harmala | DMT | 0.29 ± 0.01 | 0.72 ± 0.04 | 1.24 ± 0.18 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | 0.47 ± 0.02 | 1.07 ± 0.10 | 1.63 ± 0.27 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 0.55 ± 0.08 | 1.07 ± 0.14 | 1.42 ± 0.22 | |
| Samples | Compounds | Time | ||
|---|---|---|---|---|
| 1 h | 2 h | 4 h | ||
| P. viridis | DMT | 0.73 ± 0.00 | 1.48 ± 0.02 | 1.99 ± 0.03 |
| B. caapi | THH | ND | ND | ND |
| Harmol | ND | ND | ND | |
| Harmine | ND | ND | 1.14 ± 0.03 | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| P. harmala | THH | ND | ND | ND |
| Harmol | ND | ND | ND | |
| Harmine | 1.83 ± 0.01 | 3.08 ± 0.09 | 4.19 ± 0.03 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 3.81 ± 0.13 | 4.75 ± 0.12 | 5.63 ± 0.08 | |
| M. hostilis | DMT | ND | 1.61 ± 0.07 | 1.90 ± 0.02 |
| Commercial mixture | DMT | ND | 0.61 ± 0.02 | 0.67 ± 0.02 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | ND | ND | ND | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| P. viridis + B. caapi | DMT | ND | 0.50 ± 0.07 | 0.48 ± 0.01 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | ND | ND | 0.70 ± 0.02 | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| P. viridis + P. harmala | DMT | ND | 0.65 ± 0.00 | 0.86 ± 0.03 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | ND | 1.02 ± 0.01 | 1.26 ± 0.03 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 0.74 ± 0.00 | 0.99 ± 0.01 | 1.26 ± 0.01 | |
| M. hostilis + B. caapi | DMT | 0.78 ± 0.01 | 1.74 ± 0.01 | 2.11 ± 0.02 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | 0.77 ± 0.00 | 1.96 ± 0.01 | 2.54 ± 0.64 | |
| Harmalol | ND | ND | ND | |
| Harmaline | ND | ND | ND | |
| M. hostilis + P. harmala | DMT | 0.60 ± 0.03 | 1.57 ± 0.06 | 1.86 ± 0.02 |
| THH | ND | ND | ND | |
| Harmol | ND | ND | ND | |
| Harmine | 0.85 ± 0.03 | 2.38 ± 0.09 | 3.34 ± 0.04 | |
| Harmalol | ND | ND | ND | |
| Harmaline | 1.10 ± 0.02 | 2.21 ± 0.01 | 3.02 ± 0.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.; Castilho, M.; Rosado, T.; Luís, Â.; Restolho, J.; Fernández, N.; Gallardo, E.; Duarte, A.P. In Vitro Study of the Bioavailability and Bioaccessibility of the Main Compounds Present in Ayahuasca Beverages. Molecules 2021, 26, 5555. https://doi.org/10.3390/molecules26185555
Gonçalves J, Castilho M, Rosado T, Luís Â, Restolho J, Fernández N, Gallardo E, Duarte AP. In Vitro Study of the Bioavailability and Bioaccessibility of the Main Compounds Present in Ayahuasca Beverages. Molecules. 2021; 26(18):5555. https://doi.org/10.3390/molecules26185555
Chicago/Turabian StyleGonçalves, Joana, Miguel Castilho, Tiago Rosado, Ângelo Luís, José Restolho, Nicolás Fernández, Eugenia Gallardo, and Ana Paula Duarte. 2021. "In Vitro Study of the Bioavailability and Bioaccessibility of the Main Compounds Present in Ayahuasca Beverages" Molecules 26, no. 18: 5555. https://doi.org/10.3390/molecules26185555
APA StyleGonçalves, J., Castilho, M., Rosado, T., Luís, Â., Restolho, J., Fernández, N., Gallardo, E., & Duarte, A. P. (2021). In Vitro Study of the Bioavailability and Bioaccessibility of the Main Compounds Present in Ayahuasca Beverages. Molecules, 26(18), 5555. https://doi.org/10.3390/molecules26185555










