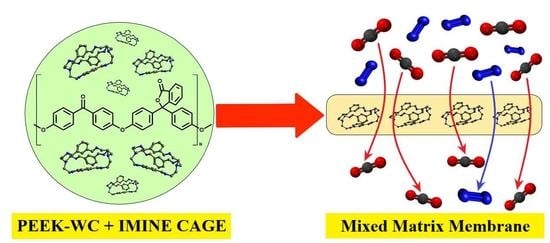
PEEK–WC-Based Mixed Matrix Membranes Containing Polyimine Cages for Gas Separation
Abstract
1. Introduction
2. Results and Discussion
2.1. Cages Preparation and Characterization
2.2. Membrane Preparation and Characterization
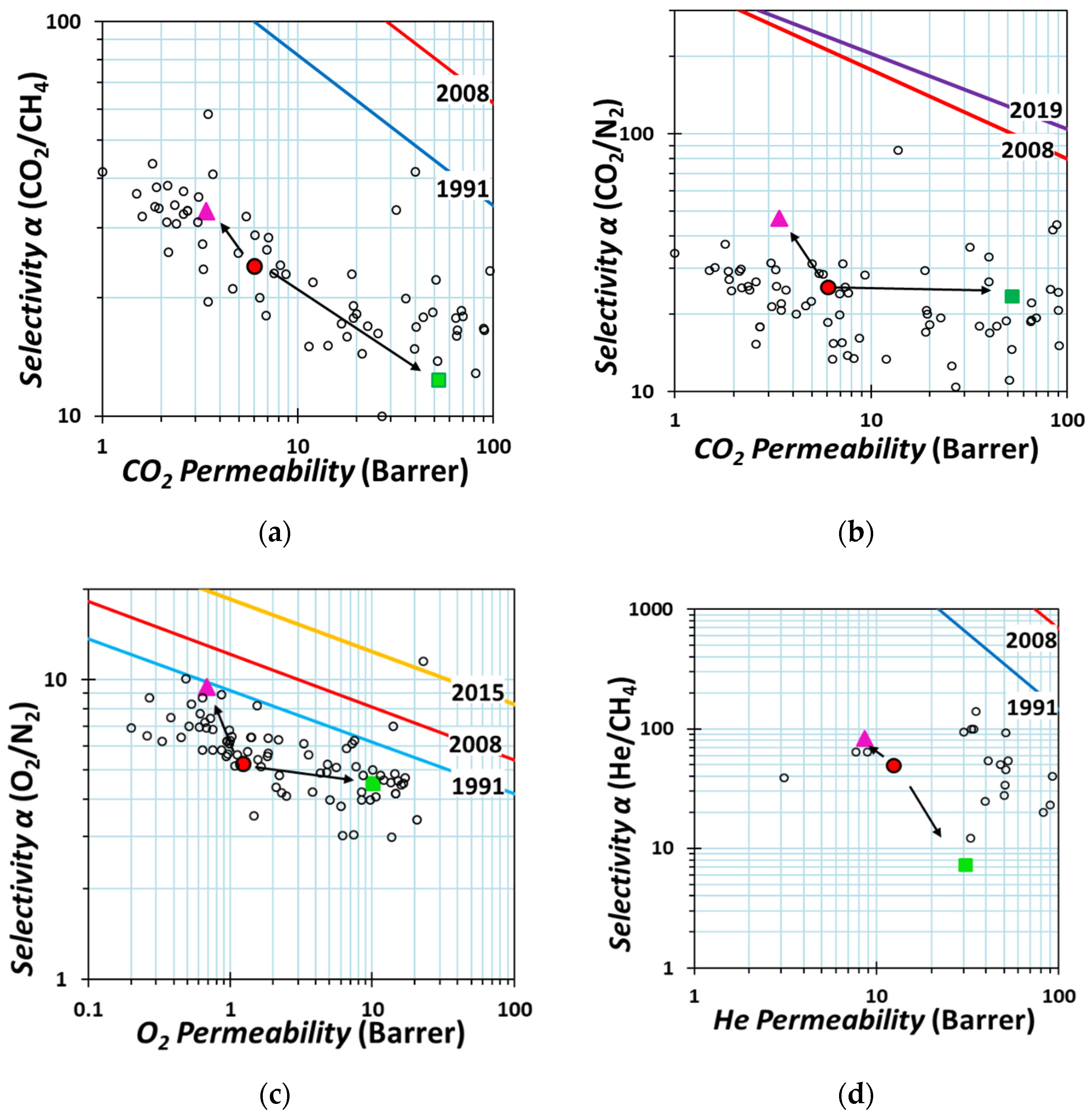
2.3. Pure Gas Transport Properties

3. Materials and Methods
3.1. Materials for Synthesis and Characterization
3.2. Syntheses and Characterization of Cages 1–4
Cages Characterization
3.3. Membranes Preparation
3.4. Membranes Characterization
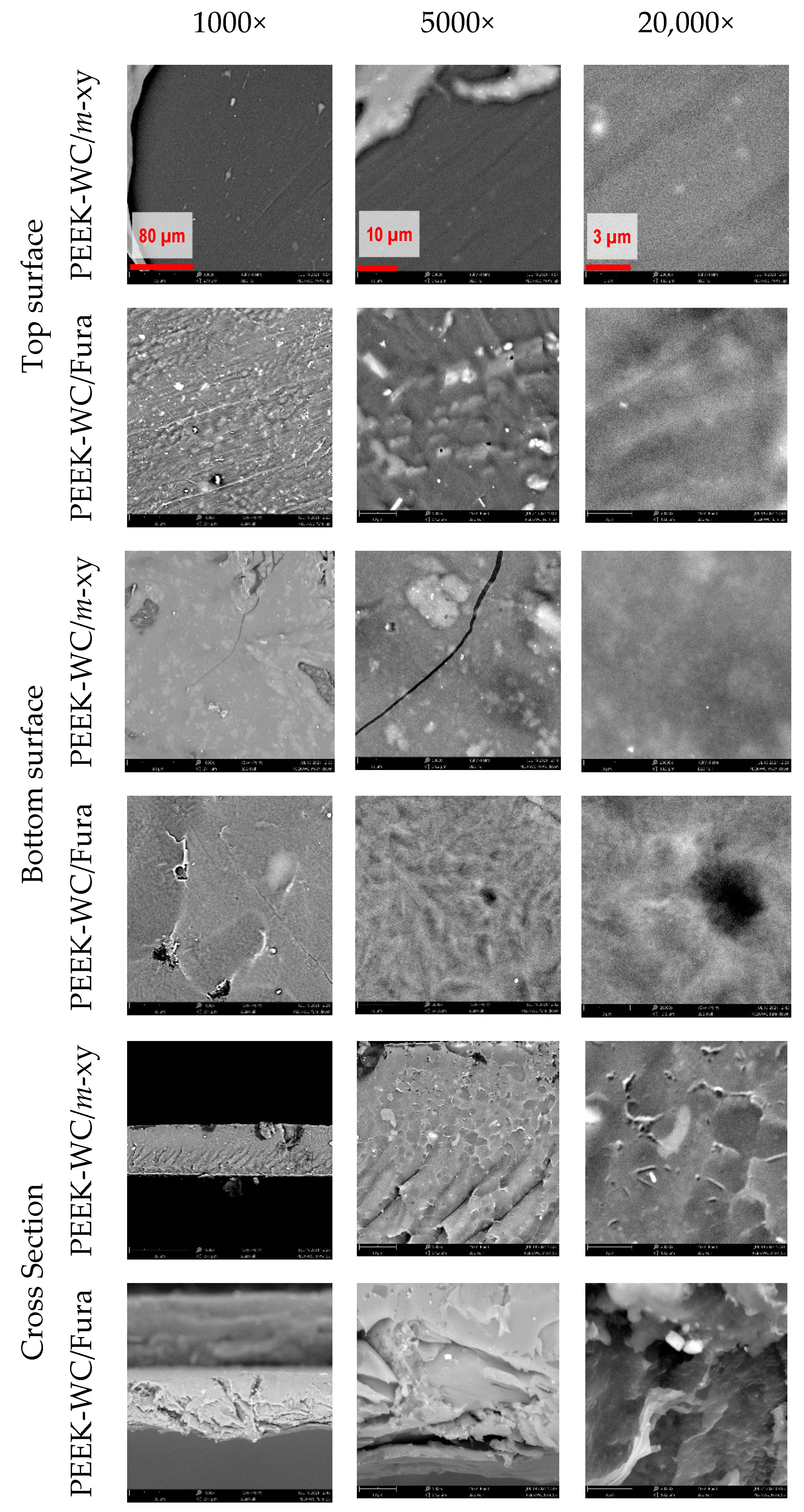
3.4.1. Morphological Characterization Membranes (SEM)
3.4.2. Single Gas Permeation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stanovsky, P.; Karaszova, M.; Petrusova, Z.; Monteleone, M.; Jansen, J.C.; Comesaña-Gándara, B.; McKeown, N.B.; Izak, P. Upgrading of raw biogas using membranes based on the ultrapermeable polymer of intrinsic microporosity PIM-TMN-Trip. J. Membr. Sci. 2021, 618, 118694. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Lu, H.T.; Li, W.; Miandoab, E.S.; Kanehashi, S.; Hu, G. The opportunity of membrane technology for hydrogen purification in the power to hydrogen (P2H) roadmap: A review. Front. Chem. Sci. Eng. 2021, 15, 464–482. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Ly, J.; Vu, J.; Wijmans, J.G.; Merkel, T.C.; Jin, J.; Haldeman, A.; Wagener, E.H.; Rue, D. Membrane-Based Oxygen-Enriched Combustion. Ind. Eng. Chem. Res. 2013, 52, 10820–10834. [Google Scholar] [CrossRef]
- Chawla, M.; Saulat, H.; Masood Khan, M.; Mahmood Khan, M.; Rafiq, S.; Cheng, L.; Iqbal, T.; Rasheed, M.I.; Farooq, M.Z.; Saeed, M.; et al. Membranes for CO2/CH4 and CO2/N2 Gas Separation. Chem. Eng. Technol. 2020, 43, 184–199. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.P.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Khdhayyer, M.; Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jiang, D.; Burrows, A.D.; Esposito, E.; Bernardo, P.; Monteleone, M.; Fuoco, A.; et al. Mixed matrix membranes based on MIL-101 metal–organic frameworks in polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2019, 212, 545–554. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Chen, S.; Duan, J.; Jin, W. Mixed-matrix membranes with soluble porous organic molecular cage for highly efficient C3H6/C3H8 separation. J. Membr. Sci. 2020, 611, 118288. [Google Scholar] [CrossRef]
- Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jones, J.T.A.; Hasell, T.; Cooper, A.I.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Nanoporous organic polymer/cage composite membranes. Angew. Chem. Int. Ed. 2013, 52, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.C.; Drioli, E. Poly(ether ether ketone) derivative membranes—A review of their preparation, properties and potential. Polym. Sci. Ser. A 2009, 51, 1355. [Google Scholar] [CrossRef]
- Esposito, E.; Bruno, R.; Monteleone, M.; Fuoco, A.; Ferrando Soria, J.; Pardo, E.; Armentano, D.; Jansen, J.C. Glassy PEEK-WC vs. Rubbery Pebax®1657 Polymers: Effect on the Gas Transport in CuNi-MOF Based Mixed Matrix Membranes. Appl. Sci. 2020, 10, 1310. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 9780470740880. [Google Scholar]
- Sanders, J.K. Supramolecular Chemistry. Concepts and Perspectives. Von J.-M. Lehn. VCH Verlagsgesellschaft, Weinheim, 1995. 271 S., geb. 128.00 DM/Broschur 58.00 DM.-ISBN 3-527-29312-4/3-527-29311-6. Angew. Chem. 1995, 107, 2617. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Cryptands and Cryptates; World Scientific Publishing Europe Ltd.: London, UK, 2017; ISBN 978-1-78634-369-7. [Google Scholar]
- Kubik, S. Anion Recognition in Aqueous Media by Cyclopeptides and Other Synthetic Receptors. Acc. Chem. Res. 2017, 50, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Ballester, P. Stabilization of reactive species by supramolecular encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Fares, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973. [Google Scholar] [CrossRef]
- Martínez-Ahumada, E.; He, D.; Berryman, V.; López-Olvera, A.; Hernandez, M.; Jancik, V.; Martis, V.; Vera, M.A.; Lima, E.; Parker, D.J.; et al. SO2 Capture Using Porous Organic Cages. Angew. Chem. Int. Ed. 2021, 60, 17556–17563. [Google Scholar] [CrossRef]
- Chen, L.; Reiss, P.S.; Chong, S.Y.; Holden, D.; Jelfs, K.E.; Hasell, T.; Little, M.A.; Kewley, A.; Briggs, M.E.; Stephenson, A.; et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages. Nat. Mater. 2014, 13, 954–960. [Google Scholar] [CrossRef]
- Hasell, T.; Cooper, A.I. Porous organic cages: Soluble, modular and molecular pores. Nat. Rev. Mater. 2016, 1, 16053. [Google Scholar] [CrossRef]
- Beuerle, F.; Gole, B. Covalent Organic Frameworks and Cage Compounds: Design and Applications of Polymeric and Discrete Organic Scaffolds. Angew. Chem. Int. Ed. 2018, 57, 4850–4878. [Google Scholar] [CrossRef] [PubMed]
- Little, M.A.; Cooper, A.I. The Chemistry of Porous Organic Molecular Materials. Adv. Funct. Mater. 2020, 30, 1909842. [Google Scholar] [CrossRef]
- Evans, J.D.; Huang, D.M.; Hill, M.R.; Sumby, C.J.; Thornton, A.W.; Doonan, C.J. Feasibility of mixed matrix membrane gas separations employing porous organic cages. J. Phys. Chem. C 2014, 118, 1523–1529. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Miljkovic, A. Azacryptands as molecular cages for anions and metal ions. Supramol. Chem. 2018, 30, 236–242. [Google Scholar] [CrossRef]
- Alibrandi, G.; Amendola, V.; Bergamaschi, G.; Fabbrizzi, L.; Licchelli, M. Bistren cryptands and cryptates: Versatile receptors for anion inclusion and recognition in water. Org. Biomol. Chem. 2015, 13, 3510–3524. [Google Scholar] [CrossRef]
- Gajula, R.K.; Kishor, R.; Prakash, M.J. Imine-Linked Covalent Organic Cage Porous Crystals for CO2 Adsorption. ChemistrySelect 2019, 4, 12547–12555. [Google Scholar] [CrossRef]
- Gayen, K.S.; Das, T.; Chatterjee, N. Recent Advances in Tris-Primary Amine Based Organic Imine Cages and Related Amine Macrocycles. Eur. J. Org. Chem. 2021, 2021, 861–876. [Google Scholar] [CrossRef]
- Wang, F.; Sikma, E.; Duan, Z.; Sarma, T.; Lei, C.; Zhang, Z.; Humphrey, S.M.; Sessler, J.L. Shape-persistent pyrrole-based covalent organic cages: Synthesis, structure and selective gas adsorption properties. Chem. Commun. 2019, 55, 6185–6188. [Google Scholar] [CrossRef] [PubMed]
- Shimekit, B.; Mukhtar, H.; Murugesan, T. Prediction of the relative permeability of gases in mixed matrix membranes. J. Membr. Sci. 2011, 373, 152–159. [Google Scholar] [CrossRef]
- Pal, R. Permeation models for mixed matrix membranes. J. Colloid Interface Sci. 2008, 317, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejski, M.; Stefankiewicz, A.R.; Lehn, J.M. Dynamic polyimine macrobicyclic cryptands-self-sorting with component selection. Chem. Sci. 2019, 10, 1836–1843. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- McDowell, D.; Nelson, J.; McKee, V. A furan-derived schiff-base crypt and incorporating the trans, trans dicarbimine link. Polyhedron 1989, 8, 1143–1145. [Google Scholar] [CrossRef]
- McKee, V.; Robinson, W.T.; McDowell, D.; Nelson, J. Solid state structure and solution conformation of a macrobicyclic cyclophane. Tetrahedron Lett. 1989, 30, 7453–7456. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Characterization and Performance Evaluation of PDMS/PSF Membrane for CO2/CH4 Separation under the Effect of Swelling. Procedia Eng. 2016, 148, 176–183. [Google Scholar] [CrossRef][Green Version]
- Haider, B.; Dilshad, M.R.; Atiq Ur Rehman, M.; Vargas Schmitz, J.; Kaspereit, M. Highly permeable novel PDMS coated asymmetric polyethersulfone membranes loaded with SAPO-34 zeoilte for carbon dioxide separation. Sep. Purif. Technol. 2020, 248, 116899. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Badieh, M.M.S.; Vatanpour, V.; Ghaemi, N. Effect of titanium dioxide nanoparticles on polydimethylsiloxane/polyethersulfone composite membranes for gas separation. Polym. Eng. Sci. 2012, 52, 2664–2674. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Badieh, M.M.S.; Vatanpour, V. Effect of coating method on gas separation by PDMS/PES membrane. Polym. Eng. Sci. 2013, 53, 1878–1885. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Drioli, E. Organic vapour transport in glassy perfluoropolymer membranes: A simple semi-quantitative approach to analyze clustering phenomena by time lag measurements. J. Membr. Sci. 2011, 367, 141–151. [Google Scholar] [CrossRef]
- Jansen, J.; Macchione, M.; Drioli, E. High flux asymmetric gas separation membranes of modified poly(ether ether ketone) prepared by the dry phase inversion technique. J. Membr. Sci. 2005, 255, 167–180. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Fuoco, A.; Rizzuto, C.; Tocci, E.; Monteleone, M.; Esposito, E.; Budd, P.M.; Carta, M.; Comesaña-Gándara, B.; McKeown, N.B.; Jansen, J.C. The origin of size-selective gas transport through polymers of intrinsic microporosity. J. Mater. Chem. A 2019, 7, 20121–20126. [Google Scholar] [CrossRef]
- Jansen, J.C.; Buonomenna, M.G.; Figoli, A.; Drioli, E. Asymmetric membranes of modified poly(ether ether ketone) with an ultra-thin skin for gas and vapour separations. J. Membr. Sci. 2006, 272, 188–197. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]
- Miljkovic, A.; La Cognata, S.; Bergamaschi, G.; Freccero, M.; Poggi, A.; Amendola, V. Towards building blocks for supramolecular architectures based on azacryptates. Molecules 2020, 25, 1733. [Google Scholar] [CrossRef]
- Yang, Z.; Lehn, J.M. Dynamic covalent self-sorting and kinetic switching processes in two cyclic orders: Macrocycles and macrobicyclic cages. J. Am. Chem. Soc. 2020, 142, 15137–15145. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.C.; Monteleone, M.; Lanč, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnáček, K.; Friess, K.; Carta, M.; McKeown, N.B.; et al. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution–Diffusion Model: A Unified Approach to Membrane Permeation. In Materials Science of Membranes for Gas and Vapor Separation; Freeman, B., Yampolskii, Y., Pinnau, I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]





| Solubility (wt/vol %) in the Given Solvents | |||||||
|---|---|---|---|---|---|---|---|
| No. | Code | Polyimine Cages | MeOH | EtOH | CHCl3 | CH2Cl2 | THF |
| 1 | Fura | furanyl-based | 0.10 | 0.08 | 0.13 | 0.10 | X |
| 2 | m-xy | m-xylyl-based | 0.13 | 0.10 | 0.40 | 0.40 | 0.20 |
| 3 | p-xy | p-xylyl-based | X | X | 0.20 | 0.13 | X |
| 4 | diphen | diphenyl-based | X | X | 0.10 | 0.07 | X |
| Membrane | Permeability [Barrer] | α(Pa/Pb) | ||||||||
| N2 | O2 | CO2 | CH4 | H2 | He | CO2/CH4 | CO2/N2 | O2/N2 | He/CH4 | |
| PEEK-WC [13] * | 0.24 | 1.24 | 6.04 | 0.25 | 13.4 | 12.5 | 24.2 | 25.2 | 5.17 | 50.0 |
| PEEK-WC/m-xy | 0.07 | 0.68 | 3.41 | 0.10 | 7.75 | 8.58 | 34.1 | 48.7 | 9.71 | 85.8 |
| PEEK-WC/Fura | 2.25 | 10.1 | 52.7 | 4.27 | 40.1 | 30.9 | 12.3 | 23.4 | 4.49 | 7.24 |
| Membrane | Diffusivity [10−12 m2 s−1] | α(Da/Db) | ||||||||
| N2 | O2 | CO2 | CH4 | H2 | He | CO2/CH4 | CO2/N2 | O2/N2 | He/CH4 | |
| PEEK-WC [13] * | 0.45 | 2.02 | 0.58 | 0.14 | 135 | 529 | 4.14 | 1.29 | 4.49 | 3779 |
| PEEK-WC/m-xy | 1.02 | 3.86 | 0.85 | 0.20 | 188 | 708 | 4.25 | 0.83 | 3.78 | 3540 |
| PEEK-WC/Fura | 28.0 | 53.0 | 15.1 | 10.5 | 455 | 776 | 1.44 | 0.54 | 1.89 | 73.9 |
| Membrane | Solubility [cm3STP cm−3 bar−1] | α(Sa/Sb) | ||||||||
| N2 | O2 | CO2 | CH4 | H2 | He | CO2/CH4 | CO2/N2 | O2/N2 | He/CH4 | |
| PEEK-WC [13] * | 0.39 | 0.46 | 7.77 | 1.33 | 0.07 | 0.02 | 5.84 | 19.9 | 1.18 | 0.015 |
| PEEK-WC/m-xy | 0.05 | 0.13 | 3.02 | 0.38 | 0.03 | 0.009 | 7.95 | 60.4 | 2.60 | 0.024 |
| PEEK-WC/Fura | 0.06 | 0.14 | 2.62 | 0.30 | 0.07 | 0.03 | 8.73 | 43.7 | 2.33 | 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteleone, M.; Mobili, R.; Milanese, C.; Esposito, E.; Fuoco, A.; La Cognata, S.; Amendola, V.; Jansen, J.C. PEEK–WC-Based Mixed Matrix Membranes Containing Polyimine Cages for Gas Separation. Molecules 2021, 26, 5557. https://doi.org/10.3390/molecules26185557
Monteleone M, Mobili R, Milanese C, Esposito E, Fuoco A, La Cognata S, Amendola V, Jansen JC. PEEK–WC-Based Mixed Matrix Membranes Containing Polyimine Cages for Gas Separation. Molecules. 2021; 26(18):5557. https://doi.org/10.3390/molecules26185557
Chicago/Turabian StyleMonteleone, Marcello, Riccardo Mobili, Chiara Milanese, Elisa Esposito, Alessio Fuoco, Sonia La Cognata, Valeria Amendola, and Johannes C. Jansen. 2021. "PEEK–WC-Based Mixed Matrix Membranes Containing Polyimine Cages for Gas Separation" Molecules 26, no. 18: 5557. https://doi.org/10.3390/molecules26185557
APA StyleMonteleone, M., Mobili, R., Milanese, C., Esposito, E., Fuoco, A., La Cognata, S., Amendola, V., & Jansen, J. C. (2021). PEEK–WC-Based Mixed Matrix Membranes Containing Polyimine Cages for Gas Separation. Molecules, 26(18), 5557. https://doi.org/10.3390/molecules26185557