Synthesis and Biological Evaluation of New N-Acyl-α-amino Ketones and 1,3-Oxazoles Derivatives
Abstract
:1. Introduction
2. Results
2.1. Chemistry
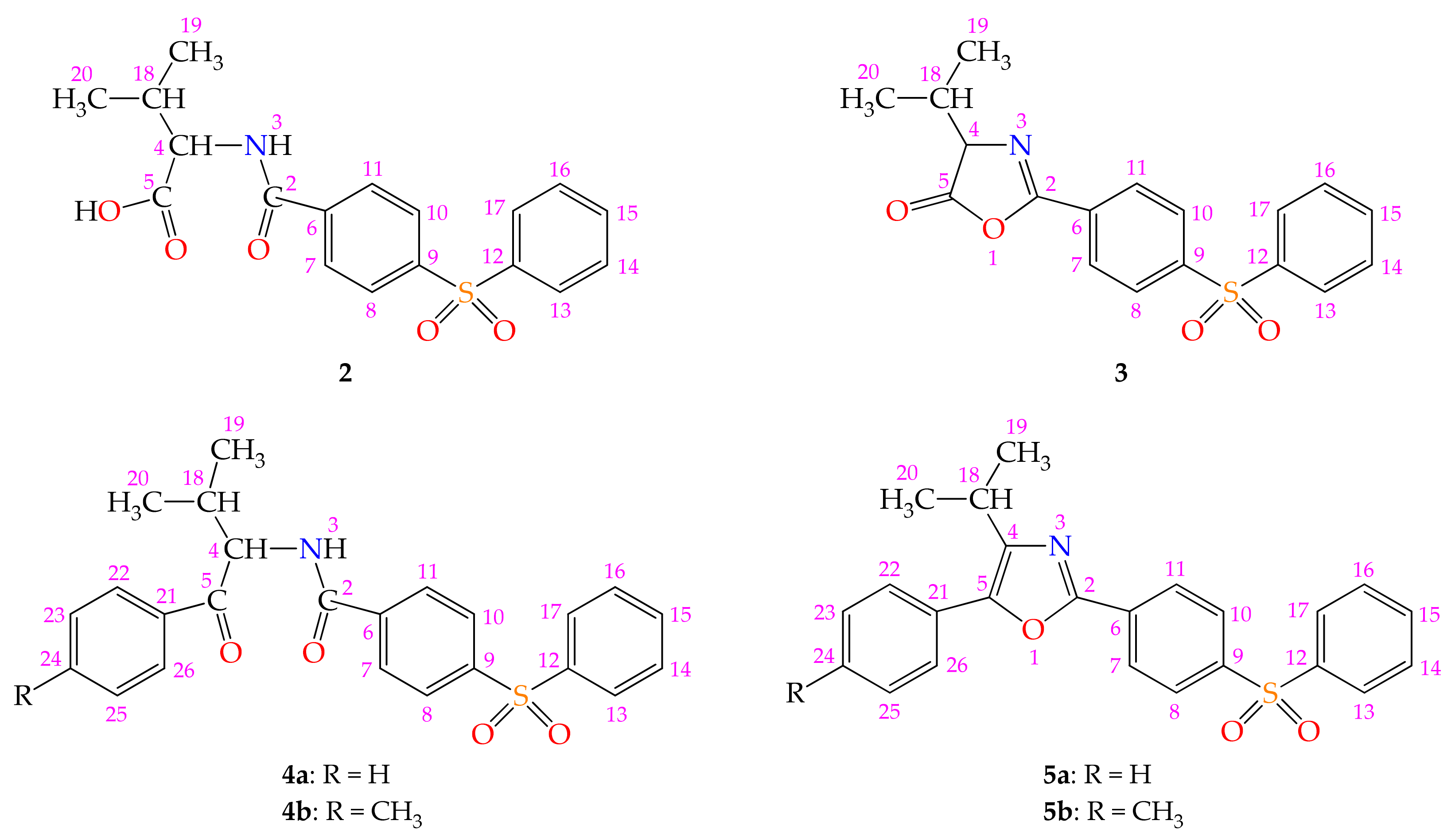
2.1.1. Chemical Synthesis
2.1.2. Spectral Characterization
UV-Vis Spectral Data
IR Spectral Data
NMR Spectral Data
1H-NMR Spectral Data
13C-NMR Spectral Data
Mass Spectral Data
2.2. Antimicrobial Activity Assessment
2.2.1. Qualitative Analysis of the Antimicrobial Activity
2.2.2. Investigation of the Influence of the Tested Compounds on the Antibiotic Susceptibility Spectrum of Enterococcus faecium E5
2.2.3. Quantitative Evaluation of Antimicrobial and Antibiofilm Activities
2.3. Daphnia Magna Toxicity Assay
2.4. Prediction of the Molecular Mechanism of Action and Toxicity
2.4.1. PASS Prediction
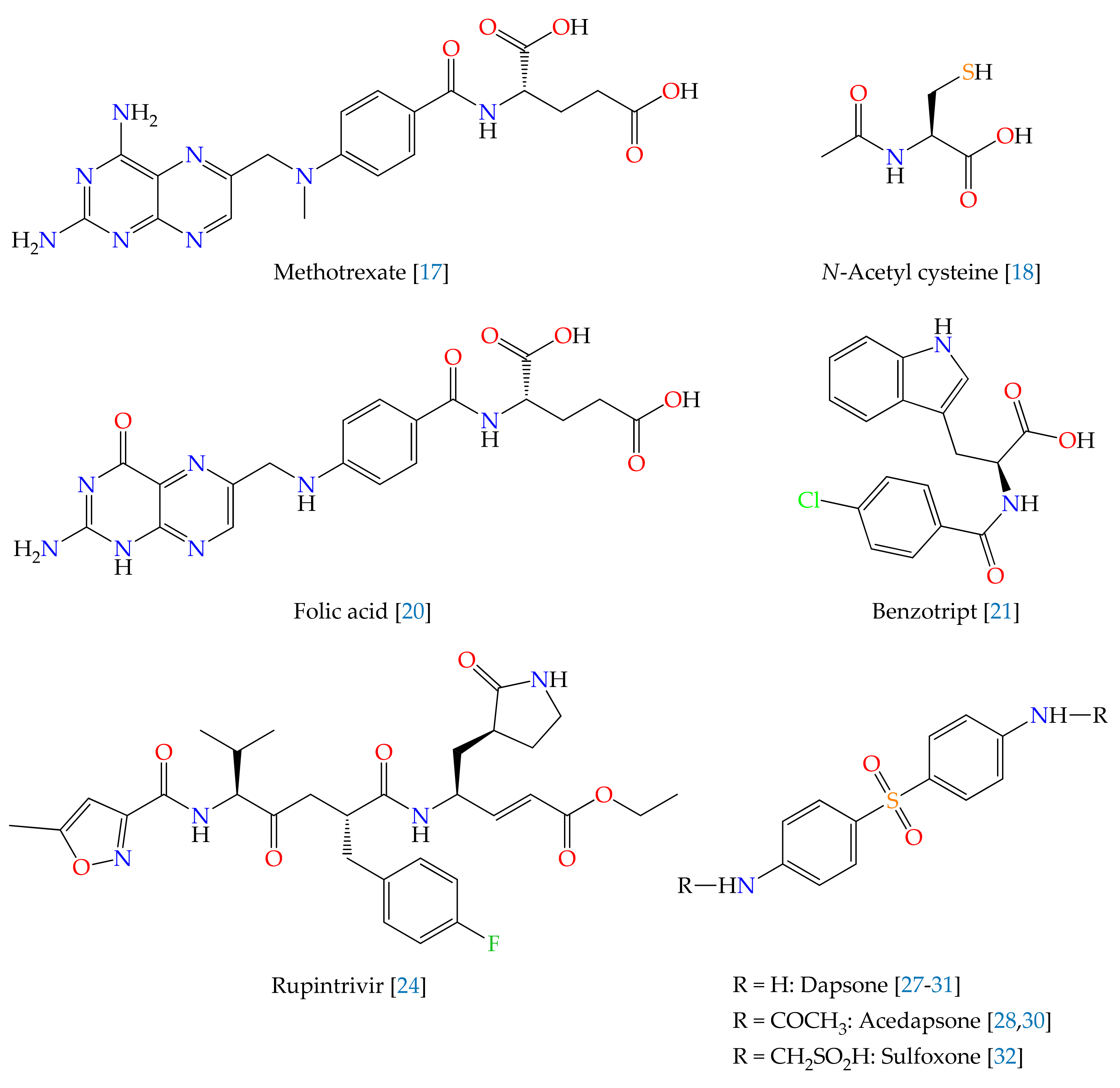
2.4.2. Structural Similarity Analysis
3. Discussion
4. Materials and Methods
4.1.General Information
4.2. Chemistry
4.2.1. Synthesis of 3-Methyl-2-[4-(phenylsulfonyl)benzamido]butanoic acid 2
4.2.2. Synthesis of 4-Isopropyl-2-[4-(phenylsulfonyl)phenyl]-1,3-oxazol-5(4H)-one 3
4.2.3. General Procedure for the Synthesis of the N-(1-Aryl-3-methyl-1-oxobutan-2-yl)-4-(phenylsulfonyl)benzamides 4a,b
N-(3-Methyl-1-oxo-1-phenylbutan-2-yl)-4-(phenylsulfonyl)benzamide 4a
N-[3-Methyl-1-oxo-1-(p-tolyl)butan-2-yl]-4-(phenylsulfonyl)benzamide 4b
4.2.4. General Procedure for the Synthesis of the 5-Aryl-4-isopropyl-2-[4-(phenylsulfonyl)phenyl]-1,3-oxazoles 5a,b
4-Isopropyl-5-phenyl-2-[4-(phenylsulfonyl)phenyl]-1,3-oxazole 5a
4-Isopropyl-2-[4-(phenylsulfonyl)phenyl]-5-(p-tolyl)-1,3-oxazole 5b
4.3. Antimicrobial Activity Assessment
4.3.1. Microbial Strains
4.3.2. Qualitative Assessment of the Antimicrobial Activity
4.3.3. Investigation of the Influence of the Tested Compounds on the Antibiotic Susceptibility Spectrum of the Studied Strain
4.3.4. Quantitative Assessment of the Antimicrobial Activity
4.3.5. Evaluation of the Antibiofilm Activity
4.4. Daphnia Magna Toxicity Assay
4.5. Prediction of the Molecular Mechanism of Action and Toxicity
4.5.1. PASS Prediction
4.5.2. Structural Similarity Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Velluti, F.; Mosconi, N.; Acevedo, A.; Borthagaray, G.; Castiglioni, J.; Faccio, R.; Back, D.F.; Moyna, G.; Rizzotto, M.; Torre, M.H. Synthesis, characterization, microbiological evaluation, genotoxicity and synergism tests of new nano silver complexes with sulfamoxole X-ray diffraction of [Ag2(SMX)2]·DMSO. J. Inorg. Biochem. 2014, 141, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, W.; Zhang, D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules 2020, 25, 1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tazehkand, A.P.; Akbarzadeh, M.; Velaie, K.; Sadeghi, M.R.; Samadi, N. The role of Her2-Nrf2 axis in induction of oxaliplatin resistance in colon cancer cells. Biomed. Pharmacother. 2018, 103, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef]
- Furihata, K.; Nagasawa, K.; Hagino, A.; Kumagai, Y. A drug-drug interaction study of a novel, selective urate reabsorption inhibitor dotinurad and the non-steroidal anti-inflammatory drug oxaprozin in healthy adult males. Clin. Exp. Nephrol. 2020, 24 (Suppl. 1), 36–43. [Google Scholar] [CrossRef] [Green Version]
- Han, C.L.; Qu, C.Z. Cardiovascular Risk and Safety Evaluation of a Dual Peroxisome Proliferator-Activated Receptor-Alpha/Gamma Agonist, Aleglitazar, in Patients With Type 2 Diabetes: A Meta-analysis. J. Cardiovasc. Pharmacol. 2020, 75, 351–357. [Google Scholar] [CrossRef]
- Sahar-Helft, S.; Chackartchi, T.; Polak, D.; Findler, M. Dental treatment in the era of new anti-thrombotic agents. Int. Dent. J. 2018, 68, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Sudo, R.T.; Do Carmo, P.L.; Trachez, M.M.; Zapata-Sudo, G. Effects of azumolene on normal and malignant hyperthermia-susceptible skeletal muscle. Basic Clin. Pharmacol. Toxicol. 2008, 102, 308–316. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N. Antibacterial, antifungal and antimycobacterial compounds from cyanobacteria. Biomed. Pharmacother. 2017, 90, 760–776. [Google Scholar] [CrossRef]
- Tilvi, S.; Singh, K.S. Synthesis of oxazole, oxazoline and isoxazoline derived marine natural products: A Review. Curr. Org. Chem. 2016, 20, 898–929. [Google Scholar] [CrossRef]
- Li, Q.; Seiple, I.B. A concise route to virginiamycin M2. Tetrahedron 2019, 75, 3309–3318. [Google Scholar] [CrossRef]
- Bai, R.; Cruz-Monserrate, Z.; Fenical, W.; Pettit, G.R.; Hamel, E. Interaction of diazonamide A with tubulin. Arch. Biochem. Biophys. 2020, 680, 108217. [Google Scholar] [CrossRef]
- Mhlongo, J.T.; Brasil, E.; de la Torre, B.G.; Albericio, F. Naturally Occurring Oxazole-Containing Peptides. Mar. Drugs 2020, 18, 203. [Google Scholar] [CrossRef]
- Giddens, A.C.; Boshoff, H.I.M.; Franzblau, S.G.; Barry, C.E., III; Copp, B.R. Antimycobacterial natural products: Synthesis and preliminary biological evaluation of the oxazole-containing alkaloid texaline. Tetrahedron Lett. 2005, 46, 7355–7357. [Google Scholar] [CrossRef]
- Pinto, I.L.; West, A.; Debouck, C.M.; Dilella, A.G.; Gorniak, J.G.; O’Donnell, K.C.; O’Shannessy, D.J.; Patel, A.; Jarvest, R.L. Novel, selective mechanism-based inhibitors of the herpes proteases. Bioorg. Med. Chem. Lett. 1996, 6, 2467–2472. [Google Scholar] [CrossRef]
- Jakeman, D.L.; Bandi, S.; Graham, C.L.; Reid, T.R.; Wentzell, J.R.; Douglas, S.E. Antimicrobial Activities of Jadomycin B and Structurally Related Analogues. Antimicrob. Agents Chemother. 2009, 53, 1245–1247. [Google Scholar] [CrossRef] [Green Version]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Page, C.; Rogliani, P.; Matera, M.G. Thiol-Based Drugs in Pulmonary Medicine: Much More than Mucolytics. Trends Pharmacol. Sci. 2019, 40, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Maternal Treatment with Captopril Persistently Alters Gut-Brain Communication and Attenuates Hypertension of Male Offspring. Hypertension 2020, 75, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Argyridis, S. Folic acid in pregnancy. Obstet. Gynaecol. Reprod. Med. 2019, 29, 118–120. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.; Siddiqui, T.I.; Singh, V.S.; Kundu, B.; Prathipati, P.; Saxena, A.K.; Dikshit, D.K.; Rastogi, L.; Dixit, C.; et al. α-Amino acid derivatives as proton pump inhibitors and potent anti-ulcer agents. Eur. J. Med. Chem. 2007, 42, 386–393. [Google Scholar] [CrossRef]
- Pohanka, M. Antidotes against Methanol Poisoning: A Review. Mini Rev. Med. Chem. 2019, 19, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Schaper, A.; Ebbecke, M. Intox, detox, antidotes—Evidence based diagnosis and treatment of acute intoxications. Eur. J. Intern. Med. 2017, 45, 66–70. [Google Scholar] [CrossRef]
- Danov, O.; Lasswitz, L.; Obernolte, H.; Hesse, C.; Braun, A.; Wronski, S.; Sewald, K. Rupintrivir reduces RV-induced TH-2 cytokine IL-4 in precision-cut lung slices (PCLS) of HDM-sensitized mice ex vivo. Respir. Res. 2019, 20, 228. [Google Scholar] [CrossRef] [Green Version]
- Semple, G.; Ashworth, D.M.; Batt, A.R.; Baxter, A.J.; Benzies, D.W.M.; Elliot, L.H.; Evans, D.M.; Franklin, R.J.; Hudson, P.; Jenkins, P.D.; et al. Peptidomimetic aminomethylene ketone inhibitors of interleukin-1β-converting enzyme (ICE). Bioorg. Med. Chem. Lett. 1998, 8, 959–964. [Google Scholar] [CrossRef]
- Deng, H.; Bannister, T.D.; Jin, L.; Babine, R.E.; Quinn, J.; Nagafuji, P.; Celatka, C.A.; Lin, J.; Lazarova, T.I.; Rynkiewicz, M.J.; et al. Synthesis, SAR exploration, and X-ray crystal structures of factor XIa inhibitors containing an α-ketothiazole arginine. Bioorg. Med. Chem. Lett. 2006, 16, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Evernden, C.; Dowhan, M.; Dabas, R.; Chaudhry, A.; Kalra, A.; Dharmani-Khan, P.; Gregson, D.; Johnson, A.; Jupp, J.; Jimenez-Zepeda, V.; et al. High incidence of Pneumocystis jirovecii pneumonia in allogeneic hematopoietic cell transplant recipients in the modern era. Cytotherapy 2020, 22, 27–34. [Google Scholar] [CrossRef]
- Bera, S.; Mondal, D. Insights of synthetic analogues of anti-leprosy agents. Bioorg. Med. Chem. 2019, 27, 2689–2717. [Google Scholar] [CrossRef]
- Mishra, M.; Mishra, V.K.; Kashaw, V.; Iyer, A.K.; Kashaw, S.K. Comprehensive review on various strategies for antimalarial drug discovery. Eur. J. Med. Chem. 2017, 125, 1300–1320. [Google Scholar] [CrossRef]
- Noordeen, S.K. History of chemotherapy of leprosy. Clin. Dermatol. 2016, 34, 32–36. [Google Scholar] [CrossRef]
- Mady, M.F.; Awad, G.E.A.; Jørgensen, K.B. Ultrasound-assisted synthesis of novel 1,2,3-triazoles coupled diaryl sulfone moieties by the CuAAC reaction, and biological evaluation of them as antioxidant and antimicrobial agents. Eur. J. Med. Chem. 2014, 84, 433–443. [Google Scholar] [CrossRef]
- Pezzella, A.T.; Fang, W. Surgical Aspects of Thoracic Tuberculosis: A Contemporary Review-Part 1. Curr. Probl. Surg. 2008, 45, 675–758. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Al-Said, M.S.; Nissan, Y.M. Dapson in Heterocyclic Chemistry, Part V: Synthesis, Molecular Docking and Anticancer Activity of Some Novel Sulfonylbiscompounds Carrying Biologically Active Dihydropyridine, Dihydroisoquinoline, 1,3-Dithiolan, 1,3-Dithian, Acrylamide, Pyrazole, Pyrazolopyrimidine and Benzochromenemoieties. Chem. Pharm. Bull. 2012, 60, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I. Shagufta. Sulfones: An important class of organic compounds with diverse biological activities. Int. J. Pharm. Pharm. Sci. 2015, 7, 19–27. [Google Scholar]
- Barbuceanu, S.F.; Saramet, G.; Bancescu, G.; Draghici, C.; Apostol, T.V.; Taran, L.; Dinu-Pirvu, C.E. Synthesis, Characterization and Antimicrobial Activity of Some Hydroxypyrazolines. Rev. Chim. 2013, 64, 355–360. [Google Scholar]
- Tipparaju, S.K.; Joyasawal, S.; Pieroni, M.; Kaiser, M.; Brun, R.; Kozikowski, A.P. In Pursuit of Natural Product Leads: Synthesis and Biological Evaluation of 2-[3-hydroxy-2-[(3-hydroxypyridine-2-carbonyl)amino]phenyl]benzoxazole-4-carboxylic acid (A-33853) and its analogues: Discovery of N-(2-Benzoxazol-2-ylphenyl)benzamides as Novel Antileishmanial Chemotypes. J. Med. Chem. 2008, 51, 7344–7347. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Al-Sanea, M.M.; Zaki, M.A.; Mohamed, E.I.A.; Khan, S.I.; Tekwani, B.L.; Chittiboyina, A.G.; Khan, I.A.; Al-Warhi, T.; Aljaeed, N.; et al. New Benzoxazole Derivatives as Antiprotozoal Agents: In Silico Studies, Synthesis, and Biological Evaluation. J. Chem. 2021, 2021, 6631868. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.L.; Ren, C.L.; Zou, W.Q.; Tian, X.Y.; Du, X.H.; Tan, C.X. Novel Pyridyl-Oxazole Carboxamides: Toxicity Assay Determination in Fungi and Zebrafish Embryos. Molecules 2021, 26, 3883. [Google Scholar] [CrossRef]
- Matio Kemkuignou, B.; Treiber, L.; Zeng, H.; Schrey, H.; Schobert, R.; Stadler, M. Macrooxazoles A-D, New 2,5-Disubstituted Oxazole-4-Carboxylic Acid Derivatives from the Plant Pathogenic Fungus Phoma macrostoma. Molecules 2020, 25, 5497. [Google Scholar] [CrossRef]
- Apostol, T.V.; Draghici, C.; Dinu, M.; Barbuceanu, S.F.; Socea, L.I.; Saramet, I. Synthesis, Characterization and Biological Evaluation of New 5-Aryl-4-methyl-2-[para-(phenylsulfonyl)phenyl]oxazoles. Rev. Chim. 2011, 62, 142–148. [Google Scholar]
- Apostol, T.V.; Saramet, I.; Draghici, C.; Barbuceanu, S.F.; Socea, L.I.; Almajan, G.L. Synthesis and Characterization of New 5-Aryl-2-[para-(4-chlorophenylsulfonyl)phenyl]-4-methyloxazoles. Rev. Chim. 2011, 62, 486–492. [Google Scholar]
- Apostol, T.V.; Barbuceanu, S.F.; Olaru, O.T.; Draghici, C.; Saramet, G.; Socea, B.; Enache, C.; Socea, L.I. Synthesis, Characterization and Cytotoxicity Evaluation of New Compounds from Oxazol-5(4H)-ones and Oxazoles Class Containing 4-(4-Bromophenylsulfonyl)phenyl Moiety. Rev. Chim. 2019, 70, 1099–1107. [Google Scholar] [CrossRef]
- Apostol, T.V.; Barbuceanu, S.F.; Socea, L.I.; Draghici, C.; Saramet, G.; Iscrulescu, L.; Olaru, O.T. Synthesis, Characterization and Cytotoxicity Evaluation of New Heterocyclic Compounds with Oxazole Ring Containing 4-(Phenylsulfonyl)phenyl Moiety. Rev. Chim. 2019, 70, 3793–3801. [Google Scholar] [CrossRef]
- Apostol, T.V.; Socea, L.I.; Drăghici, C.; Olaru, O.T.; Șaramet, G.; Enache-Preoteasa, C.; Bărbuceanu, Ș.F. Design, Synthesis, Characterization, and Cytotoxicity Evaluation of New 4-Benzyl-1,3-oxazole Derivatives Bearing 4-(4-Chlorophenylsulfonyl)phenyl Moiety. Farmacia 2021, 69, 314–324. [Google Scholar] [CrossRef]
- Apostol, T.V.; Drăghici, C.; Socea, L.I.; Olaru, O.T.; Șaramet, G.; Hrubaru, M.; Bărbuceanu, Ș.F. Synthesis, Characterization and Cytotoxicity Assessment of New 4-Benzyl-1,3-oxazole Derivatives Incorporating 4-[(4-Bromophenyl)sulfonyl]phenyl Fragment. Farmacia 2021, 69, 521–529. [Google Scholar] [CrossRef]
- Schiketanz, I.; Draghici, C.; Saramet, I.; Balaban, A.T. Aminoketone, oxazole and thiazole synthesis. Part 16. Novel 5-aryl-2-(para-benzenesulfonylphenyl)oxazoles. Rev. Roum. Chim. 2002, 47, 235–238. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Allen, L.A.T.; Raclea, R.C.; Natho, P.; Parsons, P.J. Recent advances in the synthesis of α-amino ketones. Org. Biomol. Chem. 2021, 19, 498–513. [Google Scholar] [CrossRef]
- Kakkar, S.; Narasimhan, B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Kamble, V.S.; Habade, B.M.; Patil, G.K.; Agasimundin, Y. Synthesis and Evaluation of 4-(1-Benzofuran-2-yl)-1,3-oxazole-2-amine and Its Derivatives. Int. J. Res. Pharm. Chem. 2012, 2, 32–36. [Google Scholar]
- Reddy, A.B.; Hymavathi, R.V.; Swamy, G.N. A new class of multi-substituted oxazole derivatives: Synthesis and antimicrobial activity. J. Chem. Sci. 2013, 125, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Guilhermino, L.; Celeste Lopes, M.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere 1996, 32, 727–738. [Google Scholar] [CrossRef]
- Tang, M.; Hong, M. Structure and mechanism of beta-hairpin antimicrobial peptides in lipid bilayers from solid-state NMR spectroscopy. Mol. Biosyst. 2009, 5, 317–722. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.C.; Vaja, D.V.; Joshi, S.B.; Khedkar, V.M. Synthesis and molecular docking study of pyrazole clubbed oxazole as antibacterial agents. Res. Chem. Intermed. 2021, 47, 573–587. [Google Scholar] [CrossRef]
- Patrinoiu, G.; Calderón-Moreno, J.M.; Chifiriuc, C.M.; Saviuc, C.; Birjega, R.; Carp, O. Tunable ZnO spheres with high anti-biofilm and antibacterial activity via a simple green hydrothermal route. J. Colloid Interface Sci. 2016, 462, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomater. 2013, 2013, 893970. [Google Scholar] [CrossRef] [Green Version]
- Stecoza, C.E.; Nitulescu, G.M.; Draghici, C.; Caproiu, M.T.; Olaru, O.T.; Bostan, M.; Mihaila, M. Synthesis and Anticancer Evaluation of New 1,3,4-Oxadiazole Derivatives. Pharmaceuticals 2021, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Olaru, O.T.; Venables, L.; van de Venter, M.; Nitulescu, G.M.; Margina, D.; Spandidos, D.A.; Tsatsakis, A.M. Anticancer potential of selected Fallopia Adans species. Oncol. Lett. 2015, 10, 1323–1332. [Google Scholar] [CrossRef] [Green Version]
- Zakharov, A.V.; Peach, M.L.; Sitzmann, M.; Nicklaus, M.C. A new approach to radial basis function approximation and its application to QSAR. J. Chem. Inf. Model. 2014, 54, 713–719. [Google Scholar] [CrossRef]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]



| Bacteria Tested | Diameter (mm) of the Zone of Inhibition of Growth | |||
|---|---|---|---|---|
| Ampicillin (S 1, ≥17 mm) | Penicillin (S, ≥15 mm) | Linezolid (S, ≥23 mm) | Vancomycin (S, ≥17 mm) | |
| E. faecium E5 | 24 | 14 | 27 | 19 |
| E. faecium E5 treated with compound 2 | 24 | 13 | 28 | 19 |
| E. faecium E5 treated with compound 3 | 23 | 12 | 27 | 18 |
| E. faecium E5 treated with DMSO | 22 | 0 | 23 | 18 |
| Compound | Enterococcus faecium E5 | Staphylococcus aureus ATCC 6538 | Bacillus subtilis ATCC 6683 | Pseudomonas aeruginosa ATCC 27857 | Escherichia coli ATCC 8739 | Candida albicans 393 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | |
| 2 | 500 | 15.6 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 3 | 500 | 15.6 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 4a | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 4b | >500 | >500 | 62.5 | >500 | 62.5 | >500 | >500 | >500 | 62.5 | >500 | >500 | >500 |
| 5a | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 5b | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Ciprofloxacin | 0.62 | 0.62 | 0.15 | 0.15 | <0.03 | <0.03 | 0.15 | 0.15 | 0.012 | 0.012 | - 1 | - |
| Fluconazole | - | - | - | - | - | - | - | - | - | - | <0.12 | <0.12 |
| Tested Compound | Predicted LC50 (48 h) 1 (µg/mL) | Max. L(48 h)% 2 | Determined LC50 (24 h) (µg/mL) | 95% CI 3 of LC50 (24 h) (µg/mL) | Determined LC50 (48 h) (µg/mL) | 95% CI of LC50 (48 h) (µg/mL) |
|---|---|---|---|---|---|---|
| 2 | 5.31 | 10 | ND 4 * | ND * | ND * | ND * |
| 3 | 1.92 | 10 | ND * | ND * | 21.73 | ND ** |
| 4a | 1.26 | 60 | 89.63 | 33.88 to 237.1 | 27.32 | 18.64 to 40.05 |
| 4b | 0.41 | 30 | ND * | ND * | ND * | ND * |
| 5a | 0.37 | 35 | ND * | ND * | 77.02 | 47.85 to 124.0 |
| 5b | 0.18 | 15 | ND * | ND * | ND * | ND * |
| L-Valine (control 1) | 1078.3 | 15 | ND * | ND * | ND * | ND * |
| 4-(Phenylsulfonyl)benzoic acid (control 2) | 68.2 | 20 | ND * | ND * | ND * | ND * |
| Target | 2 | 3 | 4a | 4b | 5a | 5b |
|---|---|---|---|---|---|---|
| Antibiotic glycopeptide-like | 0.1 | 0.56 | - | - | - | - |
| Antimycobacterial | 0.40 | 0.49 | 0.42 | 0.43 | 0.28 | 0.29 |
| Antituberculosis | 0.43 | 0.36 | 0.43 | 0.43 | 0.25 | 0.25 |
| Antiinfective | 0.50 | 0.32 | 0.32 | 0.36 | 0.26 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostol, T.-V.; Marutescu, L.G.; Draghici, C.; Socea, L.-I.; Olaru, O.T.; Nitulescu, G.M.; Pahontu, E.M.; Saramet, G.; Enache-Preoteasa, C.; Barbuceanu, S.-F. Synthesis and Biological Evaluation of New N-Acyl-α-amino Ketones and 1,3-Oxazoles Derivatives. Molecules 2021, 26, 5019. https://doi.org/10.3390/molecules26165019
Apostol T-V, Marutescu LG, Draghici C, Socea L-I, Olaru OT, Nitulescu GM, Pahontu EM, Saramet G, Enache-Preoteasa C, Barbuceanu S-F. Synthesis and Biological Evaluation of New N-Acyl-α-amino Ketones and 1,3-Oxazoles Derivatives. Molecules. 2021; 26(16):5019. https://doi.org/10.3390/molecules26165019
Chicago/Turabian StyleApostol, Theodora-Venera, Luminita Gabriela Marutescu, Constantin Draghici, Laura-Ileana Socea, Octavian Tudorel Olaru, George Mihai Nitulescu, Elena Mihaela Pahontu, Gabriel Saramet, Cristian Enache-Preoteasa, and Stefania-Felicia Barbuceanu. 2021. "Synthesis and Biological Evaluation of New N-Acyl-α-amino Ketones and 1,3-Oxazoles Derivatives" Molecules 26, no. 16: 5019. https://doi.org/10.3390/molecules26165019
APA StyleApostol, T.-V., Marutescu, L. G., Draghici, C., Socea, L.-I., Olaru, O. T., Nitulescu, G. M., Pahontu, E. M., Saramet, G., Enache-Preoteasa, C., & Barbuceanu, S.-F. (2021). Synthesis and Biological Evaluation of New N-Acyl-α-amino Ketones and 1,3-Oxazoles Derivatives. Molecules, 26(16), 5019. https://doi.org/10.3390/molecules26165019








