1,2,5-Thiadiazole 1,1-dioxides and Their Radical Anions: Structure, Properties, Reactivity, and Potential Use in the Construction of Functional Molecular Materials
Abstract
:1. Introduction
2. Structure and Geometry
2.1. Theoretical Investigations
2.2. NMR, UV-Vis, and IR Spectroscopy
3. Preparation Methods
4. Reactivity
4.1. Reactivity toward Nucleophiles
4.2. Radical Anion Formation and Electrochemistry
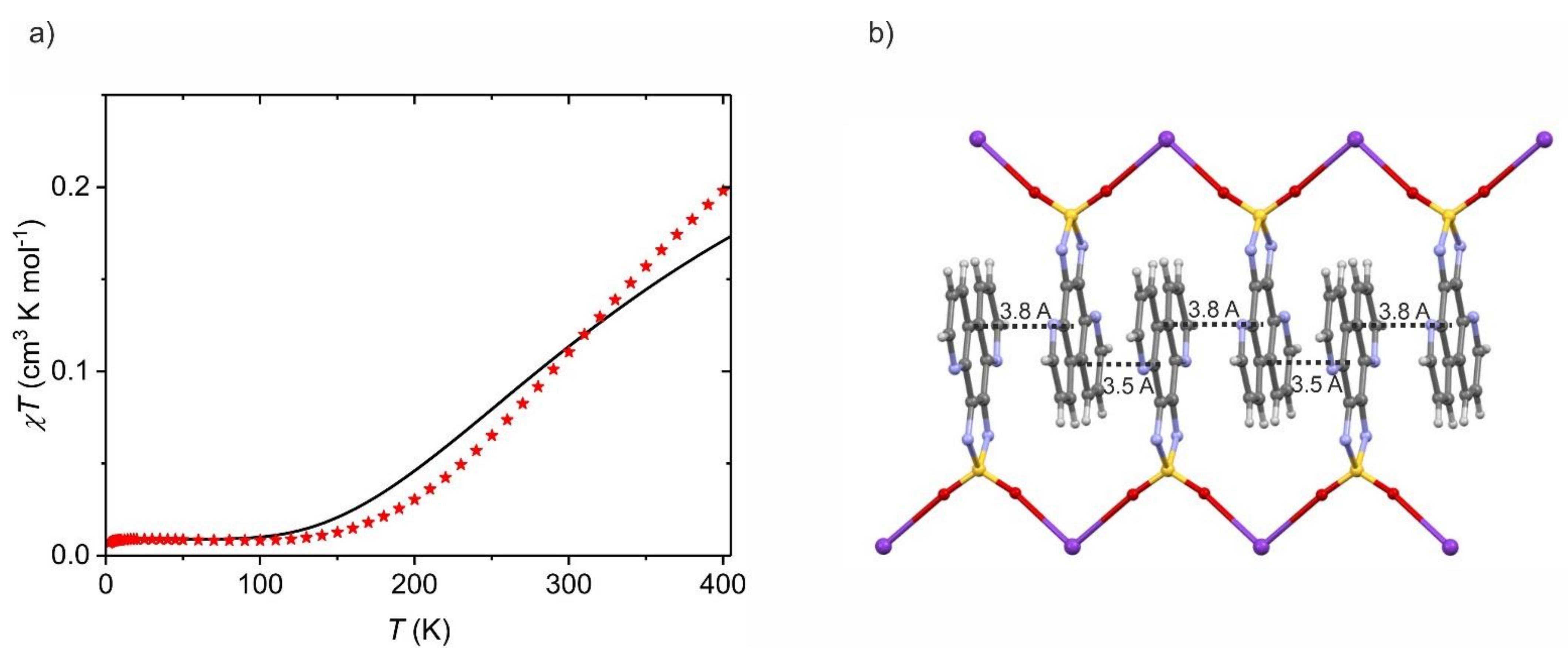
5. Magnetic Properties of Dioxothiadiazole Radical Anions
5.1. Electron Paramagnetic Resonance
5.2. Magnetometry
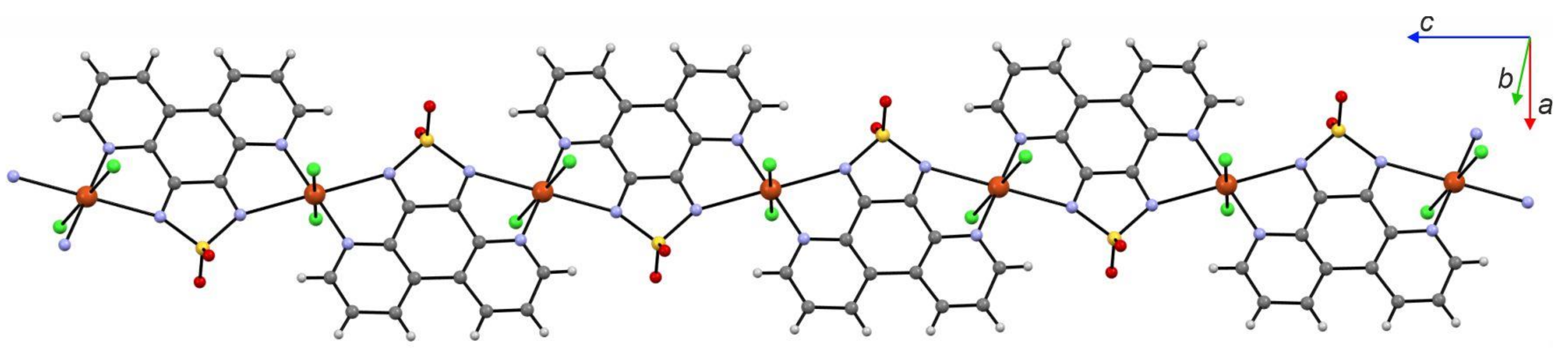
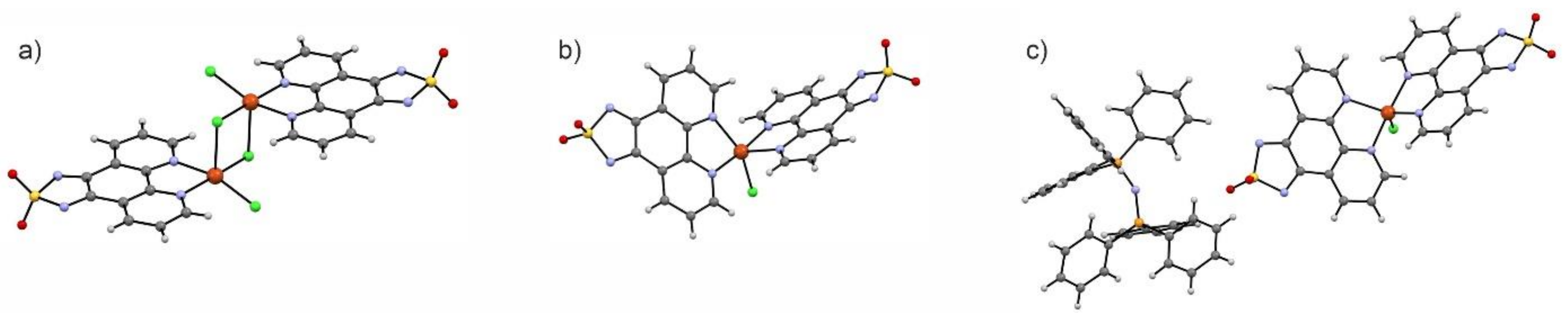
6. Magnetic Materials with Dioxothiadiazoles as Ligands
7. Other Properties and Applications
7.1. Organic Semiconductors
7.2. Medical and Other Studies
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Schmidt, R.R. Recent Developments in the Synthesis of Glycoconjugates. Pure Appl. Chem. 1989, 61, 1257–1270. [Google Scholar] [CrossRef]
- Koutentis, P.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 515–565. ISBN 9780080449920. [Google Scholar]
- Clerici, F. Thiazole and thiadiazole S-oxides. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Elsevier: Cambridge, MA, USA; Academic Press: Cambridge, MA, USA, 2002; Volume 83, pp. 71–115. ISBN 978-0-12-020783-1. [Google Scholar]
- Hu, Y.; Li, C.Y.; Wang, X.M.; Yang, Y.H.; Zhu, H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef]
- Weinstock, L.M.; Shinkai, I. 1,2,5-Thiadiazoles and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Elsevier: Oxford, UK, 1984; Volume 6–7, pp. 513–543. ISBN 9780080965192. [Google Scholar]
- Shinkai, I.; Reider, P.J. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Elsevier: Oxford, UK, 1996; Volume 4, pp. 355–377. ISBN 9780080420721. [Google Scholar]
- Mali, S.N.; Pandey, A. 1,2,5-Thiadiazole Scaffold: A review on recent progress in biological activities. Comb. Chem. High Throughput Screen. 2021, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-thiadiazole and its derivatives: A review on recent progress in biological activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Frija, L.M.T.; Pombeiro, A.J.L.; Kopylovich, M.N. Coordination chemistry of thiazoles, isothiazoles and thiadiazoles. Coord. Chem. Rev. 2016, 308, 32–55. [Google Scholar] [CrossRef]
- Pushkarevsky, N.A.; Chulanova, E.A.; Shundrin, L.A.; Smolentsev, A.I.; Salnikov, G.E.; Pritchina, E.A.; Genaev, A.M.; Irtegova, I.G.; Bagryanskaya, I.Y.; Konchenko, S.N.; et al. Radical Anions, Radical-Anion Salts, and Anionic Complexes of 2,1,3-Benzochalcogenadiazoles. Chem. Eur. J. 2019, 25, 806–816. [Google Scholar] [CrossRef]
- Beer, L.; Reed, R.W.; Brusso, J.L.; Cordes, A.W.; Haddon, R.C.; Itkis, M.E.; Kirschbaum, K.; MacGregor, D.S.; Oakley, R.T.; Pinkerton, A.A. Resonance-stabilized 1,2,3-dithiazolo-1,2,3-dithiazolyis as neutral π-radical conductors. J. Am. Chem. Soc. 2002, 124, 9498–9509. [Google Scholar] [CrossRef]
- Tang, H.; Sun, J.; Yan, X.; Wu, P. Electrochemical and adsorption behaviors of thiadiazole derivatives on the aluminum surface. RSC Adv. 2019, 9, 34617–34626. [Google Scholar] [CrossRef] [Green Version]
- Shuku, Y.; Suizu, R.; Domingo, A.; Calzado, C.J.; Robert, V.; Awaga, K. Multidimensional network structures and versatile magnetic properties of intermolecular compounds of a radical-anion ligand, [1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 1,1-dioxide. Inorg. Chem. 2013, 52, 9921–9930. [Google Scholar] [CrossRef]
- Kremer, A.; Aurisicchio, C.; Deleo, F.; Ventura, B.; Wouters, J.; Armaroli, N.; Barbieri, A.; Bonifazi, D. Walking Down the Chalcogenic Group of the Periodic Table: From Singlet to Triplet Organic Emitters. Chem. Eur. J. 2015, 21, 15377–15387. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Ma, B.B.; Peng, Y.X.; Wang, X.X.; Huang, W.; You, X.Z. Asymmetrical/symmetrical D-π-A/D-π-D thiazole-containing aromatic heterocyclic fluorescent compounds having the same triphenylamino chromophores. J. Org. Chem. 2013, 78, 8669–8679. [Google Scholar] [CrossRef]
- Godugu, K.; Shaik, S.; Mohinuddin Pinjari, M.K.; Gundala, T.R.; Chellappa Subramanyam, D.V.; Loka, S.S.; Divi, H.; Vemula, V.; Reddy Nallagondu, C.G. Solid state thiazole-based fluorophores: Promising materials for white organic light emitting devices. Dye. Pigment. 2021, 187, 109077. [Google Scholar] [CrossRef]
- Mishra, S.P.; Palai, A.K.; Kumar, A.; Srivastava, R.; Kamalasanan, M.N.; Patri, M. Highly air-stable thieno[3,2-b]thiophene-Thiophene-Thiazolo[5,4-d]thiazole- based polymers for light-emitting diodes. Macromol. Chem. Phys. 2010, 211, 1890–1899. [Google Scholar] [CrossRef]
- Biet, T.; Martin, K.; Hankache, J.; Hellou, N.; Hauser, A.; Bürgi, T.; Vanthuyne, N.; Aharon, T.; Caricato, M.; Crassous, J.; et al. Triggering Emission with the Helical Turn in Thiadiazole-Helicenes. Chem. Eur. J. 2017, 23, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Arán, V.J.; Goya, P.; Ochoa, C. Heterocycles Containing the Sulfamide Moiety. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Elsevier: Cambridge, MA, USA; Academic Press: Cambridge, MA, USA, 1988; Volume 44, pp. 81–197. ISBN 0065-2725. [Google Scholar]
- Linder, T.; Badiola, E.; Baumgartner, T.; Sutherland, T.C. Synthesis of π-extended thiadiazole (oxides) and their electronic properties. Org. Lett. 2010, 12, 4520–4523. [Google Scholar] [CrossRef]
- Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef]
- Train, C.; Norel, L.; Baumgarten, M. Organic radicals, a promising route towards original molecule-based magnetic materials. Coord. Chem. Rev. 2009, 253, 2342–2351. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-nitronyl nitroxide chains as molecular magnetic nanowires. Angew. Chem. Int. Ed. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Sun, J.; Xie, J.; Li, L.; Sutter, J.P. Single-chain magnet behavior in a 2p-3d-4f spin array with a nitronyl nitroxide biradical. Inorg. Chem. Front. 2020, 7, 1949–1956. [Google Scholar] [CrossRef]
- Ovcharenko, V.; Romanenko, G.; Polushkin, A.; Letyagin, G.; Bogomyakov, A.; Fedin, M.; Maryunina, K.; Nishihara, S.; Inoue, K.; Petrova, M.; et al. Pressure-Controlled Migration of Paramagnetic Centers in a Heterospin Crystal. Inorg. Chem. 2019, 58, 9187–9194. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Wang, K.; Xi, L.; Ma, Y.; Li, L. Slow relaxation of magnetization in unprecedented Cu-Ln-Rad hetero-tri-spin chains constructed from multidentate nitronyl nitroxide. J. Mater. Chem. C 2019, 7, 9057–9064. [Google Scholar] [CrossRef]
- Jobelius, H.; Wagner, N.; Schnakenburg, G.; Meyer, A. Verdazyls as possible building blocks for multifunctional molecular materials: A case study on 1,5-Diphenyl-3-(p-iodophenyl)-verdazyl focusing on magnetism, electron transfer and the applicability of the Sonogashira-Hagihara reaction. Molecules 2018, 23, 1758. [Google Scholar] [CrossRef]
- Brook, D.J.R.; Fleming, C.; Chung, D.; Richardson, C.; Ponce, S.; Das, R.; Srikanth, H.; Heindl, R.; Noll, B.C. An electron transfer driven magnetic switch: Ferromagnetic exchange and spin delocalization in iron verdazyl complexes. Dalton Trans. 2018, 47, 6351–6360. [Google Scholar] [CrossRef] [PubMed]
- Norel, L.; Chamoreau, L.M.; Journaux, Y.; Oms, O.; Chastanet, G.; Train, C. Verdazyl-lanthanide(iii) one dimensional compounds: Synthesis, structure and magnetic properties. Chem. Commun. 2009, 2381–2383. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-R.; Negru, B.; Van Duyne, R.P.; Harris, T.D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Chevalier, K.; Graf, M.; Schmitz, M.; Kelm, H.; Grün, A.; Zimmer, M.; Gerhards, M.; van Wüllen, C.; Krüger, H.J.; et al. Spectroscopic, Structural, and Kinetic Investigation of the Ultrafast Spin Crossover in an Unusual Cobalt(II) Semiquinonate Radical Complex. Chem. Eur. J. 2017, 23, 2119–2132. [Google Scholar] [CrossRef]
- Yee, G.T.; McLean, R.S.; Epstein, A.J.; Miller, J.S. A room-temperature molecular/organic-based magnet. Science 1991, 252, 1415–1417. [Google Scholar] [CrossRef]
- Zhang, J.; Kosaka, W.; Kitagawa, Y.; Miyasaka, H. Host–Guest Hydrogen Bonding Varies the Charge-State Behavior of Magnetic Sponges. Angew. Chem. Int. Ed. 2019, 58, 7351–7356. [Google Scholar] [CrossRef]
- Phan, H.; Herng, T.S.; Wang, D.; Li, X.; Zeng, W.; Ding, J.; Loh, K.P.; Shen Wee, A.T.; Wu, J. Room-Temperature Magnets Based on 1,3,5-Triazine-Linked Porous Organic Radical Frameworks. Chem 2019, 5, 1223–1234. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.R.; Park, J.G.; Xiao, D.J.; Harris, T.D. An azophenine radical-bridged Fe2 single-molecule magnet with record magnetic exchange coupling. J. Am. Chem. Soc. 2013, 135, 16845–16848. [Google Scholar] [CrossRef]
- Zhang, W.X.; Ishikawa, R.; Breedlove, B.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Degayner, J.A.; Wang, K.; Harris, T.D. A Ferric Semiquinoid Single-Chain Magnet via Thermally-Switchable Metal-Ligand Electron Transfer. J. Am. Chem. Soc. 2018, 140, 6550–6553. [Google Scholar] [CrossRef]
- Vaz, M.G.F.; Cassaro, R.A.A.; Akpinar, H.; Schlueter, J.A.; Lahti, P.M.; Novak, M.A. A cobalt pyrenylnitronylnitroxide single-chain magnet with high coercivity and record blocking temperature. Chem. Eur. J. 2014, 20, 5460–5467. [Google Scholar] [CrossRef]
- Cassaro, R.A.A.; Reis, S.G.; Araujo, T.S.; Lahti, P.M.; Novak, M.A.; Vaz, M.G.F. A Single-Chain Magnet with a Very High Blocking Temperature and a Strong Coercive Field. Inorg. Chem. 2015, 54, 9381–9383. [Google Scholar] [CrossRef]
- Mirífico, M.V.; Caram, J.A.; Gennaro, A.M.; Cobos, C.J.; Vasini, É.J. Radical anions containing the dioxidated 1,2,5-thiadiazole heterocycle. J. Phys. Org. Chem. 2009, 22, 964–970. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Li, Z.; Pietrzyk, P.; Rams, M. New thiadiazole dioxide bridging ligand with a stable radical form for the construction of magnetic coordination Chains. Cryst. Growth Des. 2014, 14, 4878–4881. [Google Scholar] [CrossRef]
- Shuku, Y.; Suizu, R.; Awaga, K. Monovalent and mixed-valent potassium salts of [1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 1,1-dioxide: A radical anion for multidimensional network structures. Inorg. Chem. 2011, 50, 11859–11861. [Google Scholar] [CrossRef]
- Pakulski, P.; Arczyński, M.; Pinkowicz, D. Bis(Triphenylphosphine)iminium salts of dioxothiadiazole radical anions: Preparation, crystal structures, and magnetic properties. Crystals 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Rozas, M.F.; Piro, O.E.; Castellano, E.E.; Mirífico, M.V.; Vasini, E.J. Novel synthesis of 3,4,4-trisubstituted thiadiazolines from 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide. Competition with the intra-molecular aryl-aryl cyclization of 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide. Synthesis 2002, 2002, 2399–2403. [Google Scholar] [CrossRef]
- Castellano, E.E.; Piro, O.E.; Caram, J.A.; Mirífico, M.V.; Aimone, S.L.; Vasini, E.J.; Marquez Lucero, A.; Glossman Mitnik, D. Crystallographic study and molecular orbital calculations of thiadiazole derivatives. 1. Phenanthro[9,10-c]-1,2,5-thiadiazole 1, 1-dioxide and acenaphtho[1,2-c]-1,2,5-thiadiazole 1,1-dioxide. J. Mol. Struct. 2001, 562, 157–166. [Google Scholar] [CrossRef]
- Caram, J.A.; Jiménez Macías, J.P.; Arroyo, N.R.; Martínez Suárez, J.F.; Gennaro, A.M.; Echeverría, G.A.; Piro, O.E.; Mirífico, M.V. Stability of the Monoelectronic Reduction Product from 1,2,5-Thiadiazole S,S-Dioxides. Electrochemical, Chemical, and Photoinduced Doping. ChemistrySelect 2018, 3, 8729–8739. [Google Scholar] [CrossRef]
- Dunn, P.J.; Rees, C.W.; Slawin, A.M.Z.; Williams, D.J. Interannular contraction upon oxidation of a mesoionic bicyclic imine. J. Chem. Soc. Chem. Commun. 1989, 1134–1136. [Google Scholar] [CrossRef]
- Castellano, E.E.; Piro, O.E.; Caram, J.A.; Mirifico, M.V.; Aimone, S.L.; Vasini, E.J.; Glossman, M.D. Crystallographic study and molecular orbital calculations of 1,2,5-thiadiazole 1,1-dioxide derivatives. J. Phys. Org. Chem. 1998, 11, 91–100. [Google Scholar] [CrossRef]
- Xie, Y.; Shuku, Y.; Matsushita, M.M.; Awaga, K. Thiadiazole dioxide-fused picene: Acceptor ability, anion radical formation, and n-type charge transport characteristics. Chem. Commun. 2014, 50, 4178–4180. [Google Scholar] [CrossRef] [PubMed]
- Shuku, Y.; Awaga, K. Transition metal complexes and radical anion salts of 1,10-Phenanthroline derivatives annulated with a 1,2,5-Tiadiazole and 1,2,5-Tiadiazole 1,1-Dioxide Moiety: Multidimensional crystal structures and various magnetic properties. Molecules 2014, 19, 609–640. [Google Scholar] [CrossRef]
- Arczyński, M.; Pinkowicz, D. Influence of the Increasing Number of Organic Radicals on the Structural, Magnetic, and Electrochemical Properties of the Copper(II)-Dioxothiadiazole Family of Complexes. Inorg. Chem. 2020, 59, 13489–13501. [Google Scholar] [CrossRef]
- Glossman-Mitnik, D. CHIH-DFT computational molecular characterization of acenaphto[1,2-c]-1,2,5-thiadiazole 1,1-dioxide. J. Mol. Struct. THEOCHEM 2007, 811, 373–378. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Rodríguez-Valdez, L.M.; Glossman-Mitnik, D. CHIH-DFT computational molecular characterization of phenanthro [9,10-c]-1,2,5-thiadiazole 1,1-dioxide. J. Mol. Struct. THEOCHEM 2008, 862, 60–65. [Google Scholar] [CrossRef]
- Vasini, E.J.; Mirífico, M.V.; Caram, J.A. On experimental versus theoretically calculated properties of thiadiazole derivatives. Int. J. Quantum Chem. 2011, 111, 1879–1884. [Google Scholar] [CrossRef]
- Castellano, E.E.; Piro, O.E.; Caram, J.A.; Mirífico, M.V.; Aimone, S.L.; Vasini, E.J.; Márquez-Lucero, A.; Glossman-Mitnik, D. Crystallographic study and molecular orbital calculations of thiadiazole derivatives. Part 3: 3,4-diphenyl-1,2,5-thiadiazoline 1,1-dioxide, 3,4-diphenyl-1,2,5-thiadiazolidine 1,1-dioxide and 4-ethoxy-5-methyl-3,4-diphenyl-1,2,5-thiadiazoline 1,1-dioxide. J. Mol. Struct. 2001, 597, 163–175. [Google Scholar] [CrossRef]
- Friedman, P.; Ferris, K.F. A theoretical study of the aromaticity of hypervalent sulfur heterocycles. J. Mol. Struct. THEOCHEM 1997, 418, 119–126. [Google Scholar] [CrossRef]
- Mirífico, M.V.; Caram, J.A.; Gennaro, A.M.; Cobos, C.J.; Vasini, E.J. Radical anions containing the dioxidated 1,2,5-thiadiazole heterocycle. Part II. J. Phys. Org. Chem. 2011, 24, 1039–1044. [Google Scholar] [CrossRef]
- Keller, S.N.; Bromby, A.D.; Sutherland, T.C. Optical Effect of Varying Acceptors in Pyrene Donor–Acceptor–Donor Chromophores. Eur. J. Org. Chem. 2017, 2017, 3980–3985. [Google Scholar] [CrossRef]
- Glossman-Mitnik, D. CHIH-DFT determination of the molecular structure and infrared and ultraviolet spectra of azathiophenes. Theor. Chem. Acc. 2007, 117, 57–68. [Google Scholar] [CrossRef]
- Lumma, W.C.; Anderson, P.S.; Baldwin, J.J.; Bolhofer, W.A.; Britcher, S.F.; Clineschmidt, B.V.; Denny, G.H.; Habecker, C.N.; Hirshfield, J.M. Inhibitors of gastric acid secretion: 3,4-diamino-1,2,5-thiadiazole 1-oxides and 1,1-dioxides as urea equivalents in a series of histamine H2 receptor antagonists. J. Med. Chem. 1982, 25, 207–210. [Google Scholar] [CrossRef]
- Arán, V.J.; Ruiz, J.R.; Dávila, E.; Alkorta, I.; Stud, M. Synthesis and reactivity of some amino-substituted 1,2,5-thiadiazole 1,1-dioxides. Liebigs Ann. Chem. 1988, 1988, 337–341. [Google Scholar] [CrossRef]
- Schüttler, C.; Li-Böhmer, Z.; Harms, K.; Von Zezschwitz, P. Enantioselective synthesis of 3,4-disubstituted cis- and trans-1,2,5-thiadiazolidine-1,1-dioxides as precursors for chiral 1,2-diamines. Org. Lett. 2013, 15, 800–803. [Google Scholar] [CrossRef]
- Grant, C.D.; Kang, S.O.; Hay, B.P. Synthesis of a hydrophilic naphthalimidedioxime. J. Org. Chem. 2013, 78, 7735–7740. [Google Scholar] [CrossRef]
- Martinez, A.; Castro, A.; Arán, V.J.; Cardelús, I.; Llenas, J.; Palacios, J.M. Synthesis of nonsymmetrically 3,4-disubstituted 1,2,5-thiadiazole dioxides. J. Heterocycl. Chem. 1998, 35, 297–300. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.H.; Yang, C.M. Studies on the Chemical Properties and Synthesis of Sulfamides (III): Oxidation Reaction of 4-Aryl-3-imino-1,2,5-thiadiazolidines 1,1-dioxides and 4-Aryl-3-amino-4,5-dihydro-1,2,5-thiadiazole 1,1-dioxides. J. Korean Chem. Soc. 1998, 42, 112–114. [Google Scholar]
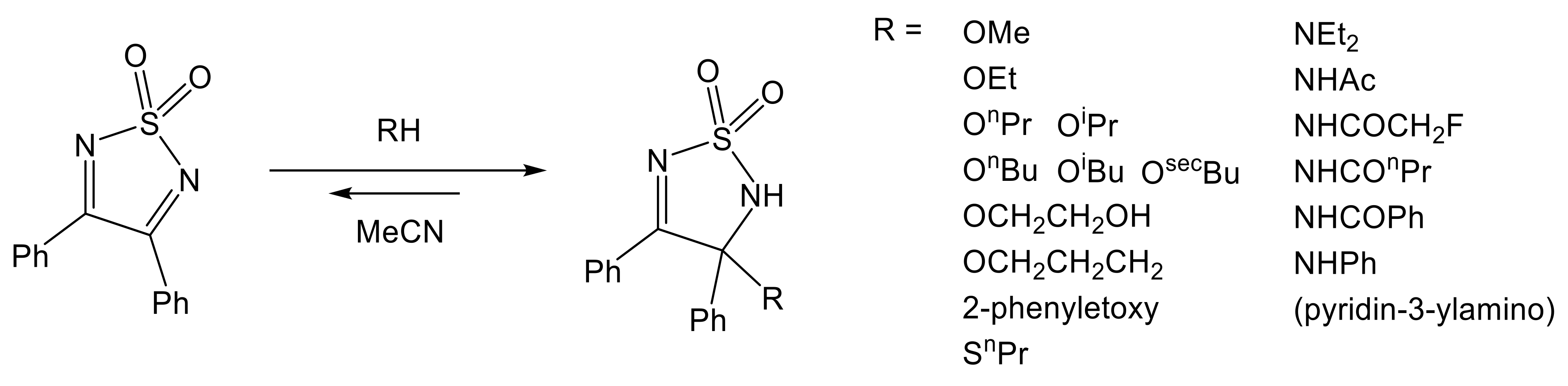
- Aimone, S.L.; Caram, J.A.; Mirífico, M.V.; Vasini, E.J. Electrochemical and spectroscopic study of the addition of several nucleophiles to 1,2,5-thiadiazole 1,1-dioxide derivatives. J. Phys. Org. Chem. 2000, 13, 272–282. [Google Scholar] [CrossRef]
- DaSilveira Neto, B.A.; Lopes, A.S.A.; Ebeling, G.; Gonçalves, R.S.; Costa, V.E.U.; Quina, F.H.; Dupont, J. Photophysical and electrochemical properties of π-extended molecular 2,1,3-benzothiadiazoles. Tetrahedron 2005, 61, 10975–10982. [Google Scholar] [CrossRef]
- Lork, E.; Mews, R.; Shakirov, M.M.; Watson, P.G.; Zibarev, A.V. Reactions of Arylthiazylamides with Internal and External Fluoro Electrophiles − Formation of Products with Unusual Structures. Eur. J. Inorg. Chem. 2001, 2001, 2123–2134. [Google Scholar] [CrossRef]
- Cillo, C.M.; Lash, T.D. Benzo[1,2-c:3,4-c′]bis[1,2,5]selenadiazole, [1,2,5]selenadiazolo-[3,4-e]-2,1,3-benzothiadiazole, furazanobenzo-2,1,3-thiadiazole, furazanobenzo-2,1,3-selenadiazole and related heterocyclic systems. J. Heterocycl. Chem. 2004, 41, 955–962. [Google Scholar] [CrossRef]
- Wen, R.Y.; Komin, A.P.; Street, R.W.; Carmack, M. Chemistry of 1,2,5-thiadiazoles. II. 3,4-Disubstituted derivatives of 1,2,5-thiadiazole 1,1-dioxide. J. Org. Chem. 1975, 40, 2743–2748. [Google Scholar] [CrossRef]
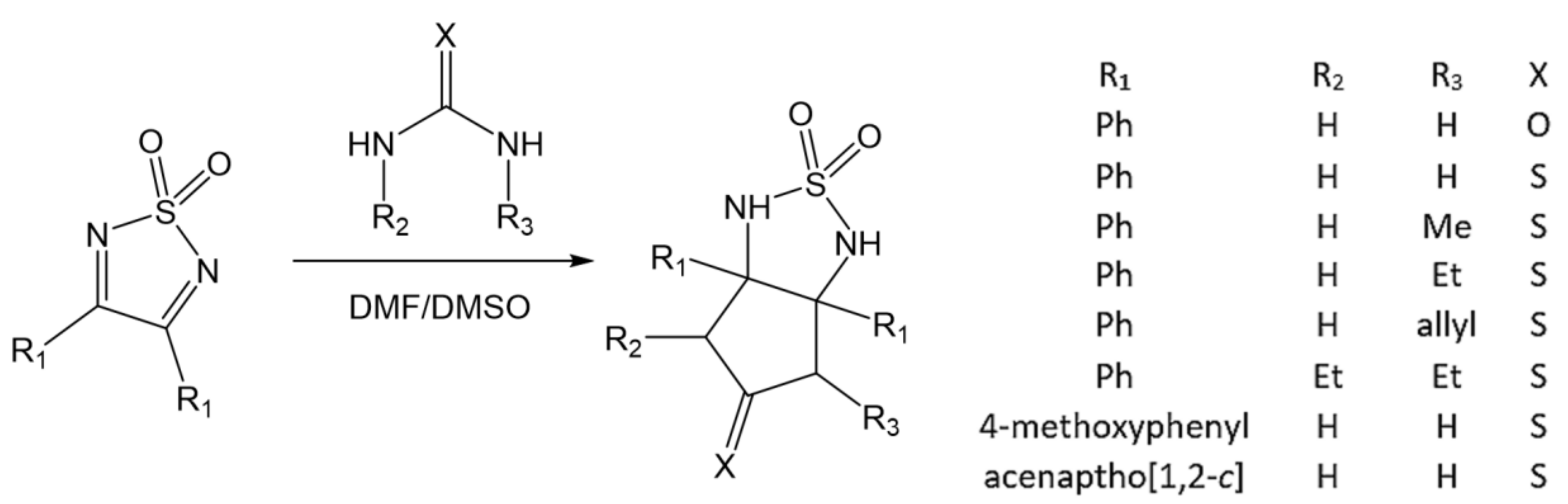
- Caram, J.A.; Mirífico, M.V.; Aimone, S.L.; Piro, O.E.; Castellano, E.E.; Vasini, E.J. The addition reaction of diamides to 1,2,5-thiadiazole 1,1 -dioxide derivatives. J. Phys. Org. Chem. 2004, 17, 1091–1098. [Google Scholar] [CrossRef]
- Arroyo, N.R.; Rozas, M.F.; Vázquez, P.; Romanelli, G.P.; Mirífico, M.V. Solvent-Free Condensation Reactions to Synthesize Five-Membered Heterocycles Containing the Sulfamide Fragment. Synthesis 2016, 48, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.B. The Reaction of Sulfamide with α- and β-Diketones. The Preparation of 1,2,5-Thiadiazole 1,1-dioxides and 1,2,6-Thiadiazine 1,1-dioxides. J. Org. Chem. 1964, 29, 1905–1909. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Xu, M.H. Rhodium-Catalyzed Enantioselective Alkenylation of Cyclic Ketimines: Synthesis of Multifunctional Chiral α,α-Disubstituted Allylic Amine Derivatives. Org. Lett. 2018, 20, 2306–2310. [Google Scholar] [CrossRef]
- Gediz Erturk, A.; Bekdemir, Y. Microwave-assisted synthesis of some substituted sulfamides. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 285–292. [Google Scholar] [CrossRef]
- Algieri, A.A.; Luke, G.M.; Standridge, R.T.; Brown, M.; Partyka, R.A.; Crenshaw, R.R. 1,2,5-Thiadizole 1-oxide and 1,1-dioxide derivatives. A new class of potent histamine H2-receptor antagonists. J. Med. Chem. 1982, 25, 210–212. [Google Scholar] [CrossRef]
- Chung, K.W.; Lee, C.H. Studies on the Chemical Properties and Synthesis of Sulfamide Derivatives (II): Oxidation Reaction of 4-Aryl-3-imino-1,2,5-thiadiazolidine 1,1-dioxides. J. Korean Chem. Soc. 1995, 39, 834–836. [Google Scholar]
- Xiao, Y.; Qian, X. Novel highly efficient fluoroionophores with a peri-effect and strong electron-donating receptors: TICT-promoted PET and signaling response to transition metal cations with low background emission. Tetrahedron Lett. 2003, 44, 2087–2091. [Google Scholar] [CrossRef]
- Ege, G.; Beisiegel, E. Eine neue Synthese aromatischer Dinitrile durch Pyrolyse benzo-kondensierter 1,2,5-Thiadiazol-1,1-dioxide. Synthesis 1974, 1974, 22–23. [Google Scholar] [CrossRef]
- Mirífico, M.V.; Caram, J.A.; Vasini, E.J.; Sicre, J.E. Interaction of 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide with proton donor solvents. J. Phys. Org. Chem. 1993, 6, 341–346. [Google Scholar] [CrossRef]
- Caram, J.A.; Mirífico, M.V.; Aimone, S.L.; Vasini, E.J. 3,4-Disubstituted derivatives of 1,2,5-thiadiazole 1,1-dioxide. Ethanol addition reactions and electroreduction of 3-methyl-4-phenyl and 3,4-dimethyl derivatives in acetonitrile and ethanol solvents. Can. J. Chem. 1996, 74, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Caram, J.A.; Mirífico, M.V.; Vasini, E.J. Electrochemistry of 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide (I) and its derivatives in ethanol-acetonitrile solutions and interactions of the dianion of I with metal cations. Electrochim. Acta 1994, 39, 939–945. [Google Scholar] [CrossRef]
- Caram, J.A.; Piro, O.E.; Castellano, E.E.; Mirífico, M.V.; Vasini, E.J. Reactions of the activated, rigid, α-diazomethine group of 1,2,5-thiadiazole 1,1-dioxides with nitrogenated nucleophiles. Part III: Aliphatic monoamines and phenylhydrazine. J. Phys. Org. Chem. 2006, 19, 229–237. [Google Scholar] [CrossRef]
- Caram, J.A.; Almone, S.L.; Mirífico, M.V.; Vasini, E.J. Reactions of 1,2,5-thiadiazole 1,1-dioxide derivatives with nitrogenated nucleophiles. Part 1—Addition of amines and amides to 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide. J. Phys. Org. Chem. 2003, 16, 220–225. [Google Scholar] [CrossRef]
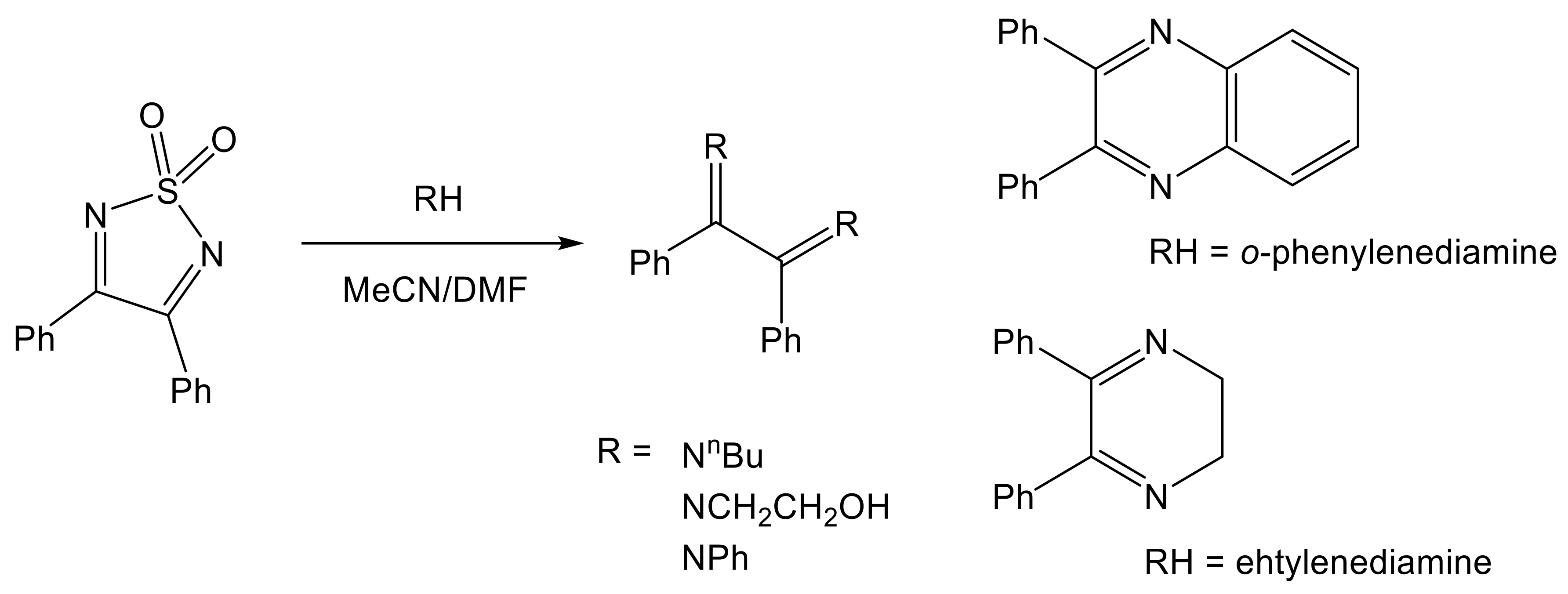
- Mirífico, M.V.; Caram, J.A.; Vasini, E.J.; Piro, O.E.; Castellano, E.E. Reactions of 1,2,5-thiadiazole 1,1-dioxide derivatives with nitrogen nucleophiles. Part IV. Addition of α-diamines. J. Phys. Org. Chem. 2009, 22, 163–169. [Google Scholar] [CrossRef]
- Mirífico, M.V.; Caram, J.A.; Piro, O.E.; Vasini, E.J. Synthesis of an α-amino nitrile and a bis α-amino nitrile derivative of thiadiazole: Reaction mechanism. J. Phys. Org. Chem. 2007, 20, 1081–1087. [Google Scholar] [CrossRef]
- Baranov, V.V.; Antonova, M.M.; Melnikova, E.K.; Kravchenko, A.N. Reductive transformation of 3-methoxy-2-methyl-3,4-diphenyl-2,3-dihydro-1,2,5-thiadiazole 1,1-dioxide in the reaction with thiourea and HC1. Russ. Chem. Bull. 2020, 69, 401–404. [Google Scholar] [CrossRef]
- Pansare, S.V.; Rai, A.N.; Kate, S.N. Stereoselective synthesis of 3,4-disubstituted 1,2,5-thiadiazolidine 1,1-dioxides and their conversion to unsymmetrical vicinal diamines. Synlett 1998, 1998, 623–624. [Google Scholar] [CrossRef]
- Rozas, M.F.; Piro, O.E.; Castellano, E.E.; Mirífico, M.V.; Vasini, E.J. Addition of aromatic nucleophiles to a C=N double bond of 1,2,5-thiadiazole 1,1-dioxide. Molecules 2000, 5, 503–504. [Google Scholar] [CrossRef]
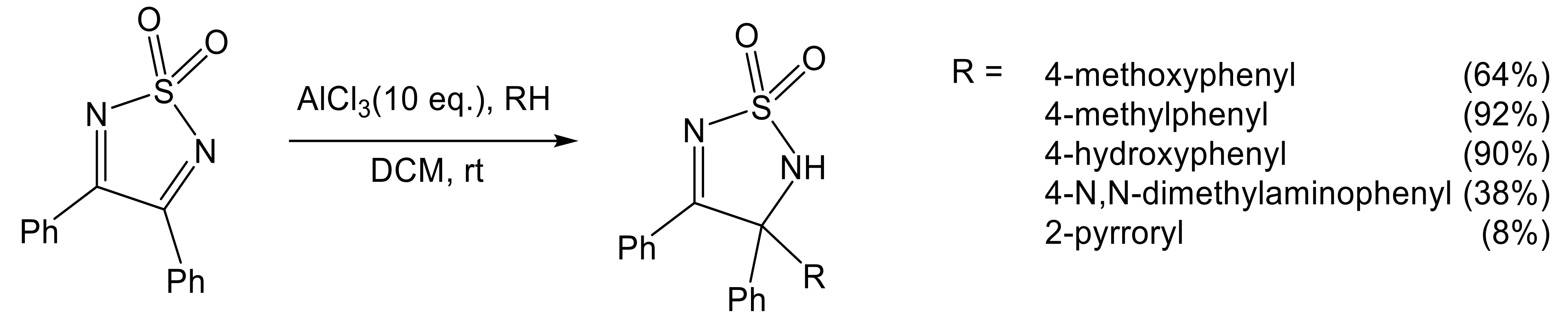
- Svartman, E.L.; Rozas, M.F.; Piro, O.E.; Castellano, E.; Mirífico, M.V. Efficient formation of polycyclic aromatic systems fused to a thiadiazole ring using strong Lewis or Brønsted acids. Synthesis 2006, 2006, 2313–2318. [Google Scholar] [CrossRef]
- Aimone, S.L.; Caram, J.A.; Mirífico, M.V.; Vasini, E.J. A new type of imine α-anion derived from 3-methyl-4-phenyl-1,2,5-thiadiazole 1,1-dioxide. Tautomerization and dimerization reactions. J. Phys. Org. Chem. 2001, 14, 217–223. [Google Scholar] [CrossRef]
- Svartman, E.L.; Caram, J.A.; Mirífico, M.V.; Vasini, E.J. Electrochemistry of 1,2,5-thiadiazole 1,1 -dioxide derivatives with 3,4 substituents presenting separated, connected, and fused π-systems. Can. J. Chem. 1999, 77, 511–517. [Google Scholar] [CrossRef]
- Boeré, R.T.; Roemmele, T.L. Electrochemistry of redox-active group 15/16 heterocycles. Coord. Chem. Rev. 2000, 210, 369–445. [Google Scholar] [CrossRef]
- Jaeger, K.; Bruenle, S.; Weinert, T.; Guba, W.; Muehle, J.; Miyazaki, T.; Weber, M.; Furrer, A.; Haenggi, N.; Tetaz, T.; et al. Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7. Cell 2019, 178, 1222–1230.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biju, P.; Taveras, A.; Yu, Y.; Zheng, J.; Chao, J.; Rindgen, D.; Jakway, J.; Hipkin, R.W.; Fossetta, J.; Fan, X.; et al. 3,4-Diamino-2,5-thiadiazole-1-oxides as potent CXCR2/CXCR1 antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kudo, T.; Lapa, C.; Buck, A.; Higuchi, T. Recent advances in radiotracers targeting norepinephrine transporter: Structural development and radiolabeling improvements. J. Neural Transm. 2020, 127, 851–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, V.K.; Knaus, E.E.; McNeill, J.H. Pyridine and reduced pyridine analogues of 1,2,5-thiadiazoles as histamine H2-receptor antagonists. Eur. J. Med. Chem. 1987, 22, 319–323. [Google Scholar] [CrossRef]
- Grillo, C.A.; Mirífico, M.V.; Morales, M.L.; Reigosa, M.A.; de Mele, M.F.L. Assessment of cytotoxic and cytogenetic effects of a 1,2,5-thiadiazole derivative on CHO-K1 cells. Its application as corrosion inhibitor. J. Hazard. Mater. 2009, 170, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
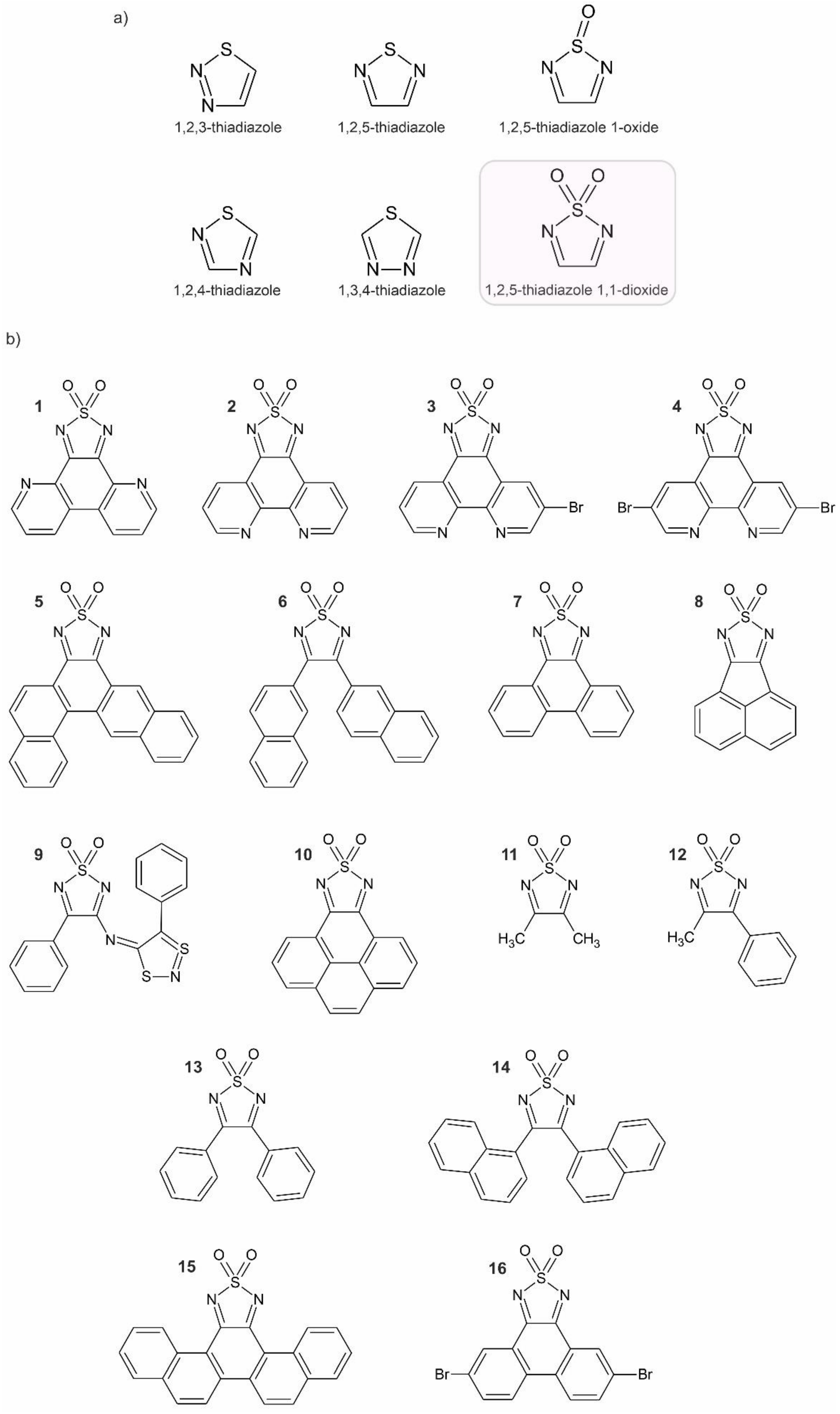


| No. in Figure 1b | Compound Name | CCDC Number (Database ID) | Ref. |
|---|---|---|---|
| 1 | [1,2,5]thiadiazolo[3,4-f][4,7]phenanthroline 2,2-dioxide (4,7-tdapO2) | 997100 (BOKXEG) | [43] |
| 2 | [1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide (1,10-tdapO2) | 819976 (NALXOP) | [44] [14] |
| 932270 (MINREI) | |||
| 3 | 5-bromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide | 1882326 (FOFFAK) | [45] |
| 4 | 5,10-dibromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide | 1882327 (FOFFEO) | [45] |
| 5 | benzo[1,2]tetrapheno[5,6-c][1,2,5]thiadiazole 8,8-dioxide | 267853 (GESRUS) | [46] |
| 6 | 3,4-di(naphthalen-2-yl)-1,2,5-thiadiazole 1,1-dioxide | 267854 (GESSAZ) | [46] |
| 7 | phenanthro[9,10-c][1,2,5]thiadiazole 2,2-dioxide | 1179881 (IGISIA) 783889 (IGISIA01) 1577093 (IGISIA02) | [47] [21] [48] |
| 8 | acenaphtho[1,2-c][1,2,5]thiadiazole 8,8-dioxide | 1179882 (IGISOG) | [47] |
| 9 | N-(1,1-dioxo-4-phenyl-1,2,5-thiadiazol-3-yl)-4′-phenyl-1′,3′,2′-dithiazole-5′-imine | 1192789 (KAMYEC) | [49] |
| 10 | pyreno[4,5-c][1,2,5]thiadiazole 10,10-dioxide | 783892 (ONIPEH) | [21] |
| 11 | 3,4-dimethyl-1,2,5-thiadiazole 1,1-dioxide | 1231828 (PEXMEK) | [50] |
| 12 | 3-methyl-4-phenyl-1,2,5-thiadiazole 1,1-dioxide | 1231842 (PEXQAK) | [50] |
| 13 | 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide | 1231843 (PEXQEO) | [50] |
| 14 | 3,4-di(naphthalen-1-yl)-1,2,5-thiadiazole 1,1-dioxide | 972953 (VITRIB) | [51] |
| 15 | piceno[13,14-c][1,2,5]thiadiazole 14,14-dioxide | 972954 (VITROH) | [51] |
| 16 | 5,10-dibromophenanthro[9,10-c][1,2,5]thiadiazole 2,2-dioxide | 1577094 (ZIMLUF) | [48] |
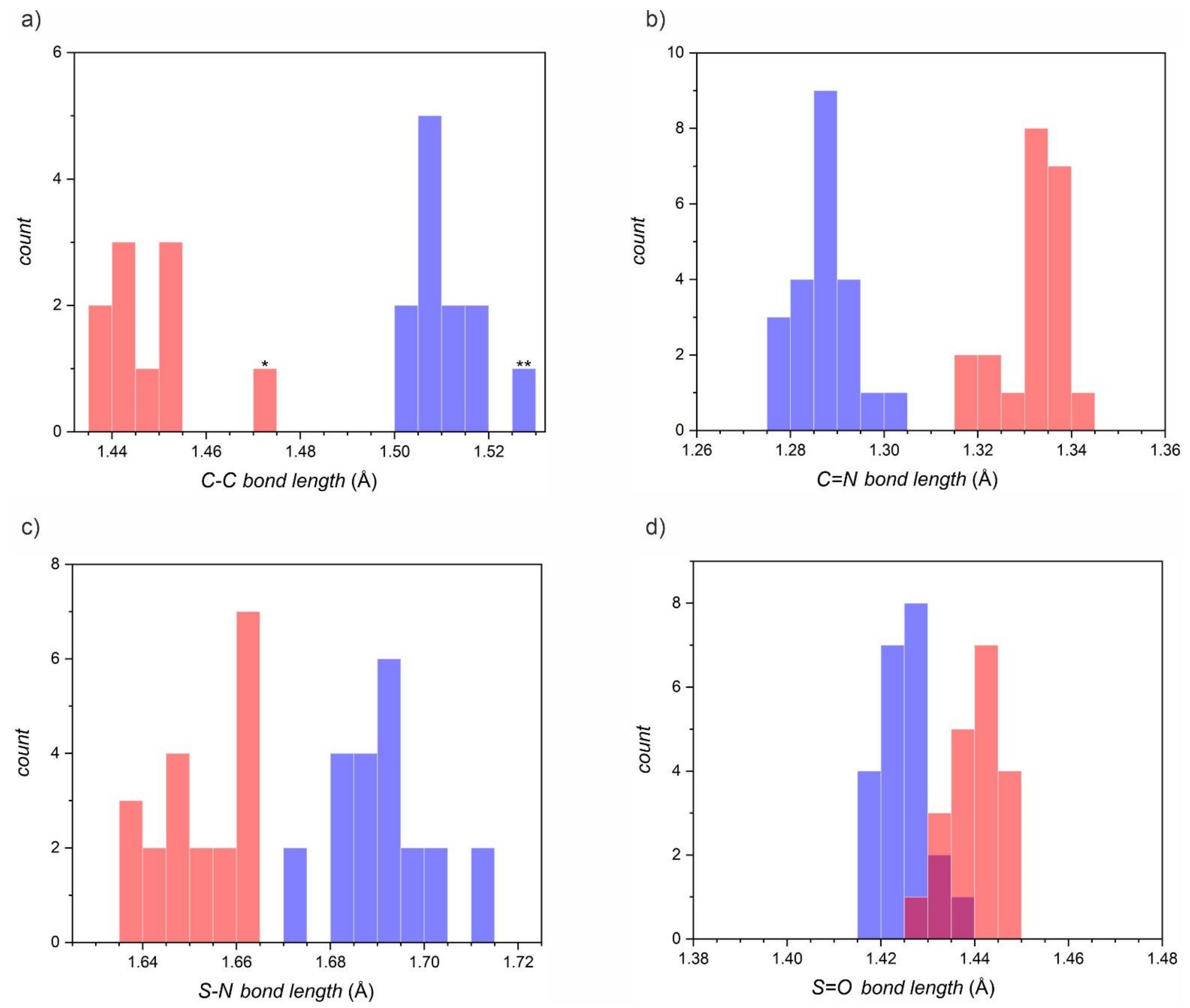
| Compound | S=O (Å) | S-N (Å) | C=N (Å) | C-C (Å) | CSD ID | T (K) |
|---|---|---|---|---|---|---|
| [1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide | 1.429 | 1.687 | 1.287 | 1.508 | NALXOP | 173 |
| 1.424 | 1.686 | 1.290 | ||||
| 1.425 | 1.684 | 1.290 | 1.507 | MINREI | 173 | |
| 1.427 | 1.690 | 1.285 | 1.510 | MINROS | 173 | |
| 1.421 | 1.684 | 1.286 | ||||
| 1.425 | 1.685 | 1.282 | 1.515 | MINRIM | 173 | |
| [CuIICl(1,10-tdapO2)](μ-Cl)2[CuIICl(1,10-tdapO2)] | 1.419 | 1.703 | 1.273 | 1.503 | DUZCIN | 120 |
| 1.419 | 1.682 | 1.287 | ||||
| 5-bromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide | 1.423 | 1.694 | 1.293 | 1.506 | FOFFAK | 120 |
| 1.429 | 1.693 | 1.285 | ||||
| 5,10-dibromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide | 1.422 | 1.693 | 1.290 | 1.514 | FOFFEO | 161 |
| 1.425 | 1.696 | 1.284 | ||||
| [1,2,5]thiadiazolo[3,4-f][4,7]phenanthroline 2,2-dioxide | 1.430 | 1.700 | 1.291 | 1.519 | BOKXEG | 100 |
| 1.427 | 1.699 | 1.287 | ||||
| pyreno[4,5-c][1,2,5]thiadiazole 10,10-dioxide | 1.417 | 1.691 | 1.295 | 1.518 | ONIPEH | 173 |
| 1.434 | 1.683 | 1.291 | ||||
| phenanthro[9,10-c][1,2,5]thiadiazole 2,2-dioxide | 1.432 | 1.686 | 1.288 | 1.510 | IGISIA01 | 173 |
| 1.417 | 1.691 | 1.294 | ||||
| piceno[13,14-c][1,2,5]thiadiazole 14,14-dioxide | 1.438 | 1.670 | 1.302 | 1.529 | VITROH | 123 |
| 1.427 | 1.674 | 1.300 | ||||
| [CuIICl(4,7-tdapO2)CuIICl] | 1.428 | 1.713 | 1.275 | 1.503 | BOKXIK | 100 |
| 1.428 | 1.713 | 1.270 | ||||
| Average in neutral species 1 | 1.426(3) | 1.691(7) | 1.287(5) | 1.512(7) | - | - |
| PPN+[CuIICl(1,10-tdapO2−)2] | 1.439 | 1.662 | 1.331 | 1.450 | DUZCUZ | 120 |
| 1.433 | 1.642 | 1.325 | ||||
| 1.431 | 1.660 | 1.320 | 1.453 | |||
| 1.434 | 1.664 | 1.321 | ||||
| PPN+[1,10-tdapO2]− | 1.439 | 1.646 | 1.333 | 1.446 | FOFFIS | 120 |
| 1.442 | 1.654 | 1.334 | ||||
| 1.442 | 1.664 | 1.343 | 1.443 | FOFFOY | 120 | |
| 1.448 | 1.664 | 1.340 | ||||
| PPN+[5,10-diBr-1,10-tdapO2]− | 1.443 | 1.646 | 1.333 | 1.452 | FOFFUE | 117 |
| 1.443 | 1.661 | 1.336 | ||||
| 1.442 | 1.660 | 1.337 | 1.435 | |||
| 1.445 | 1.656 | 1.333 | ||||
| PPN+[5-Br-1,10-tdapO2]− | 1.437 | 1.648 | 1.334 | 1.441 | FOFGAL | 120 |
| 1.432 | 1.659 | 1.332 | ||||
| K+[1,10-tdapO2]− | 1.449 | 1.646 | 1.338 | 1.441 | MINQEH | 173 |
| 1.448 | 1.639 | 1.337 | ||||
| NEt+[ptdaO2]− | 1.442 | 1.640 | 1.340 | 1.471 | VITSAU | 173 |
| 1.447 | 1.636 | 1.337 | ||||
| K+[4,7-tdapO2]− | 1.438 | 1.638 | 1.330 | 1.437 | VONZOP | 110 |
| 1.437 | 1.651 | 1.320 | ||||
| Average in radical anions 1 | 1.440(4) | 1.652(7) | 1.333(4) | 1.447(10) | - | - |
| Relative difference between neutral and radical | 1.0(0.3)% | 2.3(0.4)% | 3.5(0.3)% | 4.3(0.7)% | - | - |
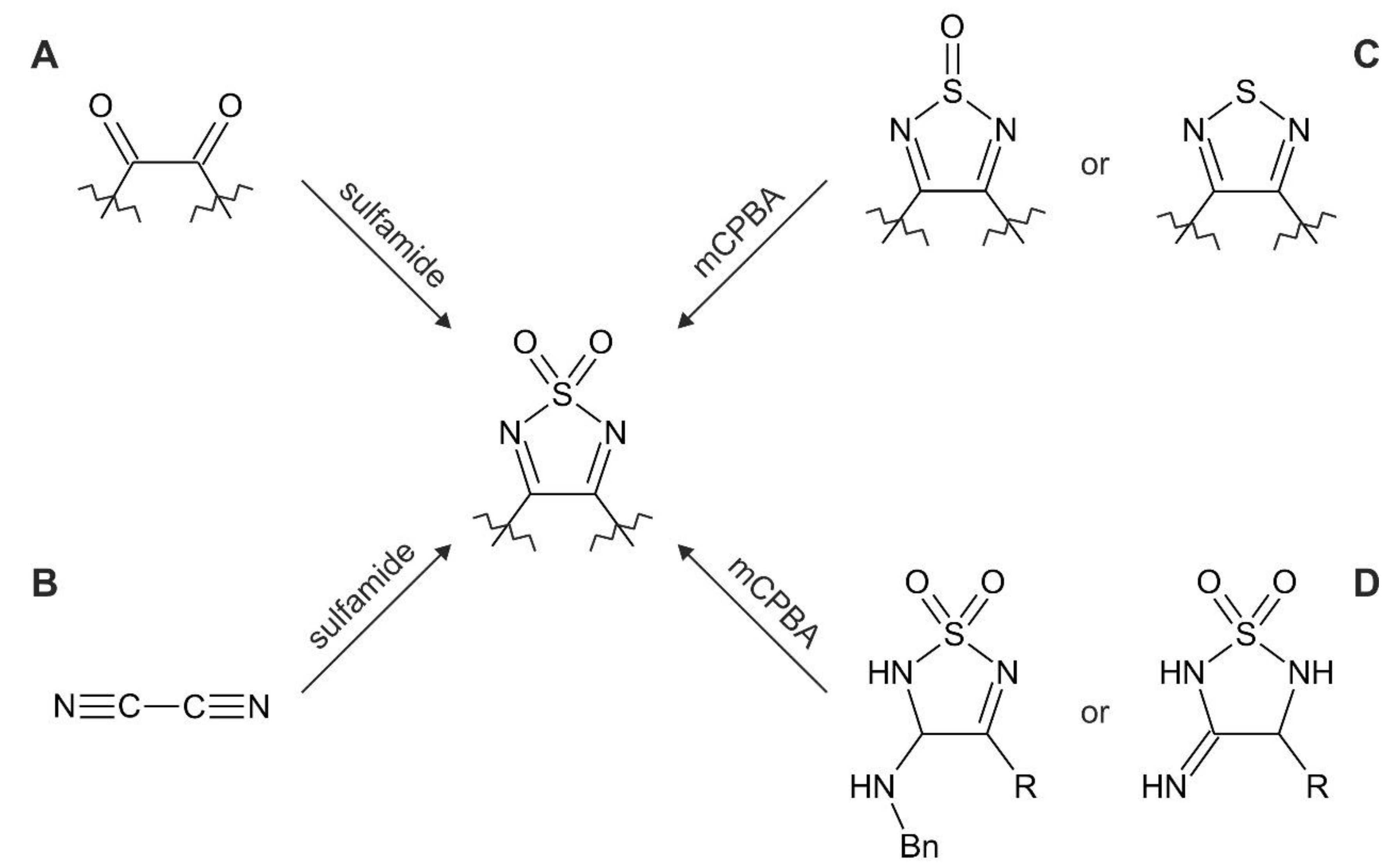
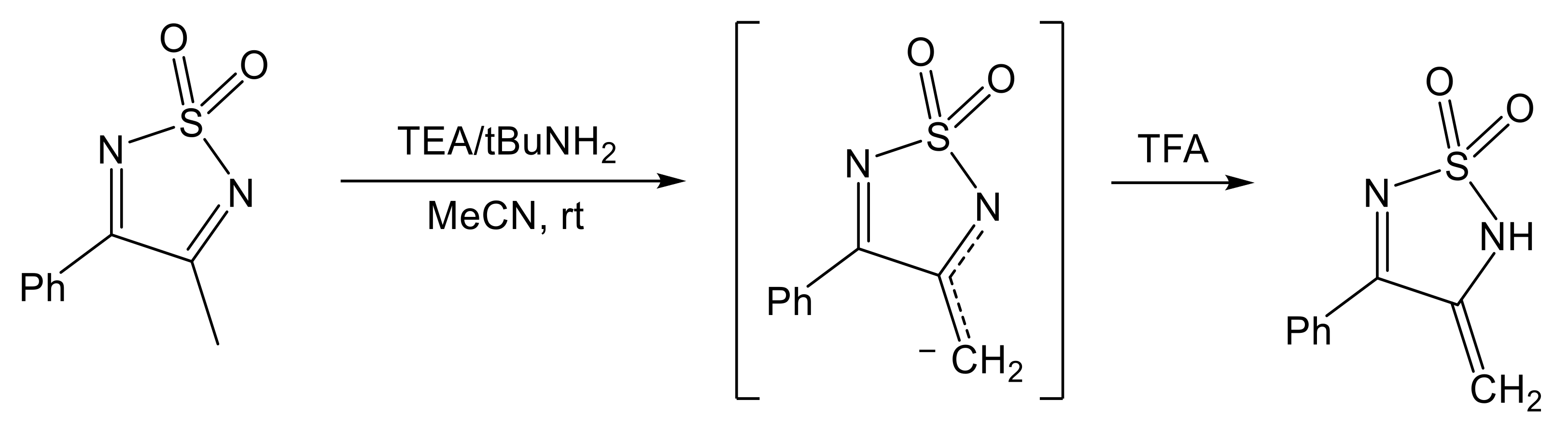
| Synthesis Type According to Figure 2 | Synthesis Details | Product | Ref. |
|---|---|---|---|
| A | ethanol, reflux, 12–168 h | [1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide [1,2,5]thiadiazolo[3,4-f][4,7]phenanthroline 2,2-dioxide 5-bromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide 5,10-dibromo-[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline 2,2-dioxide | [44] [43] [45] |
| A | ethanol, dry HCl, reflux, 2–3 h | 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide, acenaphtho[1,2-c][1,2,5]thiadiazole 8,8-dioxide | [75] |
| A | methanol, MeONa, 80 °C, overnight | sodium 4-phenyl-1,2,5-thiadiazol-3-olate 1,1-dioxide disodium 1,2,5-thiadiazole-3,4-bis(olate) 1,1-dioxide | [76] [72] |
| A | NEt3, MW (360 W), 10 min | 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide | [77] |
| A | solvent-free, molybdophosphoric acid (MPA), rt-150 °C, 3–530 h | 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide, 3-methyl-4-phenyl-1,2,5-thiadiazole 1,1-dioxide, 3,4-dimethyl-1,2,5-thiadiazole 1,1-dioxide, phenanthro[9,10-c][1,2,5]thiadiazole 2,2-dioxide, pyreno[4,5-c][1,2,5]thiadiazole 10,10-dioxide, 3,4-di(naphthalen-2-yl)-1,2,5-thiadiazole 1,1-dioxide, 3,4-di([1,1′-biphenyl]-4-yl)-1,2,5-thiadiazole 1,1-dioxide, 3,4-bis(4-chlorophenyl)-1,2,5-thiadiazole 1,1-dioxide | [74] |
| A | N,N’-bis(TMS)sulfamide, toluene, BF3·Et2O, rt, 8 h | 3,4-diphenyl-1,2,5-thiadiazole 1,1-dioxide, 3-methyl-4-phenyl-1,2,5-thiadiazole 1,1-dioxide, 3-ethyl-4-phenyl-1,2,5-thiadiazole 1,1-dioxide, 3-ethyl-4-(4-methoxyphenyl)-1,2,5-thiadiazole-1,1-dioxide, 3-Isopropyl-4-(4-methoxyphenyl)-1,2,5-thiadiazole-1,1-dioxide, 3-ethyl-4-(4-chlorophenyl)-1,2,5-thiadiazole-1,1-dioxide, 3-ethyl-4-(furan-2-yl)-1,2,5-thiadiazole-1,1-dioxide, 3-ethyl-4-(thiophen-3-yl)-1,2,5-thiadiazole-1,1-dioxide | [64] |
| B | diglyme, −60 °C, dry HCl | 3,4-diamino-1,2,5-thiadiazole 1,1-dioxide | [63] |
| C | DCM, mCPBA, reflux, overnight | phenanthro[9,10-c][1,2,5]thiadiazole 2,2-dioxide, pyreno[4,5-c][1,2,5]thiadiazole 10,10-dioxide, 3,4-dimethoxy-1,2,5-thiadiazole 1,1-dioxide | [21] [78] |
| D | THF, KO2 or DMF, NaOEt | 3-amino-4-phenyl-1,2,5-thiadiazole 1,1-dioxide, 3-amino-4-(4-methoxyphenyl)-1,2,5-thiadiazole 1,1-dioxide, 3-amino-4-(naphthalen-1-yl)-1,2,5-thiadiazole 1,1-dioxide, 3-amino-4-hexyl-1,2,5-thiadiazole 1,1-dioxide, 3-(benzylamino)-4-phenyl-1,2,5-thiadiazole 1,1-dioxide, 3-(benzylamino)-4-(4-methoxyphenyl)-1,2,5-thiadiazole 1,1-dioxide | [79] [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakulski, P.; Pinkowicz, D. 1,2,5-Thiadiazole 1,1-dioxides and Their Radical Anions: Structure, Properties, Reactivity, and Potential Use in the Construction of Functional Molecular Materials. Molecules 2021, 26, 4873. https://doi.org/10.3390/molecules26164873
Pakulski P, Pinkowicz D. 1,2,5-Thiadiazole 1,1-dioxides and Their Radical Anions: Structure, Properties, Reactivity, and Potential Use in the Construction of Functional Molecular Materials. Molecules. 2021; 26(16):4873. https://doi.org/10.3390/molecules26164873
Chicago/Turabian StylePakulski, Paweł, and Dawid Pinkowicz. 2021. "1,2,5-Thiadiazole 1,1-dioxides and Their Radical Anions: Structure, Properties, Reactivity, and Potential Use in the Construction of Functional Molecular Materials" Molecules 26, no. 16: 4873. https://doi.org/10.3390/molecules26164873
APA StylePakulski, P., & Pinkowicz, D. (2021). 1,2,5-Thiadiazole 1,1-dioxides and Their Radical Anions: Structure, Properties, Reactivity, and Potential Use in the Construction of Functional Molecular Materials. Molecules, 26(16), 4873. https://doi.org/10.3390/molecules26164873