Polyphenol Rich Ajuga bracteosa Transgenic Regenerants Display Better Pharmacological Potential
Abstract
1. Introduction
2. Results
2.1. Elemental Analysis
2.2. Qualitative Screening
2.3. Quantitative Analyses
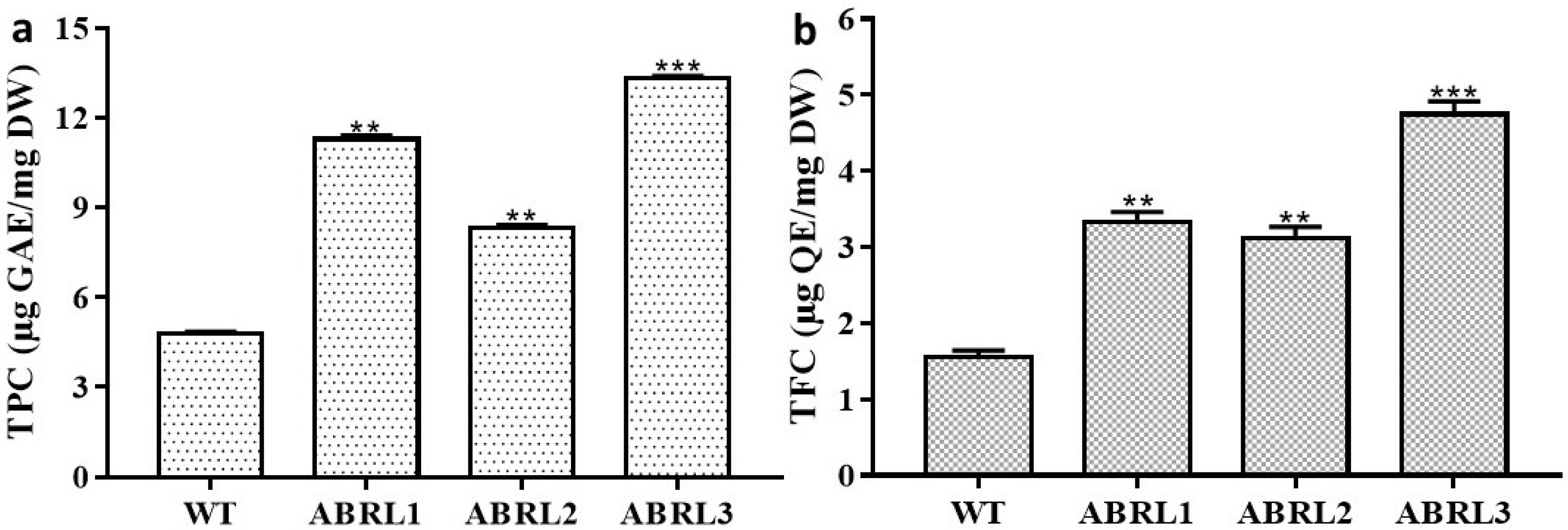
2.3.1. Determination of TPC and TFC
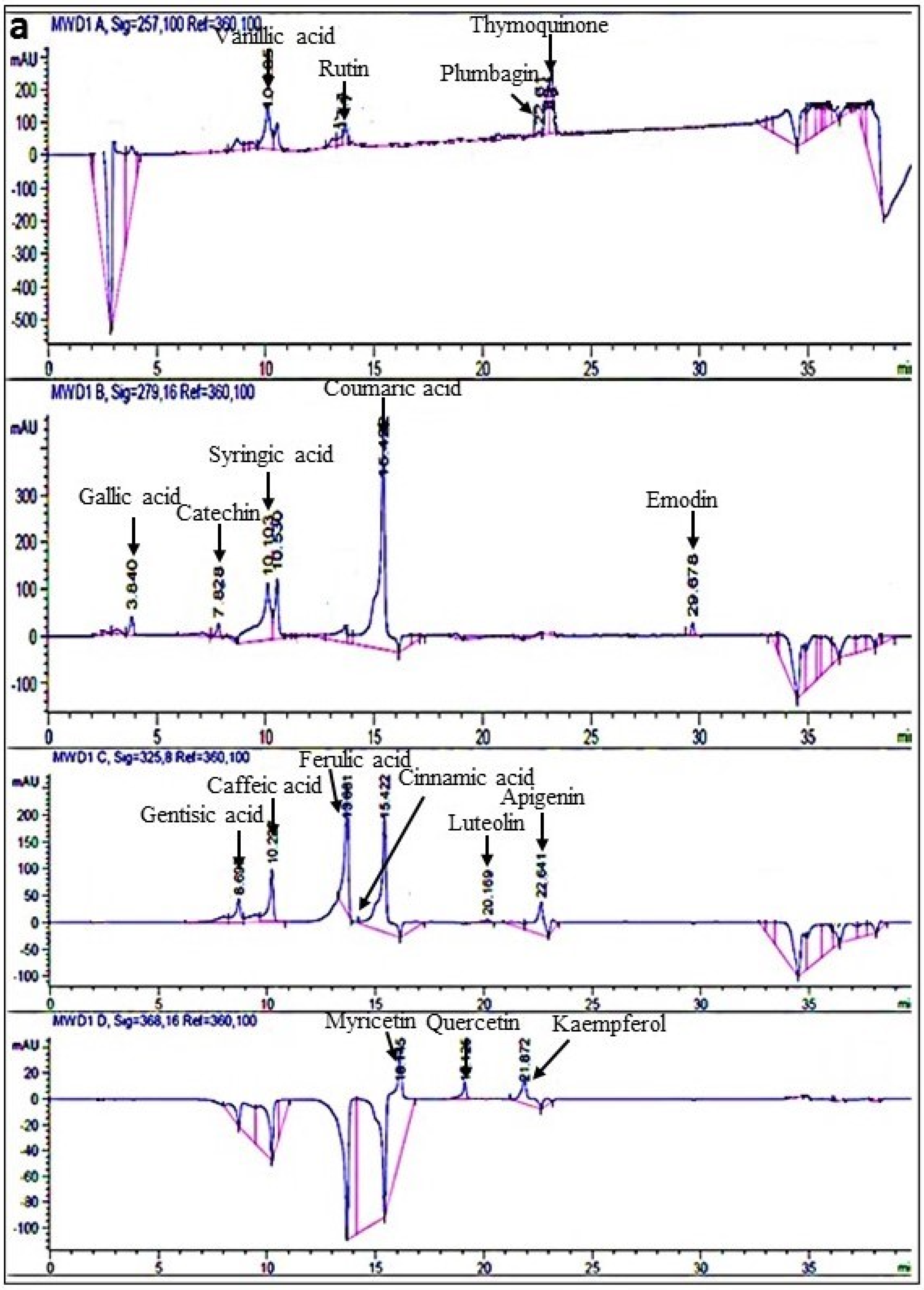
2.3.2. RP-HPLC
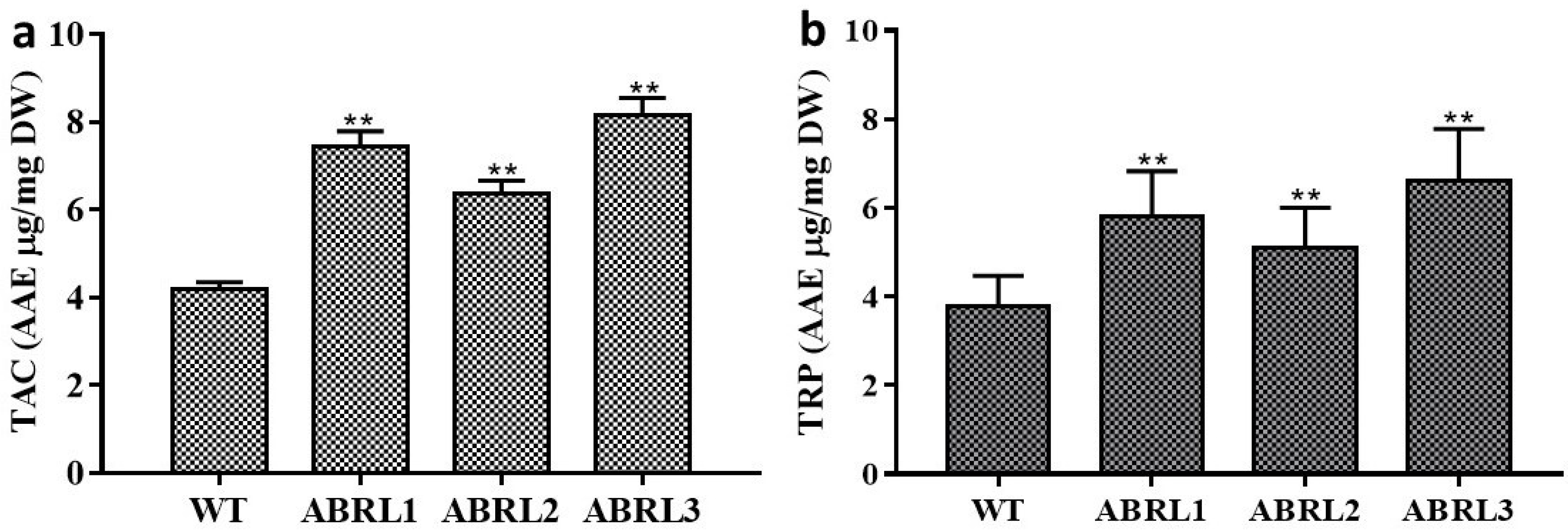
2.3.3. Total Antioxidant Capacity (TAC) and Total Reducing Power (TRP)
2.4. In Vitro Antioxidant Assays
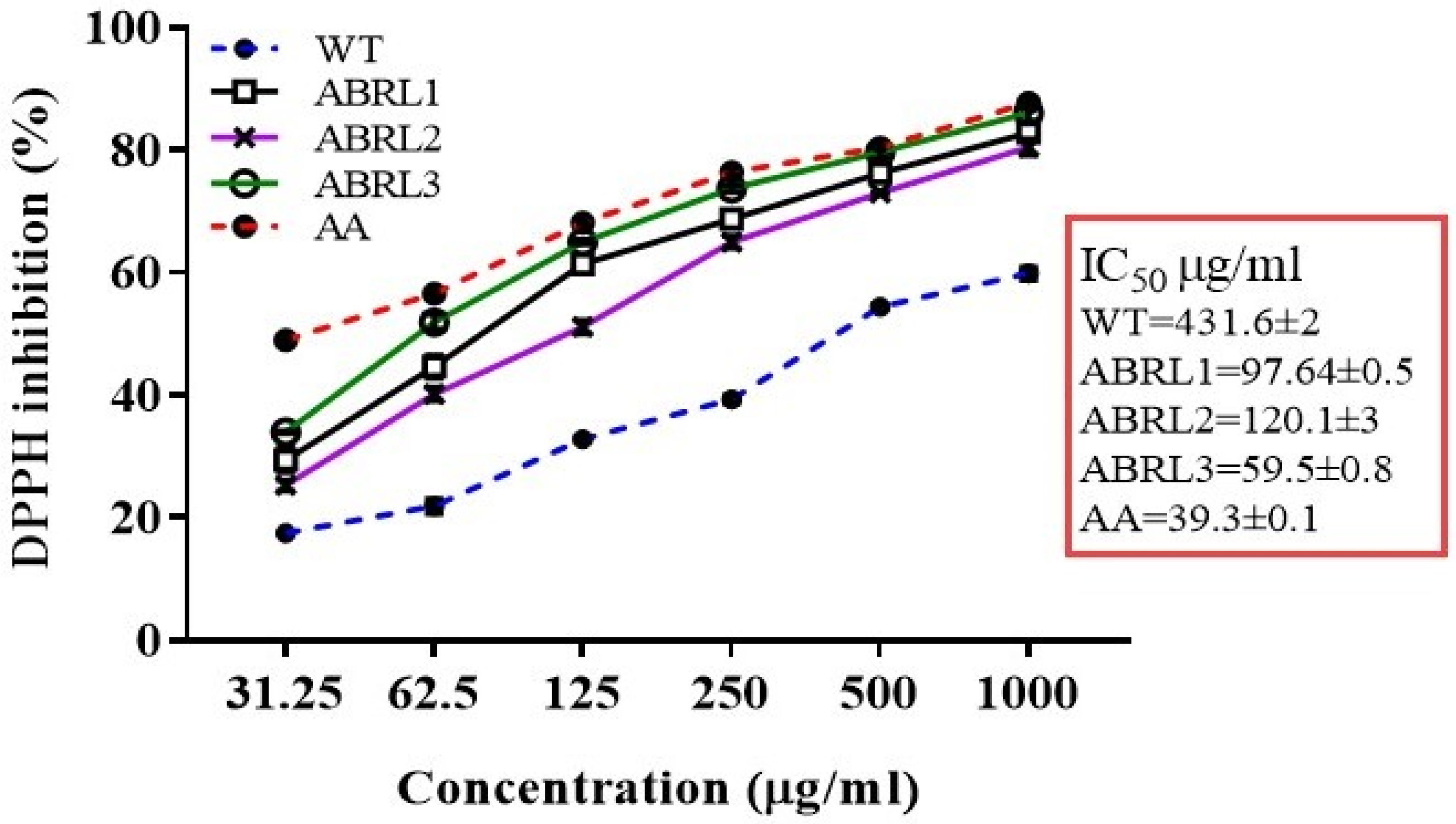
2.4.1. DPPH Radical Scavenging Assay
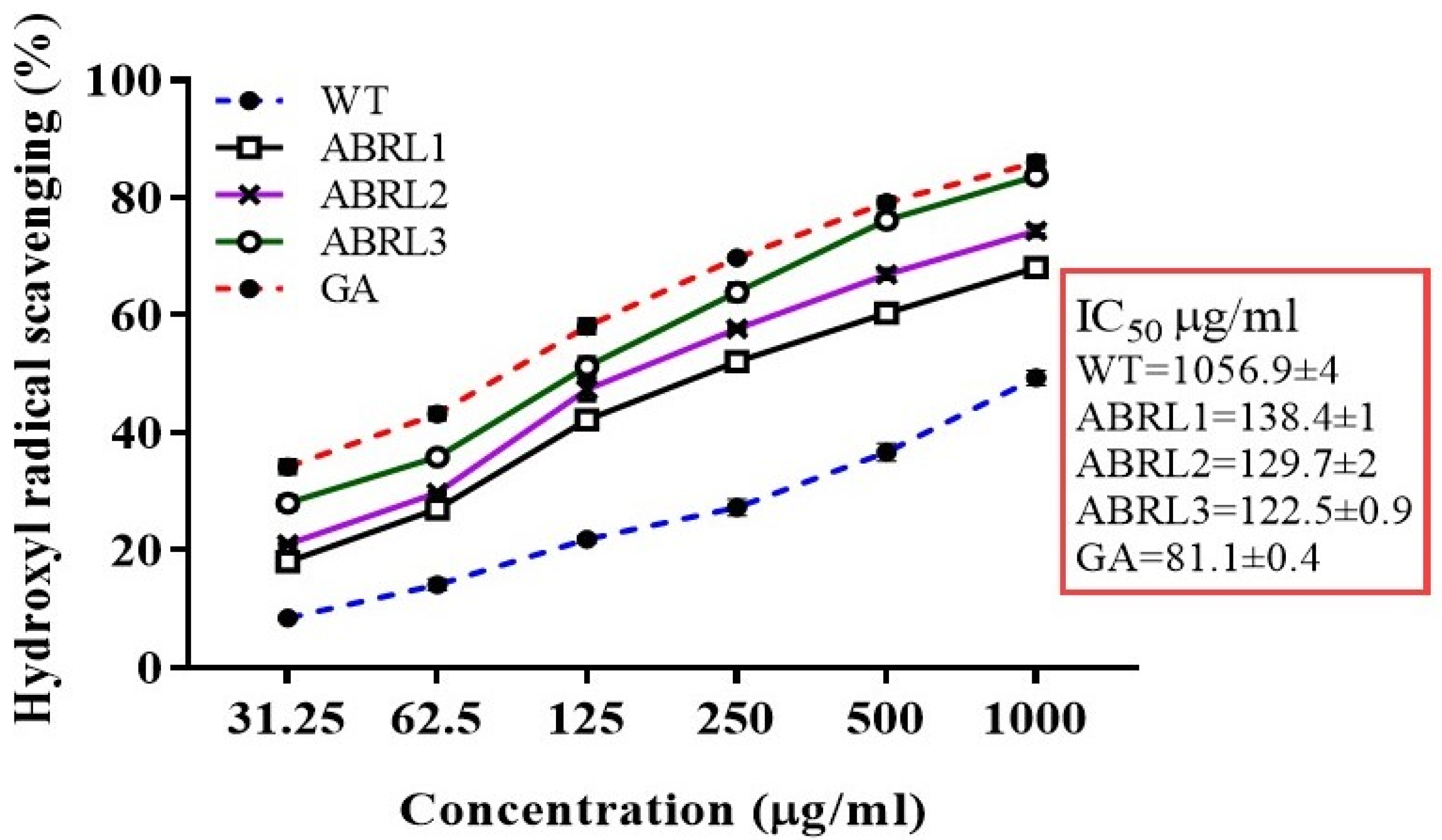
2.4.2. Hydroxyl Ion Scavenging Assay
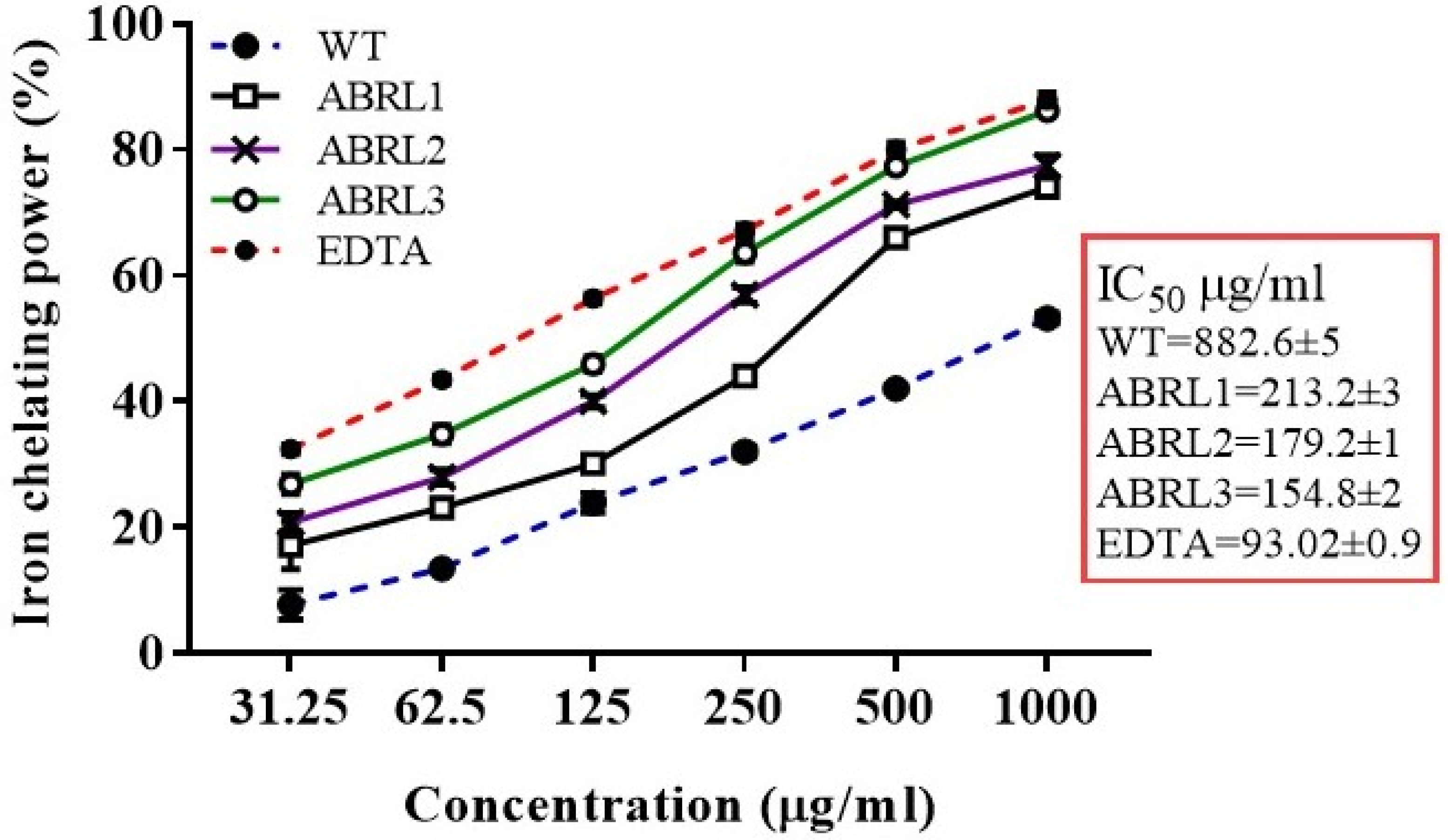
2.4.3. Ferrous Ion Chelating Activity
2.5. In Vivo Assays in BALB/c Mice
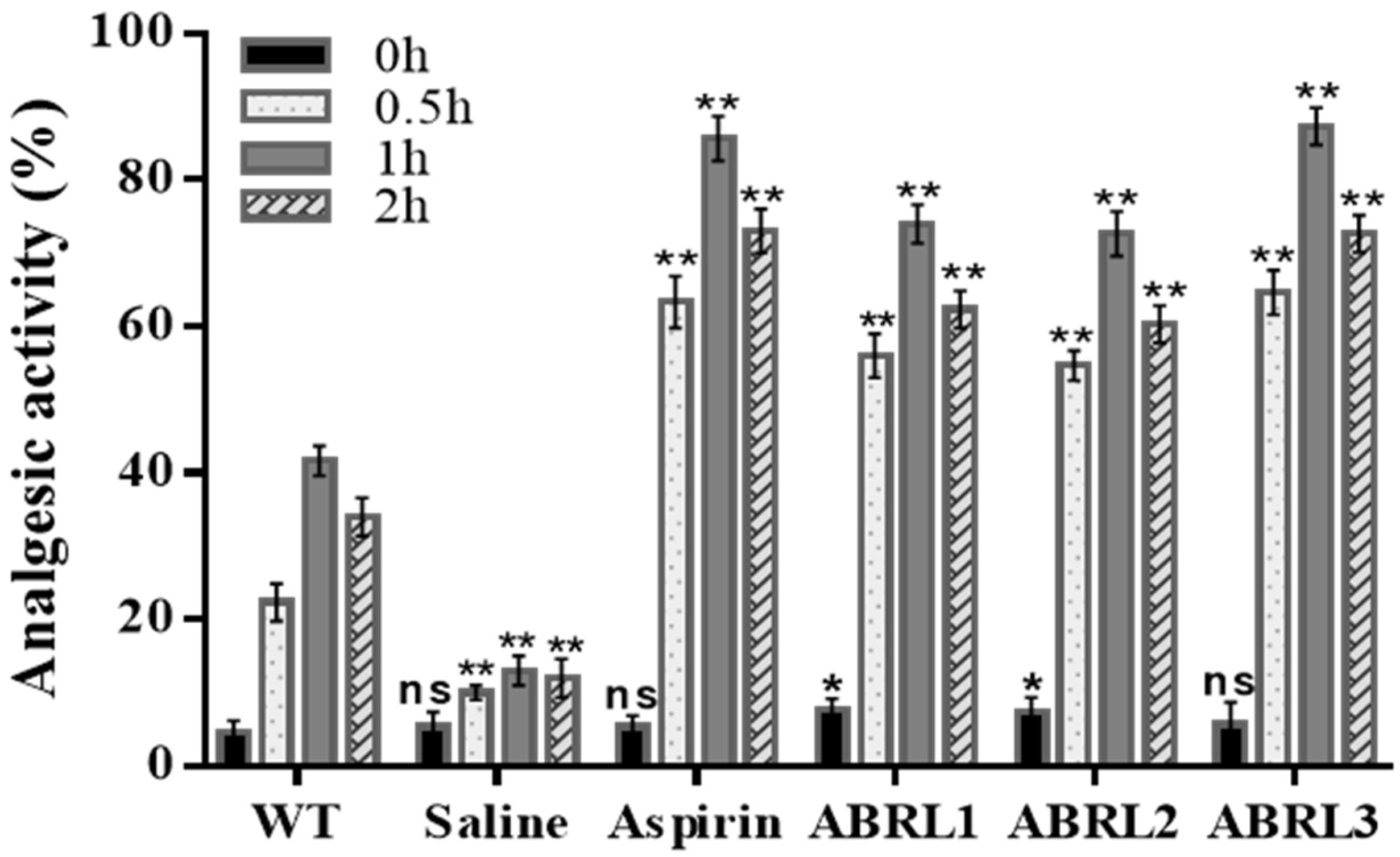
2.5.1. Analgesic Activity
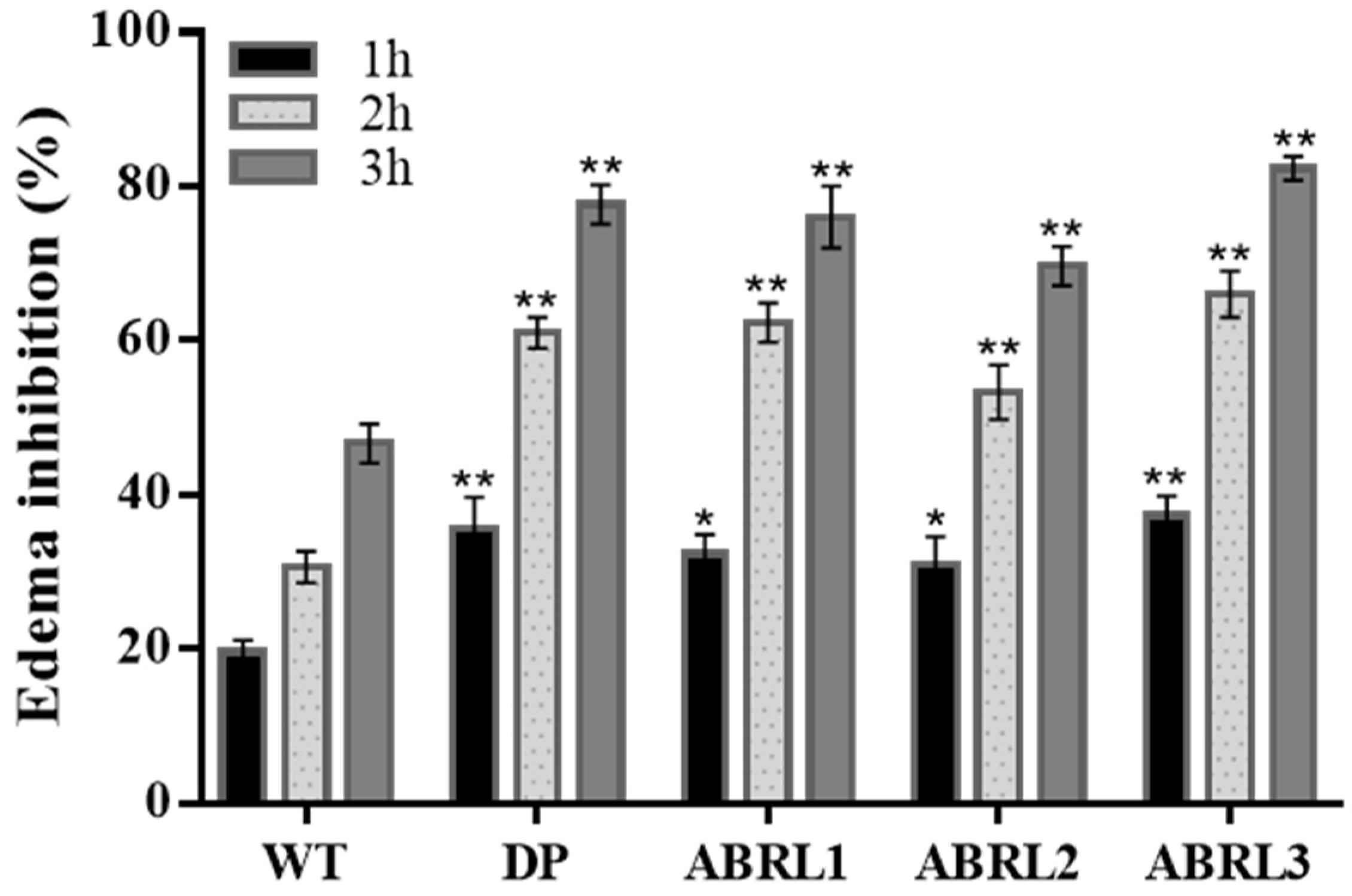
2.5.2. Anti-Inflammatory Activity
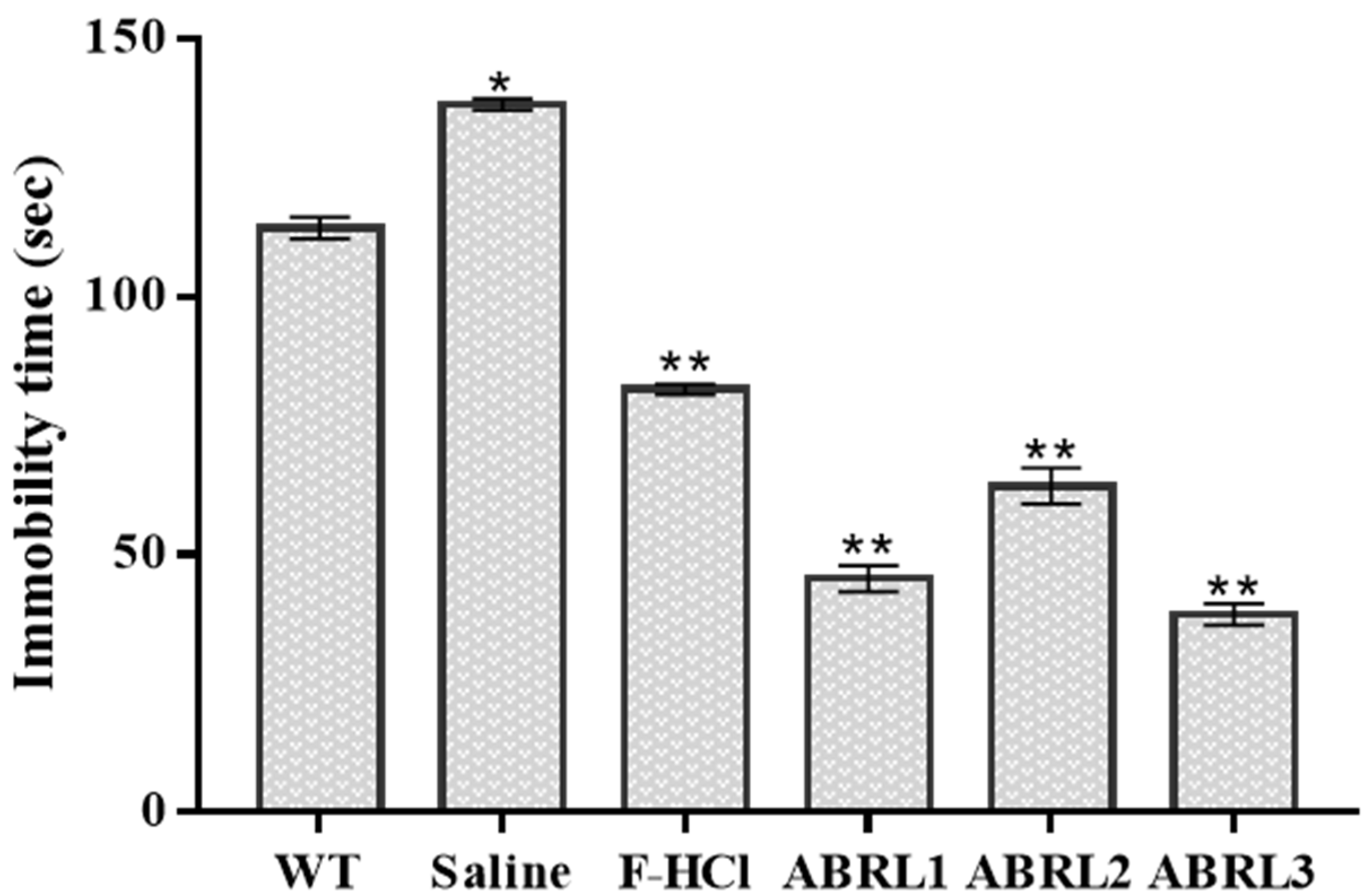
2.5.3. Antidepressant Activity
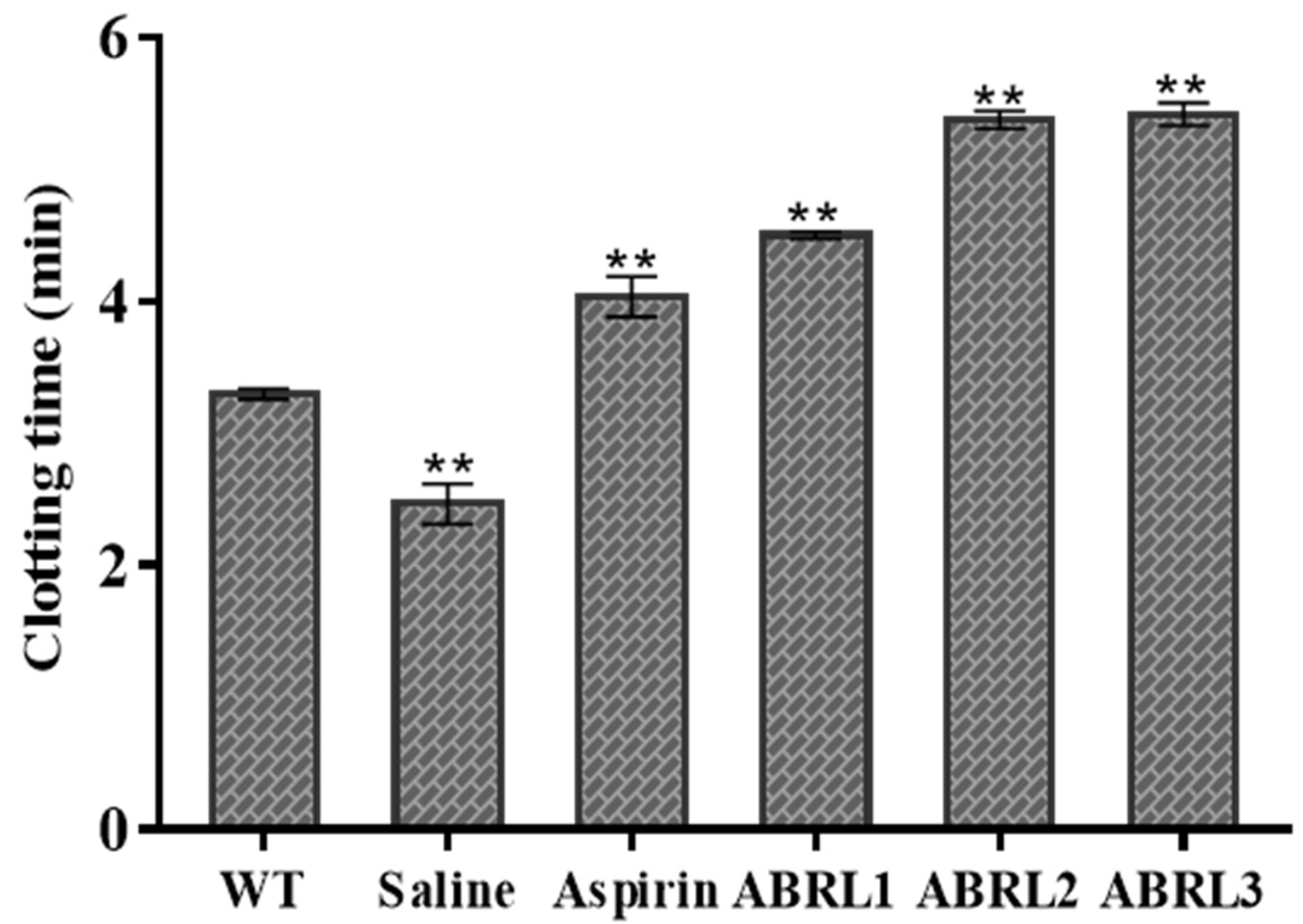
2.5.4. Anticoagulant Activity
3. Discussion
4. Materials and Methods
4.1. Source of Plant
4.2. Elemental Analysis
4.3. Crude Extracts Preparation for Biological Activities
4.4. Phytochemical Profiling
4.4.1. Qualitative Assays
4.4.2. Quantitative Assays
Estimation of Total Phenolics and Flavonoids Content
Quantification of Polyphenols by RP-HPLC
4.5. In Vitro Assays
4.5.1. Total Antioxidant Capacity Assay
4.5.2. Total Reducing Power (TRP)
4.5.3. Antioxidant Assays
Diphenyl-2-Picryl-Hydrazyl (DPPH) Radical Scavenging Activity
Hydroxyl Ion (OH) Scavenging Assay
Chelating Power Assay
4.6. In Vivo Biological Activities
4.6.1. Test Animals
4.6.2. Experimental Design
4.6.3. Acute Toxicity Test
4.6.4. Analgesic Activity (Hot-Plate Method)
4.6.5. Anti-Inflammatory Activity
4.6.6. Anti-Depressant Activity by Tail Suspension Test (TST)
4.6.7. Anticoagulant Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-Jasmonate and Salicylic Acid as Potent Elicitors for Secondary Metabolite Production in Medicinal Plants: A Review. Int. J. Pharmacogn. Phytochem. Res. 2018, 7, 7,750–757. [Google Scholar]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, A.N.; Nath, P.U.; Basumatary, B.I.; Brahma, D.U. Phytochemical, Antioxidant, and Trace Element Analysis of Anthelmintic Plants of North-East India. Int. J. Pharm. Pharm. Sci. 2017, 9, 228–232. [Google Scholar] [CrossRef]
- Kaithwas, G.; Gautam, R.; Jachak, S.M.; Saklani, A. Antiarthritic Effects of Ajuga bracteosa Wall ex Benth in Acute and Chronic Models of Arthritis in Albino Rats. Asian Pac. J. Trop. Biomed. 2012, 2, 185–188. [Google Scholar]
- Kayani, W.K.; Dilshad, E.; Ahmed, T.; Ismail, H.; Mirza, B. Evaluation of Ajuga bracteosa for Antioxidant, Anti-inflammatory, Analgesic, Antidepressant, and Anticoagulant Activities. BMC Complement. Altern. Med. 2016, 16, 375. [Google Scholar] [CrossRef]
- Qasim, S.; Uttra, A.M.; Hasan, U.H.; Batool, A. Evaluation of Anticonvulsant Potential of Aqueous Methanolic extract and Various Fractions of Ajuga bracteosa wall. J. Exp. Appl. Anim. Sci. 2017, 2, 137–146. [Google Scholar] [CrossRef]
- Zahra, S.S.; Ahmed, M.; Qasim, M.; Gul, B.; Zia, M.; Mirza, B.; Haq, I.U. Polarity Based Characterization of Biologically Active Extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC Analysis. BMC Complement. Altern. Med. 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Pal, A.; Pawar, R.S. A Study on Ajuga bracteosa wall ex. Benth for Analgesic Activity. Int. J. Curr. Biol. Med. Sci. 2011, 1, 12–14. [Google Scholar]
- Mauro, M.L.; Costantino, P.; Bettini, P.P. The Never-Ending Story of rol Genes: A Century After. Plant Cell Tiss. Org. Cult. 2017, 131, 201–212. [Google Scholar] [CrossRef]
- Bulgakov, V.P. Functions of rol Genes in Plant Secondary Metabolism. Biotechnol. Adv. 2008, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Wieczfinska, J.; Skała, E.; Śliwiński, T.; Sitarek, P. Transgenesis as a Tool for The Efficient Production of Selected Secondary Metabolites from In Vitro Plant Cultures. Plants 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Rubnawaz, S.; Kayani, W.K.; Mahmood, R.; Mirza, B. Enhanced Stress Tolerance in Transformed Ajuga bracteosa Wall. ex Benth. Regenerants by Up-Regulated Gene Expression of Metabolic Pathways. Turk. J. Bot. 2020, 44, 410–426. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of Secondary Metabolites of Various Plants: A Review. Int. J. Pharm. Sci. Res. 2019, 10, 494–498. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Karpiuk, U.V.; Al Azzam, K.M.; Abudayeh, Z.H.M.; Kislichenko, V.; Naddaf, A.; Cholak, I.; Yemelianova, O. Qualitative and Quantitative Content Determination of Macro-Minor Elements in Bryonia alba L. Roots Using Flame Atomic Absorption Spectroscopy Technique. Adv. Pharm. Bull. 2016, 6, 285. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Gundorina, S.; Vergel, K.; Grozdov, D.; Ciocarlan, A.; Aricu, A.; Dragalin, I.; Ciocarlan, N. Elemental Analysis of Lamiaceae Medicinal and Aromatic Plants Growing in The Republic of Moldova Using Neutron Activation Analysis. Phytochem. Lett. 2020, 35, 119–127. [Google Scholar] [CrossRef]
- Jimoh, F.O.; Adedapo, A.A.; Afolayan, A.J. Comparison of The Nutritional Value and Biological Activities of The Acetone, Methanol and Water Extracts of The Leaves of Solanum nigrum and Leonotis leonorus. Food Chem. Toxicol. 2010, 48, 964–971. [Google Scholar] [CrossRef]
- Sattar, A.; Baqi, A.; Suleman, M.; Shah, M.T.; Samiullah, N.K. Elemental Analysis of Medicinal Plants and Their Impacts on Human Health. Pure. Appl. Biol. 2020, 9, 249–255. [Google Scholar] [CrossRef]
- Mustafa, G.; Arif, R.; Atta, A.; Sharif, S.; Jamil, A. Bioactive Compounds from Medicinal Plants and Their Importance in Drug Discovery in Pakistan. Matrix Sci. Pharma. 2017, 1, 17–26. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal Plants: Future Source of New Drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Abbas, Z.; Khan, S.M.; Alam, J.; Khan, S.W.; Abbasi, A.M. Medicinal Plants Used by Inhabitants of The Shigar Valley, Baltistan Region of Karakorum Range-Pakistan. J. Ethnobiol. Ethnomed. 2017, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Fraga-Corral, M.; García-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and Nutritional Value of Edible Wild Plants from The Northern Spain Rich in Phenolic Compounds with Potential Health Benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Anwar, M.; Hashmi, H.F.; Khan, M.R. Evaluation of Antioxidant and Anti-inflammatory Potency of Lepidium pinnatifidum Ledeb. Clin. Phytosci. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Ismail, H.; Dilshad, E.; Waheed, M.T.; Sajid, M.; Kayani, W.K.; Mirza, B. Transformation of Lactuca sativa L. with rol C Gene Results in Increased Antioxidant Potential and Enhanced Analgesic, Anti-inflammatory and Antidepressant Activities In Vivo. 3 Biotech 2016, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Dilshad, E.; Zafar, S.; Ismail, H.; Waheed, M.T.; Cusido, R.M.; Palazon, J.; Mirza, B. Effect of rol Genes on Polyphenols Biosynthesis in Artemisia annua and Their Effect on Antioxidant and Cytotoxic Potential of The Plant. Appl. Biochem. Biotech. 2016, 179, 1456–1468. [Google Scholar] [CrossRef]
- Kiani, B.H.; Ullah, N.; Haq, I.U.; Mirza, B. Transgenic Artemisia dubia WALL Showed Altered Phytochemistry and Pharmacology. Arab. J. Chem. 2019, 12, 2644–2654. [Google Scholar] [CrossRef]
- Majidinia, M.; Karimian, A.; Alemi, F.; Yousefi, B.; Safa, A. Targeting miRNAs by Polyphenols: Novel Therapeutic Strategy for Aging. Biochem. Pharmacol. 2020, 173, 113688. [Google Scholar] [CrossRef]
- Venkatachalam, R.; Kalimuthu, K.; Chinnadurai, V.; Saravanan, M.; Radhakrishnan, R.; Shanmuganathan, R. Various Solvent Effects on Phytochemical Constituent Profiles, Analysis of Antioxidant and Antidiabetic Activities of Hopea parviflora. Process Biochem. 2020, 89, 227–232. [Google Scholar] [CrossRef]
- Zafar, S.; Dilshad, E.; Ismail, H.; Rizvi, C.B.; Mirza, B. Rol Genes Enhance Content of Artemisinin and Other Secondary Metabolites in Shennong Hybrid of Artemisia annua. Chin. Herb. Med. 2019, 11, 209–215. [Google Scholar] [CrossRef]
- Ismail, H.; Dilshad, E.; Waheed, M.T.; Mirza, B. Transformation of Lettuce with rol ABC Genes: Extracts Show Enhanced Antioxidant, Analgesic, Anti-inflammatory, Antidepressant, and Anticoagulant Activities in Rats. Appl. Biochem. Biotechnol. 2017, 181, 1179–1198. [Google Scholar] [CrossRef]
- Hafeez, K.; Andleeb, S.; Ghousa, T.; Mustafa, G.R.; Naseer, A.; Shafique, I.; Akhter, K. Phytochemical Screening, Alpha-glucosidase Inhibition, Antibacterial and Antioxidant Potential of Ajuga bracteosa extracts. Curr. Pharm. Biotechnol. 2017, 18, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Balducci, C.; Guibout, L.; Foucault, A.S.; Bakrim, A.; Kumpun, S.; Girault, J.P.; Tourette, C.; Dioh, W.; Dilda, P.J.; et al. Ecdysteroid Metabolism in Mammals: The Fate of Ingested 20-hydroxyecdysone in Mice and Rats. J. Steroid Biochem. 2021, 2, 105896. [Google Scholar] [CrossRef]
- Ismail, H.; Mirza, B. Evaluation of Analgesic, Anti-inflammatory, Anti-depressant, and Anti-coagulant Properties of Lactuca sativa (CV. Grand Rapids) Plant Tissues and Cell Suspension in Rats. BMC Complement. Altern. Med. 2015, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Tavares, L.; Jardim, C.; Costa, I.; Terrasso, A.P.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.D.; et al. Blood–Brain Barrier Transport and Neuroprotective Potential of Blackberry-Digested Polyphenols: An In Vitro Study. Eur. J. Nutr. 2019, 58, 113–130. [Google Scholar] [CrossRef]
- Welcome, M.O. Blood Brain Barrier Inflammation and Potential Therapeutic Role of Phytochemicals. Pharma Nutr. 2020, 11, 100177. [Google Scholar] [CrossRef]
- Leclerc, M.; Dudonné, S.; Calon, F. Can Natural Products Exert Neuroprotection Without Crossing the Blood–Brain Barrier? Int. J. Mol. Sci. 2021, 22, 3356. [Google Scholar] [CrossRef]
- Lü, K.; Ding, M.Y.; Li, H.X.; Liu, D.L. Determination of Ferulic Acid in Chuanxiong and in Animal Serum and Cerebrospinal Fluid by Reversed-Phase High Performance Liquid Chromatography. Chin. J. Chromatogr. 2000, 18, 518–520. [Google Scholar]
- Zafra-Gómez, A.; Luzón-Toro, B.; Jiménez-Diaz, I.; Ballesteros, O.; Navalón, A. Quantification of Phenolic Antioxidants in Rat Cerebrospinal Fluid by GC–MS After Oral Administration of Compounds. J. Pharm. Biomed. Anal. 2010, 53, 103–108. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Kaczmarek-Bak, J.; Figlus, M.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Glabinski, A.; Nowak, D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence That Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients 2020, 12, 1531. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey Through Blood-Brain Barrier Towards Neuronal Protection. Sci. Rep. 2017, 7, 1–6. [Google Scholar]
- Riaz, N.; Malik, A.; Rehman, A.U.; Nawaz, S.A.; Muhammad, P.; Chaudhary, M.I. Cholinesterase-Inhibiting Withanolides from Ajuga bracteosa. Chem. Biodiv. 2004, 1, 1289–1295. [Google Scholar] [CrossRef]
- Singh, R.; Patil, S.M.; Pal, G.; Ahmad, M. Evaluation of In Vivo and In Vitro Anti-inflammatory Activity of Ajuga bracteosa Wall ex Benth. Asian Pac. J. Trop. Dis. 2012, 2, 404–407. [Google Scholar] [CrossRef]
- Kayani, W.K.; Palazòn, J.; Cusidò, R.M.; Mirza, B. The Effect of rol Genes on Phytoecdysteroid Biosynthesis in Ajuga bracteosa Differs Between Transgenic Plants and Hairy Roots. RSC Adv. 2016, 6, 22700–22708. [Google Scholar] [CrossRef]
- Hseu, Z.Y. Evaluating Heavy Metal Contents in Nine Composts Using Four Digestion Methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef]
- Mahmood, R.; Malik, F.; S Shamas, S.; Ahmed, T.; Kausar, M.; Rubnawaz, S.; Ashfaq, M.; Hussain, S.; Green, B.D.; Mirza, B. Pharmacological Evaluation of Rhazya stricta Root Extract. Bol. Latinoam. Caribe. 2020, 19, 188–206. [Google Scholar]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total Phenolic Content and DPPH Radical Scavenging Activity of Lettuce (Lactuca sativa L.) Grown in Colorado. LWT-Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, Anti-inflammatory Activities and HPLC Analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Phatak, R.S.; Hendre, A.S. Total Antioxidant Capacity (TAC) of Fresh Leaves of Kalanchoe pinnata. J. Pharmacogn. Phytochem. 2014, 2, 32–35. [Google Scholar]
- Wintola, O.A.; Afolayan, A.J. Phytochemical Constituents and Antioxidant Activities of The Whole Leaf Extract of Aloe ferox Mill. Pharmacogn. Mag. 2011, 7, 325. [Google Scholar] [PubMed]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Hydroxyl Radical Scavenging Assay of Phenolics and Flavonoids with a Modified Cupric Reducing Antioxidant Capacity (CUPRAC) Method Using Catalase for Hydrogen Peroxide Degradation. Anal. Chim. Acta. 2008, 616, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, K.; Dorman, H.D.; Oinonen, P.P.; Darwis, Y.; Laakso, I.; Hiltunen, R. Chemical Composition and In Vitro Antioxidative Activity of a Lemon Balm (Melissa officinalis L.) Extract. LWT-Food Sci. Technol. 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic Analgesic. II. Dithienylbutenyl- and Dithienylbutylamines. J. Pharmacol. Exp. Ther. 1952, 107, 385–393. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of The Rat as An Assay for Anti-inflammatory Drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]

| Elements (µg/mg) | λmax (nm) | Slit Width (nm) | Samples | |||
|---|---|---|---|---|---|---|
| WT | ABRL1 | ABRL2 | ABRL3 | |||
| Sodium | 589.0 | 0.5 | 2.86 ± 0.2 | 3.54 ± 0.8 b | 3.07 ± 1 b | 3.94 ± 2 ab |
| Potassium | 766.5 | 1.0 | 8.06 ± 0.3 | 12.00 ± 1 bc | 12.62 ± 3 b | 13.09 ± 2 a |
| Calcium | 422.7 | 0.5 | 0.50 ± 0.06 | 1.69 ± 0.5 a | 1.32 ± 0.4 a | 1.95 ± 0.5 a |
| Magnesium | 285.2 | 0.5 | 0.91 ± 0.1 | 2.15 ± 0.3 a | 1.12 ± 0.2 b | 2.12 ± 0.7 a |
| Zinc | 213.9 | 1.0 | 0.25 ± 0.05 | 0.40 ± 0.1 b | 0.24 ± 0.04 c | 0.47 ± 0.03 a |
| Iron | 248.3 | 0.2 | 0.25 ± 0.1 | 0.33 ± 0.2 c | 0.46 ± 0.1 a | 0.44 ± 0.2 a |
| Manganese | 279.5 | 0.2 | 0.004 ± 0.01 | 0.02 ± 0.01 a | 0.01 ± 0.03 b | 0.02 ± 0.01 a |
| Nickel | 232.0 | 0.2 | 0.09 ± 0.02 | 0.18 ± 0.1 b | 0.19 ± 0.1 b | 0.23 ± 0.2 a |
| Copper | 324.8 | 0.5 | 0.005 ± 0.01 | 0.01 ± 0.02 b | 0.01 ± 0.03 b | 0.02 ± 0.01 a |
| Chromium | 357.9 | 0.2 | 0.16 ± 0.03 | 0.45 ± 0.1 a | 0.43 ± 0.2 a | 0.36 ± 0.1 b |
| Phytochemicals | Samples | |||
|---|---|---|---|---|
| WT | ABRL1 | ABRL2 | ABRL3 | |
| Alkaloids | ++ | ++ | +++ | +++ |
| Glycosides | + | +++ | ++ | +++ |
| Flavonoids | + | ++ | ++ | +++ |
| Phenols | ++ | ++ | ++ | ++ |
| Tannins | + | + | + | + |
| Saponins | + | + | ++ | ++ |
| Terpenoids | − | + | + | + |
| Coumarins | − | − | − | − |
| β-cyanins | − | − | − | − |
| Anthocyanin | − | − | − | − |
| Sterols | − | − | − | − |
| No. | Compound Name | λmax (nm) | Extracts (µg/mg Dry Extract) | |||
|---|---|---|---|---|---|---|
| WT | ABRL1 | ABRL2 | ABRL3 | |||
| 1 | Vanillic Acid | 257 | 8.98 ± 1 | 15.87 ± 3 a | 15.49 ± 2 a | 16.33 ± 1 a |
| 2 | Rutin | 257 | 0.63 ± 0.5 | 4.49 ± 1 c | 9.24 ± 2 b | 14.86 ± 2 a |
| 3 | Plumbagin | 257 | Nd | Nd | Nd | Nd |
| 4 | Thymoquinone | 257 | Nd | Nd | Nd | Nd |
| 5 | Gallic Acid | 279 | 4.59 ± 0.3 | 14.99 ± 2 ab | 15.01 ± 3 a | 16.67 ± 1 a |
| 6 | Catechin | 279 | Nd | Nd | Nd | Nd |
| 7 | Syringic Acid | 279 | 10.79 ± 0.8 | 13.93 ± 2 b | 12.41 ± 1 b | 17.78 ± 3 a |
| 8 | Coumaric Acid | 279 | 1.92 ± 0.7 | 15.39 ± 3 a | 14.02 ± 1 ab | 23.45 ± 2 a |
| 9 | Emodin | 279 | Nd | Nd | Nd | Nd |
| 10 | Gentisic Acid | 325 | Nd | Nd | Nd | Nd |
| 11 | Caffeic Acid | 325 | 13.39 ± 2 | 25.51 ± 3 b | 22.01 ± 2 b | 30.18 ± 4 a |
| 12 | Ferulic Acid | 325 | 75.55 ± 3 | 77.17 ± 4 | 76.86 ± 4 | 78.05 ± 3 |
| 13 | Cinnamic Acid | 325 | 3.19 ± 0.7 | 4.36 ± 0.5 c | 5.47 ± 0.2 b | 6.09 ± 0.3 a |
| 14 | Luteolin | 325 | Nd | Nd | Nd | Nd |
| 15 | Apigenin | 325 | 8.20 ± 2 | 20.84 ± 5 bc | 23.12 ± 3 b | 32.29 ± 4 a |
| 16 | Myricetin | 368 | 4.14 ± 0.7 | 13.66 ± 3 a | 11.6 ± 2 b | 13.37 ± 4 a |
| 17 | Quercetin | 368 | 4.68 ± 0.3 | 6.44 ± 0.8 c | 7.52 ± 1 bc | 9.19 ± 0.5 a |
| 18 | Kaempferol | 368 | 17.6 ± 2 | 83.9 ± 4 b | 78.6 ± 5 b | 101.26 ± 6 a |
| Antioxidant Activities | Correlation R2 | |
|---|---|---|
| TPC | TFC | |
| DPPH Radical Scavenging Activity | 0.9627 *** | 0.7627 ** |
| Hydroxyl Ion Scavenging Assay | 0.9312 *** | 0.8158 ** |
| Iron Chelating Power | 0.9159 *** | 0.8243 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubnawaz, S.; Kayani, W.K.; Akhtar, N.; Mahmood, R.; Khan, A.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; Alwasel, Y.A.; Mirza, B. Polyphenol Rich Ajuga bracteosa Transgenic Regenerants Display Better Pharmacological Potential. Molecules 2021, 26, 4874. https://doi.org/10.3390/molecules26164874
Rubnawaz S, Kayani WK, Akhtar N, Mahmood R, Khan A, Okla MK, Alamri SA, Alaraidh IA, Alwasel YA, Mirza B. Polyphenol Rich Ajuga bracteosa Transgenic Regenerants Display Better Pharmacological Potential. Molecules. 2021; 26(16):4874. https://doi.org/10.3390/molecules26164874
Chicago/Turabian StyleRubnawaz, Samina, Waqas Khan Kayani, Nosheen Akhtar, Rashid Mahmood, Asif Khan, Mohammad K. Okla, Saud A. Alamri, Ibrahim A. Alaraidh, Yasmeen A. Alwasel, and Bushra Mirza. 2021. "Polyphenol Rich Ajuga bracteosa Transgenic Regenerants Display Better Pharmacological Potential" Molecules 26, no. 16: 4874. https://doi.org/10.3390/molecules26164874
APA StyleRubnawaz, S., Kayani, W. K., Akhtar, N., Mahmood, R., Khan, A., Okla, M. K., Alamri, S. A., Alaraidh, I. A., Alwasel, Y. A., & Mirza, B. (2021). Polyphenol Rich Ajuga bracteosa Transgenic Regenerants Display Better Pharmacological Potential. Molecules, 26(16), 4874. https://doi.org/10.3390/molecules26164874







