Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease
Abstract
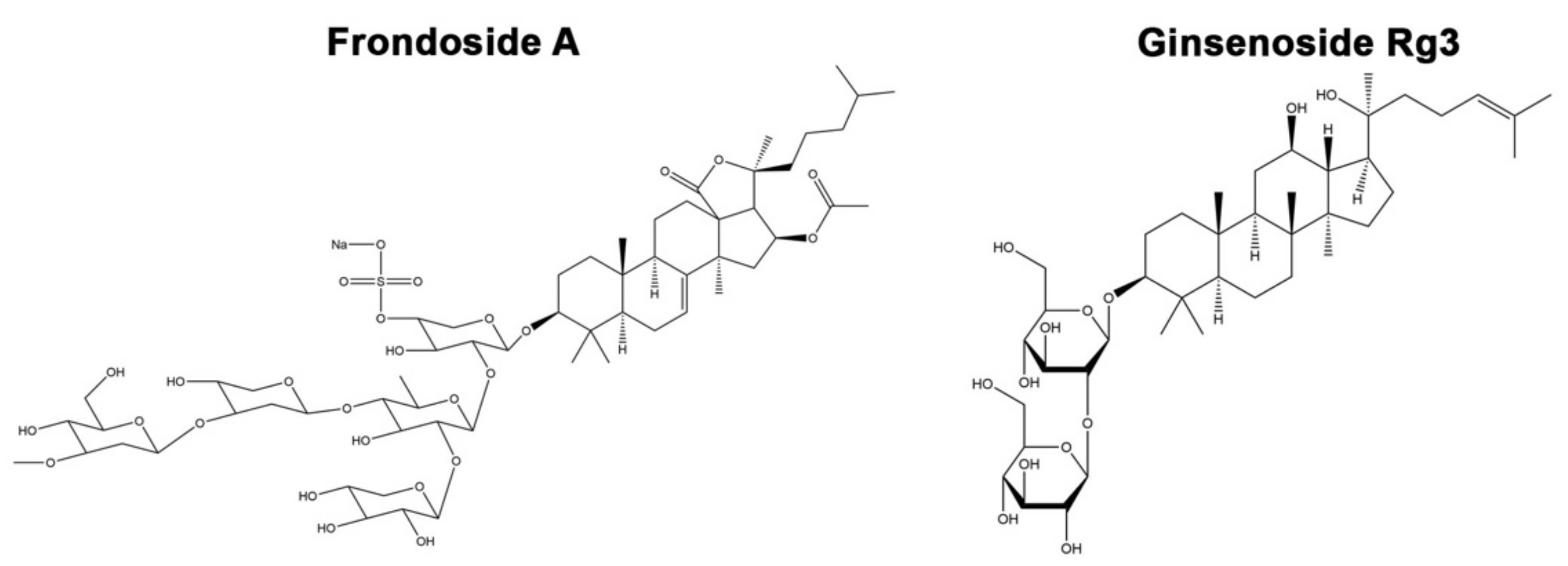
1. Introduction
2. Results
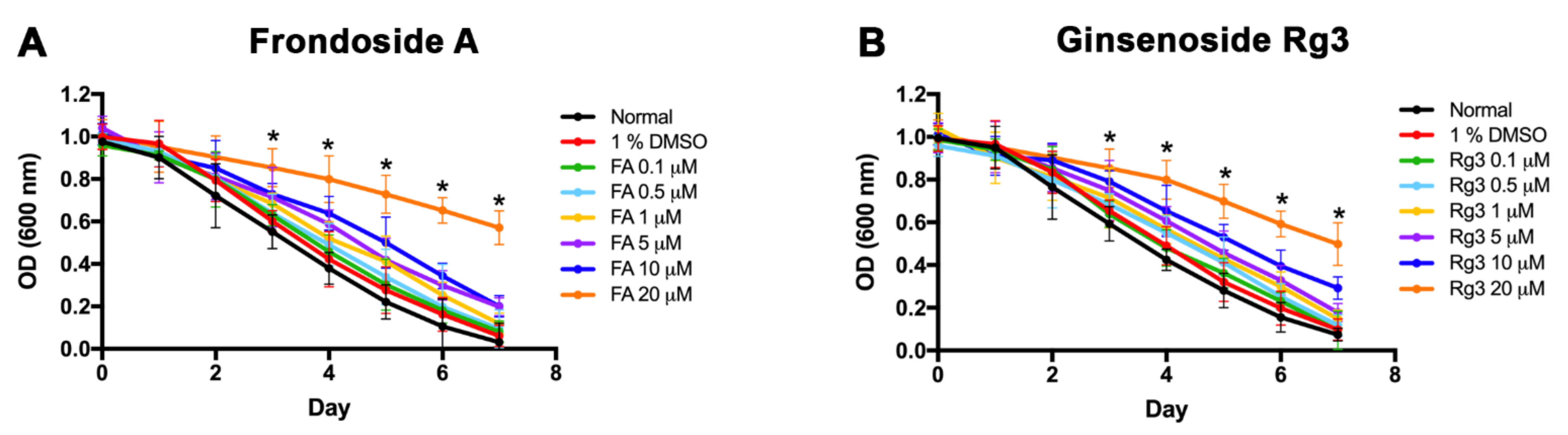
2.1. Determining Dose Range and Toxicity of FA and Rg3 on C. elegans by the Food Clearance Assay
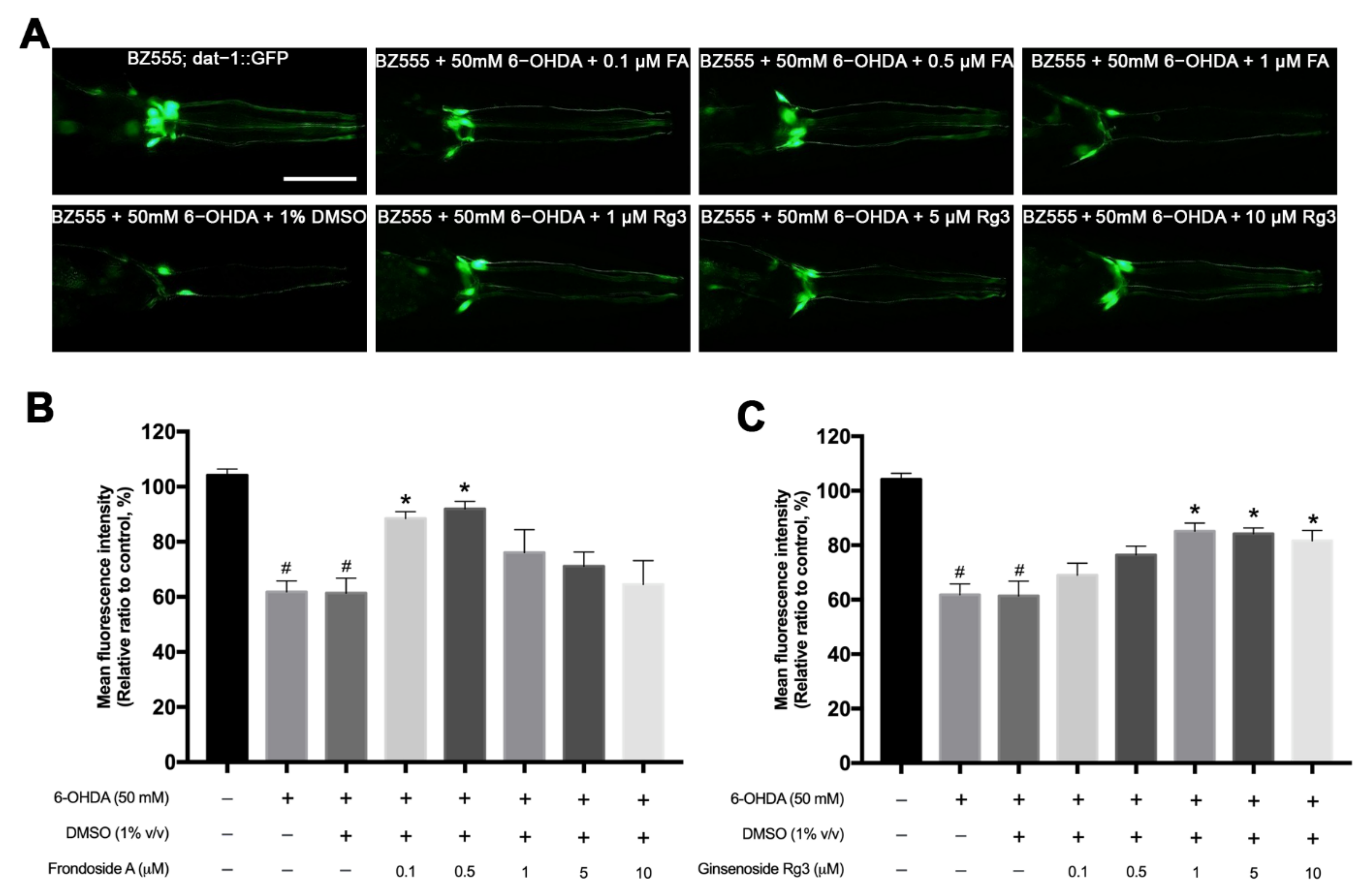
2.2. FA and Rg3 Rescue the 6-OHDA-Induced DAergic Neurodegeneration in C. elegans
2.3. FA and Rg3 Recover the Basal Slowing Rate in 6-OHDA-Induced C. elegans
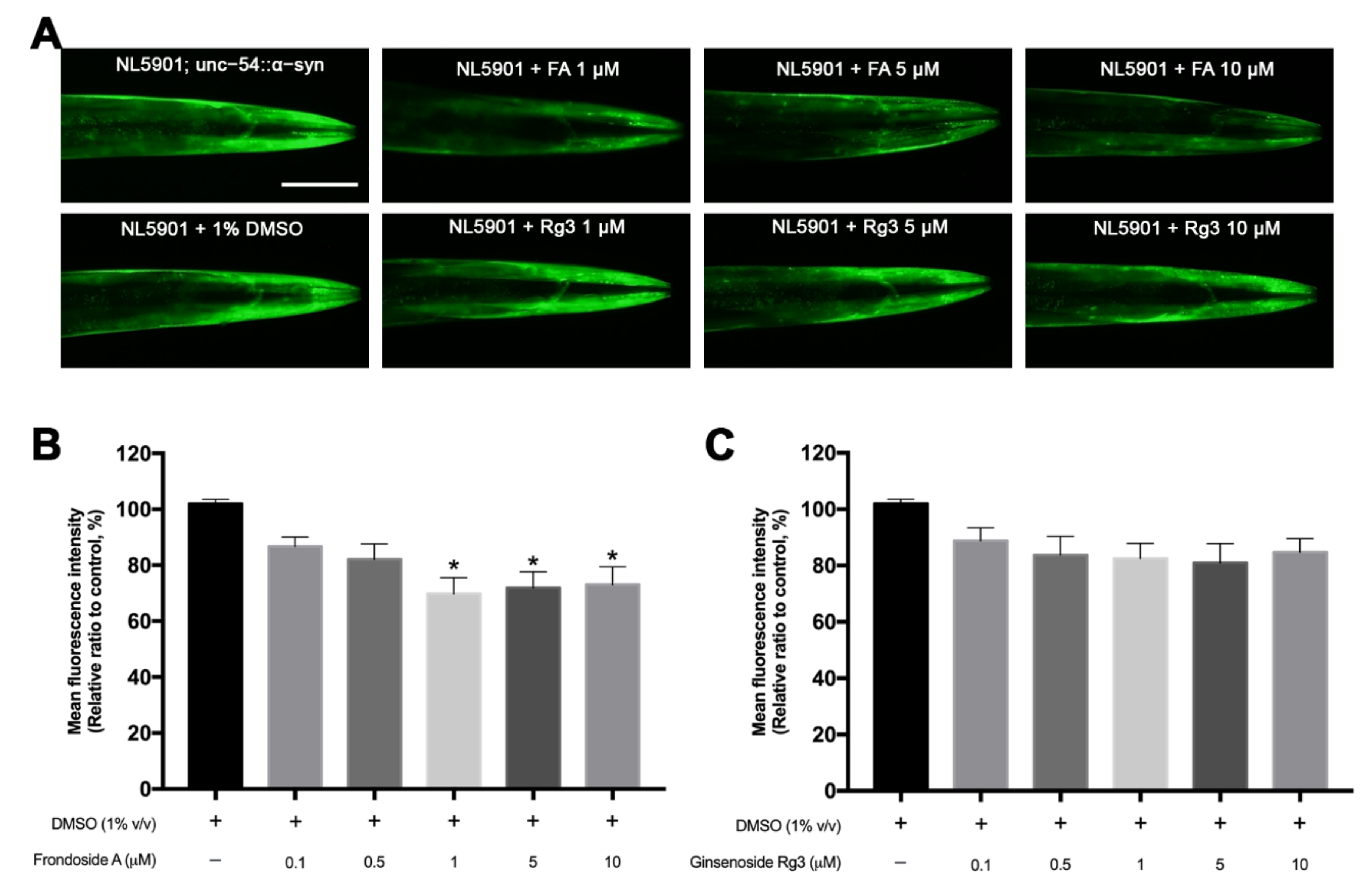
2.4. FA Reduces α-Synuclein Accumulation in Transgenic NL5901 C. elegans
2.5. FA and Rg3 Increase the Lifespan of 6-OHDA-Induced C. elegans
2.6. FA Rescues Lifespan Shortened by α-Synuclein Overexpressed C. elegans
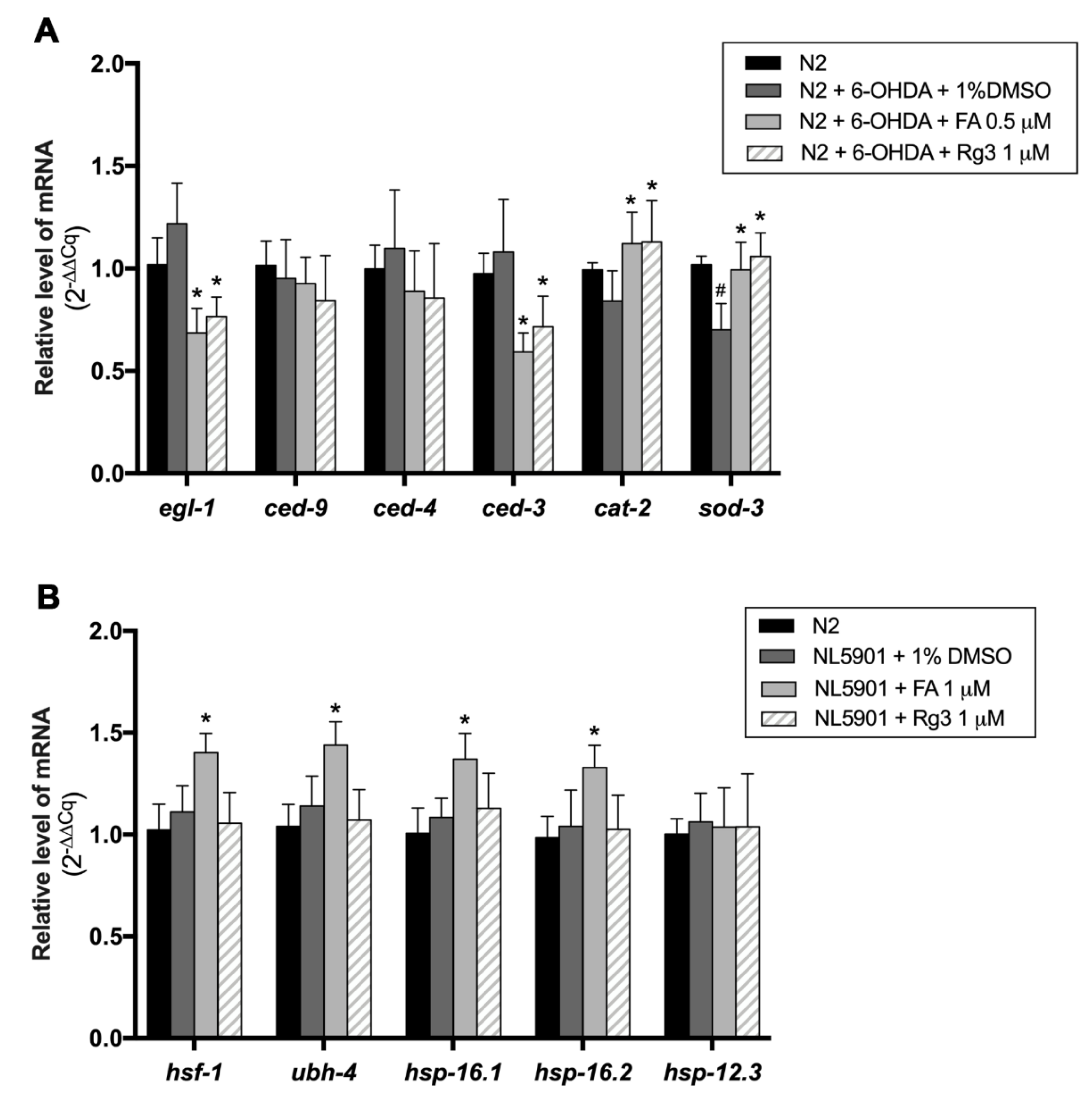
2.7. FA and Rg3 Suppress the Apoptosis Regulators, egl-1/BH-3 and ced-3/caspase-9, and Increase sod-3 in 6-OHDA-Induced C. elegans
2.8. FA Enhances Protein Degradation Regulators, hsf-1, ubh-4, hsp-16.1 and hsp-16.2, in α-Synuclein-Overexpressed C. elegans Model
3. Discussion
4. Materials and Methods
4.1. Strains, Maintenance, and Synchronization
4.2. Food Clearance Assay
4.3. Treatment of Worms with 6-OHDA, FA, and Rg3
4.4. Quantitative Assay for DAergic Neurodegeneration
4.5. Analysis of the Basal Slowing Behavior
4.6. Quantitative Assay for α-Synuclein Accumulation
4.7. Lifespan Assay
4.8. Quantitative RT-PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef] [PubMed]
- McNaught, K.S.P.; Jenner, P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 2001, 297, 191–194. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F. Plant-based compounds for antimicrobial textiles. In Antimicrobial Textiles; Sun, G., Ed.; Woodhead Publishing: Sawston, Cambridgeshire, UK, 2016; pp. 155–195. [Google Scholar]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef]
- Fujihara, K.; Shimoyama, T.; Kawazu, R.; Sasaki, H.; Koyama, K.; Takahashi, K.; Kinoshita, K. Amyloid β aggregation inhibitory activity of triterpene saponins from the cactus Stenocereus pruinosus. J. Nat. Med. 2021, 75, 284–298. [Google Scholar] [CrossRef]
- Chen, X.-C.; Zhu, Y.-G.; Zhu, L.-A.; Huang, C.; Chen, Y.; Chen, L.-M.; Fang, F.; Zhou, Y.-C.; Zhao, C.-H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Li, X.-F.; Lui, C.N.-P.; Jiang, Z.-H.; Ken, Y.K.-L. Neuroprotective effects of ginsenosides Rh1 and Rg2 on neuronal cells. Chin. Med. 2011, 6, 19. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of Alzheimer’s disease. J. Agric. Food Chem. 2019, 67, 10048–10058. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.; Ahn, K.; Park, W.-K.; Nah, S.-Y.; Rhim, H. Ginsenoside Rg3 antagonizes NMDA receptors through a glycine modulatory site in rat cultured hippocampal neurons. Biochem. Biophys. Res. Commun. 2004, 323, 416–424. [Google Scholar] [CrossRef]
- Moon, J.-H.; Lee, J.-H.; Lee, Y.-J.; Park, S.-Y. Autophagy flux induced by ginsenoside-Rg3 attenuates human prion protein-mediated neurotoxicity and mitochondrial dysfunction. Oncotarget 2016, 7, 85697–85708. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Roginsky, A.B.; Ding, X.Z.; Woodward, C.; Collin, P.; Newman, R.A.; Bell, J.R.H.; Adrian, T.E. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A. Ann. N. Y. Acad. Sci. 2008, 1138, 181–198. [Google Scholar] [CrossRef]
- Ma, X.; Kundu, N.; Collin, P.D.; Goloubeva, O.; Fulton, A.M. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res. Treat. 2012, 132, 1001–1008. [Google Scholar] [CrossRef]
- Jin, J.-O.; Shastina, V.V.; Shin, S.-W.; Xu, Q.; Park, J.-I.; Rasskazov, V.A.; Avilov, S.A.; Fedorov, S.N.; Stonik, V.A.; Kwak, J.-Y. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett. 2009, 583, 697–702. [Google Scholar] [CrossRef]
- Attoub, S.; Arafat, K.; Gélaude, A.; Al Sultan, M.A.; Bracke, M.; Collin, P.; Takahashi, T.; Adrian, T.E.; De Wever, O. Frondoside A suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS ONE 2013, 8, e53087. [Google Scholar] [CrossRef]
- Tangrodchanapong, T.; Sobhon, P.; Meemon, K. Frondoside A attenuates amyloid-β proteotoxicity in transgenic Caenorhabditis elegans by suppressing its formation. Front. Pharmacol. 2020, 11, 553579. [Google Scholar] [CrossRef]
- Harrington, A.J.; Hamamichi, S.; Caldwell, G.A.; Caldwell, K.A. C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s disease. Dev. Dyn. 2010, 239, 1282–1295. [Google Scholar]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef]
- Ishihara, L.S.; Cheesbrough, A.; Brayne, C.; Schrag, A. Estimated life expectancy of Parkinson’s patients compared with the UK population. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1304–1309. [Google Scholar] [CrossRef]
- Malaiwong, N.; Chalorak, P.; Jattujan, P.; Manohong, P.; Niamnont, N.; Suphamungmee, W.; Sobhon, P.; Meemon, K. Anti-Parkinson activity of bioactive substances extracted from Holothuria leucospilota. Biomed. Pharmacother. 2019, 109, 1967–1977. [Google Scholar] [CrossRef]
- Venderova, K.; Park, D.S. Programmed cell death in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009365. [Google Scholar] [CrossRef]
- Jones, D.R.; Moussaud, S.; McLean, P. Targeting heat shock proteins to modulate α-synuclein toxicity. Ther. Adv. Neurol. Disord. 2014, 7, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.F.; Dues, D.J.; Spielbauer, K.K.; Machiela, E.; Senchuk, M.M.; Van Raamsdonk, J.M. Delaying aging is neuroprotective in Parkinson’s disease: A genetic analysis in C. elegans models. NPJ Parkinsons Dis. 2015, 1, 15022. [Google Scholar] [CrossRef] [PubMed]
- Chalorak, P.; Jattujan, P.; Nobsathian, S.; Poomtong, T.; Sobhon, P.; Meemon, K. Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: A model for anti-Parkinson testing. Nutr. Neurosci. 2018, 21, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.-T.; Tsai, C.-W.; Liu, S.-P.; Gao, J.-X.; Kuo, Y.-H.; Chao, P.-M.; Hung, H.-S.; Shyu, W.-C.; Lin, S.-Z.; Fu, R.-H. Maackiain ameliorates 6-hydroxydopamine and SNCA pathologies by modulating the PINK1/Parkin pathway in models of Parkinson’s disease in Caenorhabditis elegans and the SH-SY5Y Cell Line. Int. J. Mol. Sci. 2020, 21, 4455. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Álvarez, E.; Hermida-Ameijeiras, Á.; Muñoz-Patiño, A.M.; Labandeira-Garcia, J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants. J. Neurochem. 2000, 74, 1605–1612. [Google Scholar] [CrossRef]
- Lettre, G.; Hengartner, M.O. Developmental apoptosis in C. elegans: A complex CEDnario. Nat. Rev. Mol. Cell Biol. 2006, 7, 97–108. [Google Scholar] [CrossRef]
- Nass, R.; Hall, D.H.; Miller, D.M., 3rd; Blakely, R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 3264–3269. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Wang, Z.; Li, W. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytother. Res. 2018, 32, 2531–2540. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, S.Y.; Lee, J.-H.; Jeong, S.M.; Yoon, I.-S.; Lee, B.-H.; Lee, J.-H.; Pyo, M.K.; Lee, S.-M.; Chung, J.-M.; et al. Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007, 1136, 190–199. [Google Scholar] [CrossRef]
- Min, J.-K.; Kim, J.-H.; Cho, Y.-L.; Maeng, Y.-S.; Lee, S.-J.; Pyun, B.-J.; Kim, Y.-M.; Park, J.H.; Kwon, Y.-G. 20(S)-Ginsenoside Rg3 prevents endothelial cell apoptosis via inhibition of a mitochondrial caspase pathway. Biochem. Biophys. Res. Commun. 2006, 349, 987–994. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Sun, B.; Xu, J.; Jiang, J.; Luo, M. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol. Med. Report. 2015, 11, 4518–4524. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Peng, J.; Stevenson, F.F.; Doctrow, S.R.; Andersen, J.K. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: Implications for Parkinson disease. J. Biol. Chem. 2005, 280, 29194–29198. [Google Scholar] [CrossRef]
- Pong, K.; Doctrow, S.R.; Baudry, M. Prevention of 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced nitration of tyrosine hydroxylase and neurotoxicity by EUK-134, a superoxide dismutase and catalase mimetic, in cultured dopaminergic neurons. Brain Res. 2000, 881, 182–189. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.X.; Lin, L.; Liu, X.N.; Ma, C.J.; Li, J.; Wang, C.B. Preparation of Ginsenoside Rg3 and protection against H2O2-induced oxidative stress in human neuroblastoma SK-N-SH cells. J. Chem. 2014, 2014, 848571. [Google Scholar] [CrossRef]
- Wei, X.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia 2012, 83, 636–642. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New perspectives on roles of alpha-synuclein in Parkinson’s disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Baranowski, D.B.; Shaw, C.A.; Kay, D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson’s disease. Exp. Gerontol. 2014, 50, 95–105. [Google Scholar] [CrossRef]
- Heng, Y.; Zhang, Q.-S.; Mu, Z.; Hu, J.-F.; Yuan, Y.-H.; Chen, N.-H. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting α-synuclein abnormalities in the substantia nigra. Toxicol. Lett. 2016, 243, 7–21. [Google Scholar] [CrossRef]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Menon, S.A.; Abul Khair, S.B.; Lu, J.-H.; Safieh-Garabedian, B.; Al-Hayani, A.A.; Eliezer, D.; Li, M.; et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol. Dis. 2015, 74, 89–101. [Google Scholar] [CrossRef]
- Yang, L.; Hao, J.; Zhang, J.; Xia, W.; Dong, X.; Hu, X.; Kong, F.; Cui, X. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J. Pharm. Pharmacol. 2009, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Bodhicharla, R.; Nagarajan, A.; Winter, J.; Adenle, A.; Nazir, A.; Brady, D.; Vere, K.; Richens, J.; O’Shea, P.; Bell, D.R.; et al. Effects of α-synuclein overexpression in transgenic Caenorhabditis elegans strains. CNS Neurol. Disord. Drug Targets 2012, 11, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, M.; Dai, Y.; Sun, Y.; Aman, Y.; Xu, Y.; Yu, P.; Zheng, Y.; Yang, J.; Zhu, X. Spermidine inhibits neurodegeneration and delays aging via the PINK1-PDR1-dependent mitophagy pathway in C. elegans. Aging 2020, 12, 16852–16866. [Google Scholar] [CrossRef] [PubMed]
- Virendra, S.; Suresh, C.P.; Deepti, Y.; Sudeep, T.; Supinder, K.; Gupta, M.M.; Aamir, N.; Rakesh, P. Iridoid compound 10-O-trans-p-coumaroylcatalpol extends longevity and reduces alpha synuclein aggregation in Caenorhabditis elegans. CNS Neurol. Disord. Drug Targets 2012, 11, 984–992. [Google Scholar]
- Kaur, S.; Nazir, A. Potential role of protein stabilizers in amelioration of Parkinson’s disease and associated effects in transgenic Caenorhabditis elegans model expressing alpha-synuclein. RSC Adv. 2015, 5, 77706–77715. [Google Scholar] [CrossRef]
- Esposito, L.A. Targeting α-synuclein as a Parkinson’s disease therapeutic. In Novel Therapeutic Approaches to the Treatment of Parkinson’s Disease: An Overview and Update; Hopkins, C.R., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 43–109. [Google Scholar]
- Grünblatt, E.; Mandel, S.; Jacob-Hirsch, J.; Zeligson, S.; Amariglo, N.; Rechavi, G.; Li, J.; Ravid, R.; Roggendorf, W.; Riederer, P.; et al. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J. Neural Transm. 2004, 111, 1543–1573. [Google Scholar] [CrossRef]
- Shruthi, K.; Reddy, S.S.; Reddy, P.Y.; Shivalingam, P.; Harishankar, N.; Reddy, G.B. Amelioration of neuronal cell death in a spontaneous obese rat model by dietary restriction through modulation of ubiquitin proteasome system. J. Nutr. Biochem. 2016, 33, 73–81. [Google Scholar] [CrossRef]
- Liangliang, X.; Yonghui, H.; Shunmei, E.; Shoufang, G.; Wei, Z.; Jiangying, Z. Dominant-positive HSF1 decreases alpha-synuclein level and alpha-synuclein-induced toxicity. Mol. Biol. Rep. 2010, 37, 1875–1881. [Google Scholar] [CrossRef]
- Fujikake, N.; Nagai, Y.; Popiel, H.A.; Okamoto, Y.; Yamaguchi, M.; Toda, T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J. Biol. Chem. 2008, 283, 26188–26197. [Google Scholar] [CrossRef]
- Fonte, V.; Kipp, D.R.; Yerg, J., III; Merin, D.; Forrestal, M.; Wagner, E.; Roberts, C.M.; Link, C.D. Suppression of in vivo β-amyloid peptide toxicity by overexpression of the HSP-16.2 small chaperone protein. J. Biol. Chem. 2008, 283, 784–791. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Y.; Lin, Y.; Wang, X.; Hu, L.; Cao, Y.; Chen, Y. Antistress and anti-aging activities of Caenorhabditis elegans were enhanced by Momordica saponin extract. Eur. J. Nutr. 2021, 60, 1819–1832. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, F.; Liu, Q.; Qian, C.; Thu, P.M.; Wang, Y.; Zheng, Z.-G.; Yang, H.; Li, P.; Xu, X. Evaluation of structure–activity relationships of ginsenosides against amyloid β induced pathological behaviours in transgenic Caenorhabditis elegans. RSC Adv. 2017, 7, 40095–40104. [Google Scholar] [CrossRef]
- Kim, S.K.; Himaya, S.W.A. Triterpene Glycosides from Sea Cucumbers and Their Biological Activities. Adv. Food Nutr. Res. 2012, 65, 297–319. [Google Scholar]
- Park, J.-I.; Bae, H.-R.; Kim, C.G.; Stonik, V.A.; Kwak, J.-Y. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem. 2014, 2, 77. [Google Scholar] [CrossRef]
- Aminin, D.L.; Silchenko, A.S.; Avilov, S.A.; Stepanov, V.G.; Kalinin, V.I. Immunomodulatory action of monosulfated triterpene glycosides from the sea cucumber Cucumaria okhotensis: Stimulation of activity of mouse peritoneal macrophages. Nat. Prod. Commun. 2010, 5, 1877–1880. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Inamoto, T.; Fuchida, M.; Kyogoku, Y. Marine natural products. XIV. Structures of Echinosides A and B, antifungal lanostane-oligosides from the sea cucumber Actinopyga echinites (Jaeger). Chem. Pharm. Bull. 1985, 33, 5214–5224. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Gomez-Amaro, R.L.; Valentine, E.R.; Carretero, M.; LeBoeuf, S.E.; Rangaraju, S.; Broaddus, C.D.; Solis, G.M.; Williamson, J.R.; Petrascheck, M. Measuring food intake and nutrient absorption in Caenorhabditis elegans. Genetics 2015, 200, 443. [Google Scholar] [CrossRef]
- Chalorak, P.; Dharmasaroja, P.; Meemon, K. Downregulation of eEF1A/EFT3-4 enhances dopaminergic neurodegeneration after 6-OHDA exposure in C. elegans model. Front. Neurosci. 2020, 14, 303. [Google Scholar] [CrossRef]

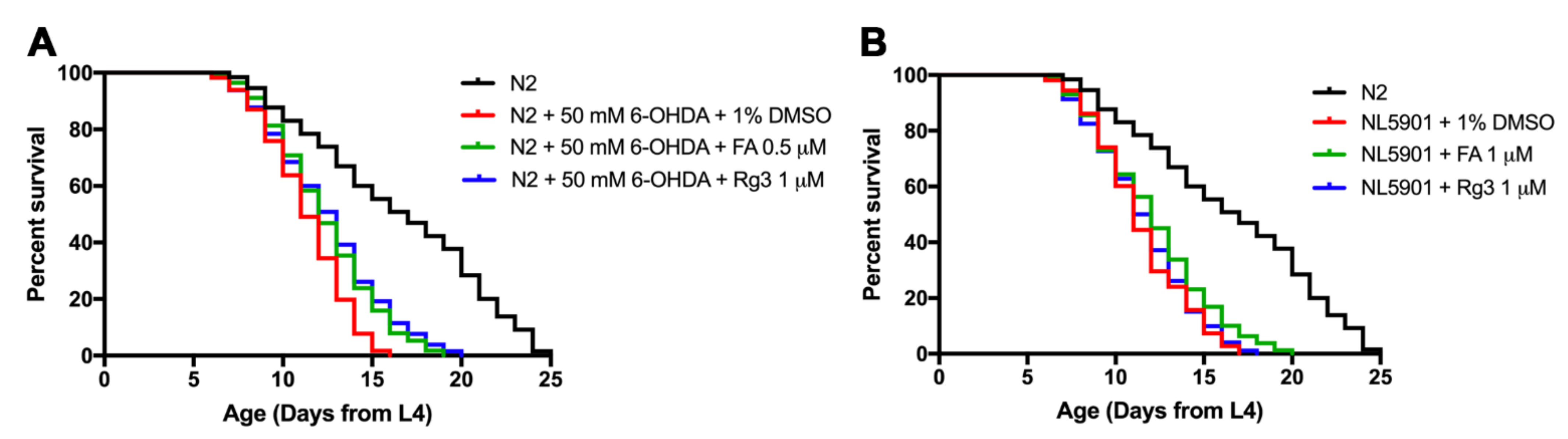
| Treatment | Mean Lifespan (Day) | Maximum Lifespan | % Increase Lifespan | Significance (p-Value) |
|---|---|---|---|---|
| N2 | 16.49 ± 0.49 | 25 | ||
| N2 + 50 mM 6-OHDA + 1% DMSO | 11.31 ± 0.95 | 16 | −31.37 (compared with N2) | **** p < 0.0001 |
| N2 + 50 mM 6-OHDA + 0.5 μM FA | 12.34 ± 0.97 | 19 | 6.22 (compared with 6-OHDA + DMSO) | *** (p = 0.004) |
| N2 + 50 mM 6-OHDA + 1 μM Rg3 | 12.46 ± 0.54 | 20 | 6.97 | **** p < 0.0001 |
| Treatment | Mean Lifespan (Day) | Maximum Lifespan | % Increased Lifespan | Significance (p-Value) |
|---|---|---|---|---|
| N2 | 16.49 ± 0.49 | 25 | ||
| NL5901 + 1% DMSO | 11.37 ± 0.80 | 20 | −31.05 (compared with N2) | **** p < 0.0001 |
| NL5901 + 1 μM FA | 12.11 ± 0.88 | 23 | 6.58 (compared with NL5901 + 1% DMSO) | * (p = 0.011) |
| NL5901 + 1 μM Rg3 | 11.51 ± 0.96 | 24 | 1.29 | Not significant (p = 0.477) |
| Foward (5′→3′) | Reverse (5′→3′) | |
|---|---|---|
| Apoptosis mediators | ||
| egl-1 | CTAGCAGCAATGTGCGATGAC | GGAA GCATGGGCCGAGTAG |
| ced-9 | TGCTCAGGACTTGCCATCAC | TTGACTCTCCGATGGACATTCTT |
| ced-4 | AAGTCGAGGATTAGTCGGTGTTG | AGAGCCATTGCGAGTGACTTG |
| ced-3 | TCAACGCGGCAAATGCT | GCCTGCACAAAAACGATTTTC |
| Antioxidant mediators | ||
| sod-3 | AGCATCATGCCACCTACGTGA | CACCACCATTGAATTTCAGCG |
| Dopamine synthesis | ||
| cat-2 | GCACGTTTTGAGTTGGGTCC | ATGCAGCCAGGATTGTCGAA |
| Protein degradation mediators | ||
| hsf-1 | ATGCAGCCAGGATTGTCGAA | GCACGTTTTGAGTTGGGTCC |
| ubh-4 | GCACTTGTTCCAAACCGCAA | GACGTCGGCGATTGTTTTCC |
| hsp-16.1 | GCAGAGGCTCTCCATCTGAA | GCTTGAACTGCGAGACATTG |
| hsp-16.2 | GTCACTTTACCACTATTTCCGT | CAATCTCAGAAGACTCAGATGG |
| hsp-12.3 | GCCATTCCAGAAAGGAGATG | CGTTTGGCAAGAAGTTGTGA |
| Housekeeping gene | ||
| act-1 | AGGTTGCCGCTCTTGTTGTA | CGTGGTCTTCCGACAATGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalorak, P.; Sanguanphun, T.; Limboonreung, T.; Meemon, K. Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease. Molecules 2021, 26, 4843. https://doi.org/10.3390/molecules26164843
Chalorak P, Sanguanphun T, Limboonreung T, Meemon K. Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease. Molecules. 2021; 26(16):4843. https://doi.org/10.3390/molecules26164843
Chicago/Turabian StyleChalorak, Pawanrat, Tanatcha Sanguanphun, Tanapol Limboonreung, and Krai Meemon. 2021. "Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease" Molecules 26, no. 16: 4843. https://doi.org/10.3390/molecules26164843
APA StyleChalorak, P., Sanguanphun, T., Limboonreung, T., & Meemon, K. (2021). Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease. Molecules, 26(16), 4843. https://doi.org/10.3390/molecules26164843






