Abstract
A series of 16 new derivatives of harmine N9-Cinnamic acid were synthesized and fully characterized using NMR and MS. The in vitro antibacterial evaluation revealed that most of the synthesized harmine derivatives displayed better antibacterial activities against Gram-positive strains (S. aureus, S. albus and MRSA) than Gram-negative strains (E. coli and PA). In particular, compound 3c showed the strongest bactericidal activity with a minimum inhibitory concentration of 13.67 μg/mL. MTT assay showed that compound 3c displayed weaker cytotoxicity than harmine with IC50 of 340.30, 94.86 and 161.67 μmol/L against WI-38, MCF-7 and HepG2 cell lines, respectively. The pharmacokinetic study revealed that the distribution and elimination of 3c in vivo were rapid in rats with an oral bioavailability of 6.9%.
1. Introduction
With the increasing use of antibiotics in humans and agriculture, the resistance has spread worldwide. Meanwhile, the decreasing effectiveness of antibiotics in treating common infections has quickened [1,2,3]. After the golden age of the antibiotic discovery between 1929 and the 1970s, the development of new antibiotics has stalled. Since then, few new antibiotics have reached the market [4,5,6,7,8,9]. As a consequence, the development of novel and efficient antibiotics is of great significance.
Harmine (Figure 1), a naturally existing tricyclic β-carboline alkaloid, has been discovered and isolated from the seeds of Peganum harmala L. by Gobel in 1841 [10,11,12]. Harmine displays a wide range of pharmacological activities, including antimicrobial, antitumor, anti-inflammatory, antidepressant, antioxidant, neuroprotective effects and so on [13,14]. It is reported that the extracts of P. harmala seeds and roots exhibit good antibacterial activities [15,16]. The literature indicated that harmine and its derivatives insert into DNA of microorganism as a highly aromatic planar alkaloid [11]. At present, harmine derivatives are known for their antibacterial activity and researchers are focusing on studying new antibacterial compounds which are more effective than the drugs now in use [17,18].
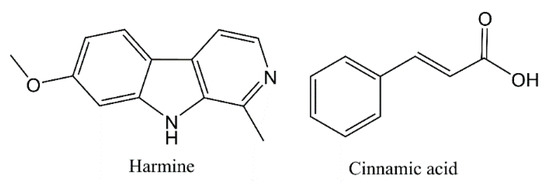
Figure 1.
Chemical structure of harmine and cinnamic acid.
Cinnamic acid (Figure 1) is isolated from cinnamon or benzoin resin. Cinnamic acid showed good antibacterial activities in vivo and in vitro [19,20,21,22]. It is reported that cinnamic acid exerted its antibacterial activity mainly by an action mode of membrane disruption [21,23]. Because of its low molecular weight and low price, cinnamic acid is often combined with other compounds to exert antibacterial activity synergistically in the design of new antibacterial drugs [21,24,25]. Using the molecular assembly method, a series novel pleuromutilin derivations bearing cinnamic acids moieties were synthesized which showed remarkable antibacterial activity [25]. Narasimhan and coauthors’ research indicated that the antibacterial activity of synthesized ester derivatives of cinnamic acid was stronger than that of amide [26]. Furthermore, it is reported that the different substituents on the benzene ring of cinnamic acid had an important effect on its antibacterial activity [27].
In this work, based on the combination principle, a series of harmine derivatives with diverse length linkers were synthesized by covalent bonding between N9 of harmine and carboxylic acid of cinnamic acids. We further preliminarily investigated the antibacterial activities and cytotoxic activity of the synthesized compounds in vitro. Finally, we performed pharmacokinetics on one of the screened compounds.
2. Result and Discussion
2.1. Chemistry
The synthesis route of target compounds is shown in Scheme 1. Firstly, in order to explore the effect of chain length on biological activity, dibromoalkanes with different carbon chain lengths used as a electrophilic agent, easily attacked the N9 position of harmine (1) to yield intermediates 2a–d. Subsequently, intermediates 2a–d were combined with substituted cinnamic acid by a nucleophilic substitution reaction to obtain 16 new target compounds (3a–d, 4a–d, 5a–d, 6a–d).
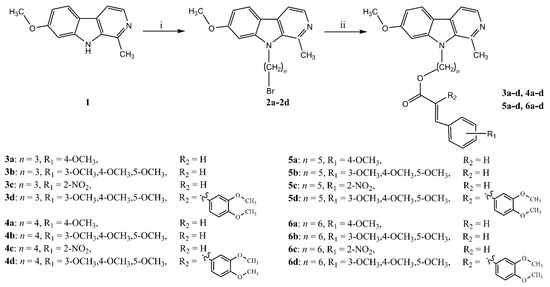
Scheme 1.
A facile route to the synthesis of target compounds. Reagents and conditions: (i) NaH (1.5 equiv.), Br(CH2)nBr (3 equiv.), dry DMF, dry THF, reflux, 3 h, 66.1–70.5%; (ii) Substituted cinnamic acid (0.33 equiv.), Et3N (5–10 drops), acetone, rt, 3 h, 21.1–52.1%.
The structure information of all synthesis compounds were confirmed by nuclear magnetic resonance spectra (NMR), including 13C and 1H-NMR, and electrospray ionization high-resolution mass spectrometry (ESI-HRMS) (13C and 1H-NMR spectra of compounds 3a–d, 4a–d, 5a–d, 6a–d had been added in Supplementary Material, Figures S1–S16). Furthermore, a colorless crystal of compound 4a was obtained by slow evaporation of a mixed solution (ethyl acetate:petroleum ether = 1:4). The crystal structures of compound 4a are shown in Figure 2. Crystal data for compound 4a were collected in Tables S1–S3.
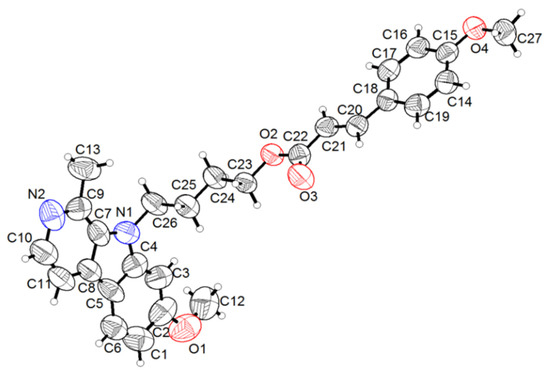
Figure 2.
ORTEP diagram for compound 4a with ellipsoids set at 75% probability.
2.2. Evaluation of the Antibacterial Activity
According to microplate dilution method, all the prepared compounds were screened for their in vitro antibacterial activity. The investigation included three representative Gram-positive strains, including Staphylococcus aureus CICC 10384 (S. aureus), Staphylococcus albus CICC 20237 (S. albus) and methicillinresistant Staphylococcus aureus ATCC 43300 (MRSA), and two representative Gram-negative strains, including Escherichia coli CICC 10389 (E. coli) and Pseudomonas aeruginosa CVCC 3359 (PA). Harmine and cinnamic acid were used as reference drugs. The results of minimum inhibitory concentration (MICs) are presented in Table 1. It showed that most of the synthesized harmine derivatives displayed better antibacterial activities against Gram-positive strains (S. aureus, S. albus and MRSA) than Gram-negative strains (E. coli and PA). Notably, Compounds 3c and 5a displayed attractive antibacterial activities than other derivatives.

Table 1.
Minimum inhibitory concentration of the synthesized harmine derivatives.
Because compounds 3c and 5a displayed promising antibacterial activity, we further evaluated their in vitro time-kill assay.The bactericidal properties of 3c and 5a were compared at 1 × MIC and 6 × MIC against S. aureus (the MICs of 3c, 5a and harmine were 13.67, 15.63, 31.25 μg/mL, respectively) (Figure 3). As shown in Figure 3, 3c and 5a showed outstanding antibacterial activity and displayed a concentration-dependent effect. Compound 3c with 1 × MIC concentration achieved a 4-log10 reduction in about 4 h and 1 × MIC of compound 5a showed a 4-log10 reduction in about 24 h. Notably, Compound 3c with 6 × MIC concentration completely killed the bacteria within the first 0.5 h and 6 × MIC of compound 5a completely killed the bacteria within 12 h. However, 1 × MIC of harmine just slowed bacterial propagation and its 6 × MIC achieved a 4-log10 reduction in about 24 h. In general, compared to harmine, 3c and 5a showed more rapid bactericidal kinetics against S. aureus with the same concentration (1 × MIC).
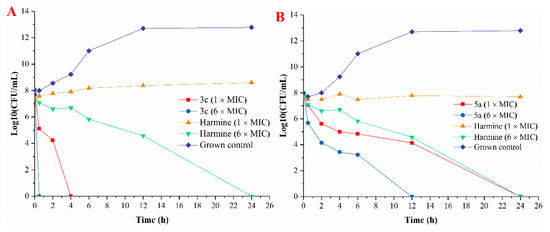
Figure 3.
Time-kill kinetics of compound 3c (A) and 5a (B) at 1 × and 6 × MIC against S. aureus (CICC 10384).
2.3. In Vitro Cytotoxic Activity
In vitro cytotoxic activity of 3c was evaluated using MTT (3-(4,5)-dimethylthi ahiazo (-2)-3,5-diphenytetrazoliiumromide) assay using WI-38 (Human embryonic lung fibroblasts), MCF-7 (human breast cancer cells) and HepG2 (human liver carcinoma cells). Harmine was used as a control. The calculated IC50 (half maximal inhibitory concentration) values of 3c and harmine are presented in Table 2. It was observed that all the tested compounds showed weaker cytotoxic activity against normal cell than cancer cell lines. Notably, the IC50 of compound 3c was higher than that of harmine against three cell lines, which means the cytotoxic activity of compound 3c was weaker than that of harmine. These combined results suggested that although a nitro group existed in compound 3c, its cytotoxic activity had not been improved, which showed that the further study of 3c is meaningful.

Table 2.
Inhibition effect of target compounds in three cell lines. (n = 3; Mean ± SD).
2.4. In Vivo Pharmacokinetic Study
Considering the excellent antibacterial activity, we further evaluated the in vivo pharmacokinetics of compound 3c. The mean plasma concentration time curves of 3c after intravenous (i.v.) and intragastric (i.g.) administration are shown in Figure 4. Additionally, the estimated non-compartmental parameters are displayed in Table 3.
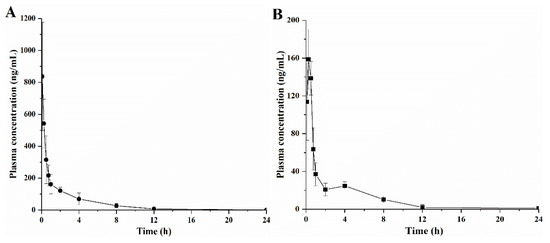
Figure 4.
Mean plasma concentration-time profile after a single intravenous administration of 10 mg/kg 3c solution to rats (A) and after a single oral dose of 40 mg/kg 3c solution (B) (n = 6, mean ± SE).

Table 3.
The non-compartmental model pharmacokinetic parameters in rats after a single intravenous injection (10 mg/kg) and intragastric administration (40 mg/kg) of compound 3c. (n = 6; Mean ± SD).
After a single i.v. and i.g. administration of 3c, the plasma concentration of the drug declined quickly (Figure 4). After 12 h, the compound 3c was almost completely eliminated. These data demonstrated that the distribution and elimination of 3c in vivo were relatively fast. Additionally, compound 3c displayed great individual differences among animals in vivo. Furthermore, the AUC0→∞ of compound 3c after a single i.g. administration (290.25 ng·h/mL) was only 6.9% of the same dose of i.v. administration (1053.24 ng·h/mL). These results indicated that compound 3c had a significant first pass effect, which need to be paid attention in the further research. In addition, the pharmacokinetic data showed that the plasma concentration of 3c was much lower than its MIC. It reminded us that we should increase the dosage in the follow-up pharmacodynamics study to achieve an effective plasma concentration.
3. Materials and Methods
3.1. General Information
All chemical reagents were purchased from Energy Reagent Co., Ltd. (Anqing, China) or Shanghai Huangdi Chemical Co., Ltd. (Shanghai, China). Unless otherwise noted, all commercially available reagents were used directly after they were received. In order to monitor the reaction, thin-layer chromatography (TLC) was performed on silica gel plate (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) and visualized by UV light. All melting points were determined on a digital X4 melting point analyzer (Henan Ruike Medical Instrument Co., Ltd., Xinxiang, China) using open capillary tubes and were uncorrected. HRMS were obtained with a ESI-Brucker APEX Ⅱ49e mass spectrometer (Bruker, Billerica, MA, USA). 1H-NMR and 13C-NMR spectra were recorded with JNM-ECS (JEOL Co., Ltd., Tokyo, Japan) and Bruke AM-400 spectrometer (Bruker, Billerica, MA, USA), respectively. The solvents used were deuterated reagents (DMSO-d6 and CDCl3). Unless otherwise noted, further purification of products was performed via column chromatography on silica gel (200–300 mesh, Gansu Yihua chemical Glass Instrument Co., Lanzhou, China) and the products were eluted in an appropriate solvent mixture under air pressure. The crystal of the typical compound was obtained by solvent evaporation method and determined by Super Nova single crystal diffractometer (Agilent, Santa Clara, CA, USA).
3.2. Synthesis
General procedure for synthesis of compounds 2a–d. To a round-bottom flask 7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (1) (5 mmol) was added in the mixed solvent of DMF and THF (DMF:17 mL, THF:15 mL), stirred at room temperature until clear. NaH (7.5 mmol) was added in batches, stirred for 10 min at room temperature. Then, dibromoalkanes (15 mmol) were added to the mixture, followed by heating and refluxing. The reaction was monitored by TLC during the whole process. After the mixture fully reacted, the mixture was cooled with ice water, and extracted three times with ethyl acetate. The mixture was washed with ether and saturated NaCl solution, after being dried over anhydrous Na2SO4, the mixture was filtered and concentrated. The crude product was purified by silica gel column to obtain 2a–d (dichloromethane:methanol = 10:1, triethylamine 1‰).
9-(3-Bromopropyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2a). Compound 2a was prepared according to the general procedure from 7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (1) and 1,3-dibromopropane. White solid, 68.8% yield. 1H-NMR (400 MHz, CDCl3) δ: 8.67 (d, J = 5.4 Hz, 1H), 8.02 (d, J = 8.7 Hz, 1H), 7.87 (d, J = 5.4 Hz, 1H), 6.86–6.79 (m, 2H), 4.36 (t, J = 7.4 Hz, 2H), 3.91 (s, 3H), 3.51 (t, J = 6.4 Hz, 2H), 2.98 (s, 3H), 1.98–1.87 (m, 2H).
9-(4-Bromobutyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2b). Compound 2b was prepared according to the general procedure from 7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (1) and 1,4-dibromobutane. White solid, 70.1% yield.1H-NMR (400 MHz, CDCl3) δ: 8.21 (d, J = 5.2 Hz, 1H), 7.98 (d, J = 8.6 Hz, 1H), 7.77 (d, J = 5.2 Hz, 1H), 6.97–6.80 (m, 2H), 4.54 (t, J = 7.5 Hz, 2H), 3.95 (s, 3H), 3.49 (t, J = 6.4 Hz, 2H), 3.01 (s, 3H), 1.96–1.81 (m, 4H).
9-(5-Bromopentyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2c). Compound 2c was prepared according to the general procedure from 7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (1) and 1,5-dibromopentane. White solid, 68.3% yield. 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 4.8 Hz, 1H), 7.99 (d, J = 8.6 Hz, 1H), 7.74 (d, J = 5.4 Hz, 1H), 6.95–6.81 (m, 2H), 4.49 (t, J = 7.4 Hz, 2H), 3.96 (s, 3H), 3.41 (t, J = 6.4 Hz, 2H), 3.02 (s, 3H), 1.98–1.82 (m, 4H), 1.59–1.44 (m, 2H).
9-(6-Bromohexyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2d). Compound 2d was prepared according to the general procedure from 7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (1) and 1,6-dibromohexane. White solid, 66.1% yield. 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.4 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.74 (d, J = 4.0 Hz, 1H), 6.96–6.79 (m, 2H), 4.48 (t, J = 7.5 Hz, 2H), 3.96 (s, 3H), 3.40 (t, J = 6.4 Hz, 2H), 3.02 (s, 3H), 1.89–1.78 (m, 4H), 1.51–1.39 (m, 4H).
General procedure for synthesis of compounds 3a–d, 4a–d, 5a–d, 6a–d. To a round-bottom flask with a magnetic stirrer substituted cinnamic acid (1 mmol) in acetone (8 mL), triethylamine were added dropwise (5–10 drops), and stirred until completely dissolved. Compound 2a–d (3 mmol) was dissolved and dropped into the above reaction solution, stirred at room temperature for 3 h. The reaction was monitored by TLC during the whole process. After the mixture fully reacted, aqueous HCl was added to the solution to adjust pH to 3–5. The mixture was extracted three times with ethyl acetate. The organic phase was collected, washed twice with saturated NaHCO3 solution and saturated NaCl solution, respectively. The organic phase was then dried over anhydrous NaSO4, filtered and concentrated. Compounds 3a–d, 4a–d, 5a–d, 6a–d were obtained by further purification using silica gel column chromatography (dichloromethane:methanol = 10:1, triethylamine 1‰).
3-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl)propyl(E)-3-(4-methoxyphenyl) acrylate (3a). Compound 3a was prepared according to the general procedure from (E)-3-(4-methoxyphenyl)acrylic acid and 9-(3-bromopropyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2a). Yellow solid. 52.1% yield; mp 133.2–133.9 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.2 Hz, 1H), 7.96 (d, J = 8.4 Hz, 1H), 7.73 (d, J = 5.2 Hz, 1H), 7.65 (d, J = 16.0 Hz, 1H), 7.49 (d, J = 8.8 Hz, 2H), 6.94–6.87 (m, 4H), 6.29 (d, J = 16.0 Hz, 1H), 4.65 (t, J = 7.6 Hz, 2H), 4.28 (t, J = 5.8 Hz, 2H), 3.91 (s, 3H), 3.86 (s, 3H), 3.06 (s, 3H), 2.28–2.22 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 166.97, 161.53, 160.89, 144.97, 142.95, 140.47, 138.48, 135.12, 129.76, 129.48, 122.35, 115.21, 114.79, 114.33, 112.22, 108.82, 93.21, 61.42, 55.58, 55.34, 41.79, 29.86, 23.35; ESI-MS: calcd. for C26H26N2O4 [M + H]+ 431.1965; found 431.1955.
3-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) propyl (E)-3-(3,4,5-trimethoxyphenyl) acrylate (3b). Compound 3b was prepared according to the general procedure from (E)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(3-bromopropyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2a). White solid. 49.2% yield; m.p.: 102.6–104.2 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.2 Hz, 1H), 7.96 (d, J = 9.2 Hz, 1H), 7.73 (d, J = 5.2 Hz, 1H), 7.58 (d, J = 16.0 Hz, 1H), 6.90 (s, 2H), 6.76 (s, 2H), 6.31 (d, J = 16.0 Hz, 1H), 4.64 (t, J = 7.4 Hz, 2H), 4.29 (t, J = 5.8 Hz, 2H), 3.92 (s, 9H), 3.90 (s, 3H), 3.05 (s, 3H), 2.28–2.22 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 166.75, 161.05, 153.56, 145.42, 143.12, 140.54, 140.44, 138.59, 135.28, 129.73, 122.54, 116.68, 115.41, 112.40, 108.90, 105.41, 93.48, 61.80, 61.08, 56.28, 55.77, 41.97, 31.82, 29.95, 29.36, 23.45; ESI-MS: calcd. for C28H30N2O6 [M + H]+ 491.2177; found 491.2171.
4-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) propyl(E)-3-(2-nitrophenyl) acrylate (3c). Compound 3c was prepared according to the general procedure from (E)-3-(2-nitrophenyl) acrylic acid and 9-(3-bromopropyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2a). Yellow solid. 44.2% yield; m.p.: 128.6–129.3 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.2 Hz, 1H), 8.14 (d, J = 16.0 Hz, 1H), 8.08 (d, J = 7.2 Hz, 1H), 7.97 (d, J = 9.2 Hz, 1H), 7.73–7.64 (m, 3H), 7.60–7.56 (m, 1H), 6.90–6.87 (m, 2H), 6.33 (d, J = 16.0 Hz, 1H), 4.67 (t, J = 7.4 Hz, 2H), 4.33–4.29 (m, 2H), 3.92 (s, 3H), 3.05 (s, 3H), 2.30–2.24 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 165.67, 160.75, 148.18, 142.94, 140.40, 139.88, 138.10, 135.16, 130.41, 130.19, 129.26, 128.98, 124.79, 123.03, 122.25, 115.09, 112.14, 108.53, 93.31, 64.62, 55.60, 44.66, 28.47, 23.31; ESI-MS: calcd. for C25H23N3O5 [M + H]+ 446.1710; found 446.1716.
3-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) propyl (E)-2-(3,4-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl) acrylate (3d). Compound 3d was prepared according to the general procedure from (E)-2-(3,4-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(3-bromopropyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2a). White solid. 21.14% yield; m.p.: 173.6–175.2 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.28 (d, J = 5.2 Hz, 1H), 7.95 (d, J = 8.4 Hz, 1H), 7.71 (d, J = 5.2 Hz, 1H), 7.67 (s, 1H), 6.95 (d, J = 8.4 Hz, 1H), 6.89–6.83 (m, 2H), 6.80 (d, J = 9.2 Hz, 2H), 6.36 (s, 2H), 4.56 (t, J = 7.6 Hz, 2H), 4.33 (t, J = 5.8 Hz, 2H), 3.90 (s, 3H), 3.89 (s, 3H), 3.83 (s, 6H), 3.60 (s, 6H), 2.95 (s, 3H), 2.24–2.19 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 167.73, 161.00, 152.65, 149.46, 148.86, 143.00, 140.64, 140.37, 139.16, 138.38, 135.11, 130.86, 129.63, 128.40, 122.50, 122.23, 115.32, 112.92, 112.33, 111.68, 108.62, 108.17, 93.45, 62.51, 60.88, 55.75, 55.69, 41.99, 29.78, 23.24; ESI-MS: calcd. for C36H38N2O8 [M + H]+ 627.2701; found 627.2701.
4-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) butyl (E)-3-(4-methoxyphenyl) acrylate (4a). Compound 4a was prepared according to the general procedure from (E)-3-(4-methoxyphenyl) acrylic acid and 9-(4-bromobutyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2b). White solid. 43.6% yield; m.p.: 118.7–120.2 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.2 Hz, 1H), 7.98 (d, J = 9.6 Hz, 1H), 7.74 (d, J = 5.2 Hz, 1H), 7.63 (d, J = 16.0 Hz, 1H), 7.46 (d, J = 8.8 Hz, 2H), 6.91–6.88 (m, 4H), 6.29 (d, J = 16.0 Hz, 1H), 4.54 (t, J = 7.6 Hz, 2H), 4.25 (t, J = 6.4 Hz, 2H), 3.94 (s, 3H), 3.84 (s, 3H), 3.03 (s, 3H), 2.01–1.94 (m, 2H), 1.867–1.80 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 167.21, 161.44, 160.88, 144.76, 142.95, 140.44, 138.38, 135.21, 129.75, 129.42, 126.95, 122.39, 115.23, 115.12, 114.30, 112.26, 108.66, 93.37, 63.47, 55.68, 55.67, 44.38, 27.18, 26.13, 23.41; ESI-MS: calcd. for C27H28N2O4 [M + H]+ 445.2111; found 445.2116.
4-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) butyl (E)-3-(3,4,5-trimethoxyphenyl) acrylate (4b). Compound 4b was prepared according to the general procedure from (E)-3- (3,4,5-trimethoxyphenyl) acrylic acid and 9-(4-bromobutyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2b). Yellow solid. 49.0% yield; m.p.: 95.7–96.3 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.2 Hz, 1H), 7.99 (d, J = 9.2 Hz, 1H), 7.75 (d, J = 5.2 Hz, 1H), 7.59 (d, J = 16.0 Hz, 1H), 6.91–6.89 (m, 2H), 6.74 (s, 2H), 6.31 (d, J = 16.0 Hz, 1H), 4.55 (t, J = 7.8 Hz, 2H), 4.26 (t, J = 6.2 Hz, 2H), 3.94 (s, 3H), 3.89 (s, 9H), 3.03 (s, 3H), 2.02–1.95 (m, 2H), 1.87–1.80 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 166.83, 160.87, 153.40, 145.08, 142.95, 140.41, 140.19, 138.40, 135.21, 129.69, 129.44, 122.42, 116.87, 115.26, 112.28, 108.61, 105.23, 93.43, 63.67, 60.93, 56.13, 55.69, 44.37, 27.19, 26.12, 23.42; ESI-MS: calcd. for C29H32N2O6 [M + H]+ 505.2333; found 505.2339.
4-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) butyl (E)-3-(2-nitrophenyl) acrylate (4c). Compound 4c was prepared according to the general procedure from (E)-3-(2-nitrophenyl) acrylic acid and 9-(4-bromobutyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2b). White solid. 43.6% yield; m.p.: 130.9–132.2 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.28 (d, J = 5.2 Hz, 1H), 8.11 (d, J = 15.6 Hz, 1H), 8.04 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 9.2 Hz, 1H), 7.73 (d, J = 5.2 Hz, 1H), 7.67–7.60 (m, 2H), 7.56–7.52(m, 1H), 6.90–6.87 (m, 2H), 6.34 (d, J = 16.0 Hz, 1H), 4.54 (t, J =7.8 Hz, 2H), 4.27 (t, J = 6.4 Hz, 2H), 3.94 (s, 3H), 3.03 (s, 3H), 2.01–1.93 (m, 2H), 1.87–1.80 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 165.62, 160.88, 148.22, 142.94, 140.41, 140.38, 138.36, 135.17, 133.49, 130.40, 130.33, 129.43, 129.08, 124.88, 122.73, 122.37, 115.20, 112.24, 108.69, 93.34, 64.07, 55.67, 44.32, 27.16, 26.03, 23.38; ESI-MS: calcd. for C26H25N3O5 [M + H]+ 460.1867; found 460.1865.
4-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) butyl (E)-2-(3,4-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl) acrylate (4d). Compound 4d was prepared according to the general procedure from (E)-2-(3,4-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(4-bromobutyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2b). White solid. 27.2% yield; m.p.: 95.7–97.4 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.6 Hz, 1H), 7.98 (d, J = 8.8 Hz, 1H), 7.73 (d, J = 5.2 Hz, 1H), 7.69 (s, 1H), 6.89 (dd, J = 2.0, 8.8 Hz, 1H), 6.83–6.81 (m, 2H), 6.76 (dd, J = 2.0, 8.4 Hz, 1H), 6.71 (d, J = 2.0 Hz, 1H), 6.34 (s, 2H), 4.47–4.43 (m, 2H), 4.29 (t, J = 6.2 Hz, 2H), 3.92 (s, 3H), 3.81 (s, 3H), 3.80 (s, 3H), 3.70 (s, 3H), 3.56 (s, 6H), 2.99 (s, 3H), 1.94–1.89 (m, 2H), 1.87–1.78 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 167.84, 161.86, 152.62, 149.30, 148.72, 144.00, 140.48, 139.07, 135.45, 134.70, 131.03, 129.65, 128.42, 122.97, 122.18, 119.25, 114.68, 112.86, 111.54, 109.93, 108.11, 105.32, 93.29, 64.26, 60.86, 55.78, 55.72, 44.52, 27.42, 26.18, 21.46; ESI-MS: calcd. for C37H40N2O8 [M + H]+ 641.2857; found 641.2844.
5-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) amyl (E)-3-(4-methoxyphenyl) acrylate (5a). Compound 5a was prepared according to the general procedure from (E)-3-(4-methoxyphenyl) acrylic acid and 9-(5-bromopentyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2c). White solid. 46.2% yield; m.p.: 91.1–92.3 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 8.17 (d, J = 5.2 Hz, 1H), 8.08 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 5.2 Hz, 1H), 7.66 (d, J = 8.8 Hz, 2H), 7.57 (d, J = 16.0 Hz, 1H), 7.20 (s, 1H), 6.98 (d, J = 8.4 Hz, 2H), 6.87 (d, J = 8.6 Hz, 1H), 6.44 (d, J = 16.0 Hz, 1H), 4.57 (t, J = 7.6 Hz, 2H), 4.13 (t, J = 6.6 Hz, 2H), 3.91 (s, 3H), 3.81 (s, 3H), 2.96 (s, 3H), 1.82–1.74 (m, 2H), 1.72–1.69 (m, 2H); 1.51–1.43 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 167.22, 161.33, 160.84, 144.44, 143.00, 140.31, 138.03, 135.15, 129.66, 129.40, 126.99, 122.33, 115.31, 115.10, 114.24, 112.21, 108.64, 93.32, 63.79, 55.63, 55.30, 44.66, 30.23, 28.50, 23.38, 23.20; ESI-MS: calcd. for C28H30N2O4 [M + H]+ 459.2278; found 459.2288.
5-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) amyl (E)-3-(3,4,5-trimethoxyphenyl) acrylate (5b). Compound 5b was prepared according to the procedure from (E)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(5-bromopentyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2c). White solid. 48.9% yield; m.p.: 103.7–104.7 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 8.16 (d, J = 5.1 Hz, 1H), 8.08 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 5.4 Hz, 1H), 7.57 (d, J = 15.9 Hz, 1H), 7.20 (s, 1H), 7.07 (s, 2H), 6.86 (d, J = 8.7 Hz, 1H), 6.64 (d, J = 15.9 Hz, 1H), 4.56 (t, J = 7.4 Hz, 2H), 4.14 (t, J = 6.5 Hz, 2H), 3.91 (s, 3H), 3.83 (s, 6H), 3.70 (s, 3H), 2.95 (s, 3H), 1.81–1.69 (m, 4H), 1.53–1.44 (m, 2H); 13C-NMR (100 MHz, DMSO-d6) δ: 166.88, 160.99, 153.55, 145.16, 143.21, 140.97, 139.93, 138.19, 135.02, 130.08, 128.90, 122.81, 117.76, 114.68, 112.67, 109.54, 106.40, 94.12, 64.19, 60.56, 56.50, 56.04, 44.41, 30.39, 28.47, 23.53, 23.20; ESI-MS: calcd. for C30H34N2O6 [M + H]+ 519.2490; found 519.2474.
5-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) amyl (E)-3-(2-nitrophenyl) acrylate (5c). Compound 5c was prepared according to the procedure from (E)-3-(2-nitrophenyl) acrylic acid and 9-(5-bromopentyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2c). Yellow solid. 44.2% yield; m.p.: 127.4–130.0 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.28 (d, J = 5.2 Hz, 1H), 8.11 (d, J = 16.0 Hz, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 9.2 Hz, 1H), 7.73 (d, J = 5.6 Hz, 1H), 7.69–7.62 (m, 2H), 7.54–7.58 (m, 1H), 6.89–6.86 (m, 2H), 6.34 (d, J = 16.0 Hz, 1H), 4.53–4.49 (m, 2H), 4.24 (t, J = 6.4 Hz, 2H), 3.95 (s, 3H), 3.03 (s, 3H), 1.95–1.87 (m, 2H), 1.82–1.75 (m, 2H), 1.59–1.50 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 165.82, 158.08, 148.23, 144.12, 140.01, 138.76, 136.94, 133.49, 130.58, 130.22, 129.14, 127.31, 124.87, 123.16, 118.24, 115.39, 113.62, 108.74, 104.15, 99.94, 64.73, 62.57, 59.60, 33.71, 32.46, 31.74, 24.40; ESI-MS: calcd. for C27H27N3O5 [M + H]+ 474.2023; found 474.2011.
5-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) pentyl (E)-2-(3,4-dimethoxyphenyl)-3- (3,4,5-trimethoxyphenyl) acrylate (5d). Compound 5d was prepared according to the procedure from (E)-2-(3,4-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(5-bromopentyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2c). White solid. 28.2% yield; m.p.: 117.4–119.9 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.28 (d, J = 5.2 Hz, 1H), 7.97 (d, J = 8.8 Hz, 1H), 7.73 (d, J = 5.2 Hz, 1H), 7.67 (s, 1H), 6.89 (d, J = 2.0 Hz, 1H), 6.87 (s, 1H), 6.85 (s, 1H), 6.77–6.75 (m, 2H), 6.35 (s, 2H), 4.39 (t, J = 7.8 Hz, 2H), 4.22 (t, J = 6.4 Hz, 2H), 3.94 (s, 3H), 3.86 (s, 3H), 3.82 (s, 3H), 3.79 (s, 3H), 3.58 (s, 6H), 2.96 (s, 3H), 1.88–1.84 (m, 2H), 1.76–1.73 (m, 2H), 1.51–1.43 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 167.69, 162.39, 160.81, 152.47, 149.15, 148.54, 142.98, 140.20, 139.91, 138.86, 137.94, 135.10, 135.09, 131.21, 129.69, 129.37, 128.42, 122.14, 115.02, 112.95, 112.87, 112.15, 111.33, 108.54, 107.92, 93.33, 64.53, 60.72, 55.86, 55.58, 44.60, 30.08, 28.33, 23.26, 23.26, 23.08; ESI-MS: calcd. for C38H42N2O8 [M + H]+ 665.3014; found 655.3018.
6-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) hexyl (E)-3-(4-methoxyphenyl) acrylate (6a). Compound 6a was prepared according to the general procedure from (E)-3-(4-methoxyphenyl) acrylic acid and 9-(6-bromohexyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2d). Yellow solid. 37.6% yield; m.p.: 88.7–90.4 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.4 Hz, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.73 (d, J = 5.1 Hz, 1H), 7.63 (d, J = 16.2 Hz, 1H), 7.46 (d, J = 8.5 Hz, 2H), 6.84–6.90(m, 4H), 6.29 (d, J = 15.9 Hz, 1H), 4.45 (t, J = 7.8 Hz, 2H), 4.18 (t, J = 6.7 Hz, 2H), 3.94 (s, 3H), 3.82 (s, 3H), 3.02 (s, 3H), 1.90–1.81 (m, 2H), 1.74–1.66 (m, 2H), 1.50–1.45 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ: 167.25, 161.27, 160.76, 144.30, 142.95, 140.37, 138.08, 135.16, 129.60, 129.28, 127.00, 122.28, 115.42, 115.11, 114.21, 112.15, 108.51, 93.31, 64.03, 55.60, 55.26, 44.68, 30.47, 28.60, 26.52, 25.76, 23.30; ESI-MS: calcd. for C29H32N2O4 [M + H]+ 473.2435; found 473.2433.
6-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) hexyl (E)-3-(3,4,5-trimethoxyphenyl) acrylate (6b). Compound 6b was prepared according to the general procedure from (E)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(6-bromohexyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2d). Yellow solid. 38.1% yield; m.p.: 73.7–73.9 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.29 (d, J = 5.2 Hz, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.74 (d, J = 5.2 Hz, 1H), 7.59 (d, J = 15.6 Hz, 1H), 6.91–6.86 (m, 2H), 6.75 (s, 2H), 6.33 (d, J = 16.0 Hz, 1H), 4.48 (t, J = 7.8 Hz, 2H), 4.20 (t, J = 6.6 Hz, 2H), 3.95 (s, 3H), 3.88 (s, 9H), 3.02 (s, 3H), 1.90–1.83 (m, 2H), 1.74–1.69 (m, 2H), 1.50–1.47 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ: 166.95, 160.86, 153.36, 144.70, 143.08, 140.30, 140.05, 137.94, 135.19, 129.81, 129.46, 122.40, 117.21, 115.15, 112.26, 108.57, 105.15, 93.44, 64.24, 60.91, 56.10, 55.68, 44.76, 30.50, 28.61, 26.55, 25.77, 23.19; ESI-MS: calcd. for C31H36N2O6 [M + H]+ 533.2646; found 533.2648.
6-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) hexyl(E)-3-(2-nitrophenyl) acrylate (6c). Compound 6c was prepared according to the general procedure from (E)-3-(2-nitrophenyl) acrylic acid and 9-(6-bromohexyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2d). White solid. 33.9% yield; m.p.: 117.3–119.9 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 8.16 (d, J = 5.1 Hz, 1H), 8.08 (d, J = 8.7 Hz, 2H), 7.95–7.90 (m, 2H), 7.87 (d, J = 5.1 Hz, 1H), 7.78 (t, J = 7.5 Hz, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.18 (s, 1H), 6.86 (d, J = 8.7 Hz, 1H), 6.62 (d, J = 15.9 Hz, 1H), 4.55 (t, J = 7.4 Hz, 2H), 4.16 (t, J = 6.5 Hz, 2H), 3.90 (s, 3H), 2.95 (s, 3H), 1.78–1.71 (m, 2H), 1.66–1.60 (m, 2H), 1.45–1.40 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ: 165.67, 160.75, 148.18, 142.94, 140.40, 139.88, 138.10, 135.16, 133.40, 130.41, 130.19, 129.26, 128.98, 124.79, 123.03, 122.25, 115.09, 112.14, 108.53, 93.31, 64.62, 55.60, 44.66, 30.48, 28.47, 26.51, 25.76, 23.31; ESI-MS: calcd. for C28H29N3O5 [M + H]+ 488.2180; found 488.2201.
6-(7-Methoxy-1-methyl-9H-pyridine [3,4-b] indole-9-yl) hexyl (E)-2-(3,4-dimethoxyphenyl)-3– (3,4,5-trimethoxyphenyl) acrylate (6d). Compound 6d was prepared according to the general procedure from (E)-2-(3,4-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl) acrylic acid and 9-(6-bromohexyl)-7-methoxy-1-methyl-9H-pyridine [3,4-b] indole (2d). White solid. 27.3% yield; m.p.: 113.1–115.0 °C; 1H-NMR (400 MHz, CDCl3) δ: 8.27 (d, J = 5.6 Hz, 1H), 7.99 (d, J = 8.8 Hz, 1H), 7.82 (d, J = 5.2 Hz, 1H), 7.68 (s, 1H), 6.93 (d, J = 8.8 Hz, 1H), 6.84 (s, 2H), 6.79–6.74 (m, 2H), 6.34 (s, 2H), 4.43 (t, J = 7.6 Hz, 2H), 4.18 (t, J = 6.6 Hz, 2H), 3.95 (s, 3H), 3.81 (s, 3H), 3.79 (s, 3H), 3.77 (s, 3H), 3.55 (s, 6H), 3.11 (s, 3H), 1.83–1.78 (m, 2H), 1.70–1.65 (m, 2H), 1.45–1.36 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ: 167.90, 161.62, 153.13, 152.62, 149.25, 148.67, 143.88, 140.03, 138.95, 134.89, 131.39, 129.83, 128.61, 122.86, 122.28, 114.83, 113.03, 112.62, 111.47, 109.55, 108.04, 105.24, 93.42, 64.84, 60.86, 55.95, 55.72, 44.85, 30.50, 28.50, 26.46, 25.67; ESI-MS: calcd. for C39H44N2O8 [M + H]+ 669.3170; found 669.3196.
3.3. Evaluation of the Antibacterial Activity
3.3.1. MIC Testing
The antibacterial efficiency of all prepared compounds was determined using the microplate dilution method [28,29]. All compounds were prepared into different concentrations of stock solution according to their solubility. The compounds were added to the 96 well plate and serially diluted in Mueller-Hinton broth. Three Gram-positive strains (S. aureus, S. albus and MRSA) and two Gram-negative strains (E. coli and PA) were cultivated and added to the 96 well plate. The initial concentration of bacteria should be higher than 105 CFU/mL. The samples were incubated at 37 °C for 18–24 h. The MIC was determined for compounds by observing change of clarity in the broth. The same procedure was repeated in triplicate.
3.3.2. Bactericidal Time–Kill Kinetics
The bacteria S. aureus was prepared in Muellere Hinton broth at 37 °C for 6 h with shaking. The solution of 3c (1 × MIC and 6 × MIC), 5a (1 × MIC and 6 × MIC), harmine (1 × MIC and 6 × MIC) and saline (served as a control group) were separately added to the bacterial suspension so that the final bacterial concentration were 106~107 CFU/mL. At the predetermined time point (0, 0.5, 2, 4, 6, 12 and 24 h), 20 mL of aliquots were serially diluted in normal saline, plated on sterile Muellere Hinton agar plates, and incubated at 37 °C for 24 h. After incubation, the viable colonies were counted and represented as log10 (CFU/mL). The same procedure was repeated in triplicate.
3.4. In Vitro Cytotoxic Activity
The in vitro cytotoxicity of 3c was determined using MTT assay. WI-38 (Human embryonic lung fibroblasts), MCF-7 (Human breast cancer cells) and HepG2 (human liver carcinoma cells) were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were maintained using Dulbecco’s modified eagle’s medium (DMEM, Sangon Biotech, Shanghai, China) supplemented with 10% fetal bovine serum (FBS, Sijiqing Bioengineering Material Co., Ltd., Hangzhou, China), 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified 37 °C incubator under 5% CO2. 100 μL culture medium at a concentration of 1 × 104 cells/mL was added to 96-well plate and incubated in a humidified 37 °C incubator under 5% CO2. After 24 h, 10 µL solution of 3c and harmine dissolved in DMSO and PBS and then diluted with DMEM under a concentration gradient, which was added in the wells for final concentrations of 1000, 500, 250, 125, 62.5 µmol/L of compounds in WI-38 and 160, 80, 40, 20, 10 µmol/L of compounds in MCF-7 and Hep-G2. After 48 h incubation, 10 μL MTT solution (5 mg/mL) was added into each well and incubated in a humidified 37 °C incubator under 5% CO2. After 4 h, the supernatant was removed and 150 μL DMSO was added. The absorbance of each well was determined by an enzyme-linked immunosorbent assay reader (Thermo Fisher Scientific Inc., Rochester, NY, USA) at wavelengths of 570 nm. The experiments were performed in triplicate.
3.5. In Vivo Pharmacokinetic Study
3.5.1. Animals and Ethics Statement
Adult males and females Sprague Dawley rats, with average weight of 200–250 g, were purchased from the Experiment Animal Center of Lanzhou University (Lanzhou, China). The rats were housed under an environmentally controlled room for one week before staring the experiment. All procedures were performed according to the guidelines issued by the Ethical Committee of Lanzhou University (Permit Number: SYXK(Gan)-2018-0002).
3.5.2. Pharmacokinetic Experiment
After 7 days acclimatization, rats were randomly divided into two groups with half male and half female in each group (n = 6). All animals were fasted for 12 h with access to water prior to dosing. One group was intravenously administered with 3c at dose of 10 mg/kg. Another group was oral administered with 3c at dose of 40 mg/kg. Approximately 0.3 mL of blood samples were collected into heparinized tubes from the orbital vein at 0.083, 0.25, 0.5, 0.75, 1, 2, 4, 8, 12 and 24 h after the administration. The heparinized blood samples were immediately centrifuged at 4000 rpm for 10 min and the upper plasma was separated and immediately stored at −20 °C used for UPLC-MS/MS analysis.
Plasma samples were processed as follows. First, 100 μL of plasma samples and 50 μL of tinidazole (1000 ng/mL, internal standard) were mixed by vortex for 1 min. Next, 250 μL of acetonitrile were added to the mixture and vortexed for 10 min. After centrifugation at 10,000 rpm for 10 min, 200 μL of the upper organic layer were removed into 1.5 mL centrifuge tube and dried with nitrogen. Finally, the residue was reconstituted in 100 μL of 50% acetonitrile, vortexed for 1 min, centrifuged at 10,000 rpm for 10 min, and then the supernatant was used for UPLC-MS/MS analysis.
3.5.3. Statistical Analysis
The obtained data were analyzed using a non-compartment open model with PKsolver Software [30,31]. Pharmacokinetic parameters and the experiment data were expressed as the mean ± standard deviation (SD). A paired student’s t-test was used for statistical analysis, with p < 0.05 considered the minimum level of significance.
4. Conclusions
In summary, 16 novel harmine derivatives were synthesized by covalent bonding between N9 of harmine and carboxylic acid of cinnamic acid derivatives. Most of the synthesized harmine derivatives displayed better antibacterial activities against Gram-positive strains (S. aureus, S. albus and MRSA) than Gram-negative strains (E. coli and PA) in vitro. Notably, compound 3c, the most effective compound, showed excellent bactericidal activity against S. aureus and S. albus in time–kill assay. In MTT assay, it showed that compound 3c had weaker cytotoxicity than harmine with IC50 of 340.30, 94.86 and 161.67 μmol/L against WI-38, MCF-7 and HepG2 cell lines, respectively. Pharmacokinetic studies showed that compound 3c showed rapid absorption and excretion with an oral bioavailability of 6.9% in vivo.
Supplementary Materials
The following are available online at, Figures S1–S16: The 1H-NMR and 13C-NMR spectrum of compound 3a–d, 4a–d, 5a–d and 6a–d; Tables S1–S3: Crystal data of compound 4a.
Author Contributions
Experiment design—Y.L. and Z.W.; synthesis of the experiment—Y.L., D.H. and L.T.; antibacterial activity and pharmacokinetics—D.Z. and J.L.; original draft and supervision—Y.L. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Committee of Lanzhou University (SYXK(Gan)-2018-0002 and 11 January 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
We gratefully acknowledge the School of Pharmacy, Lanzhou University, for the financial support and synthesis assay. We also thank the Ministry of Agriculture and Rural Affairs/Lanzhou Institute of Husbandry and Pharmaceutical Sciences of Chinese Academy of Agriculture Sciences, for the test of the antibacterial activity.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Laxminarayan, R.; Dune, A.; Wattal, C. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2014, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Abushaheen, M.A.; Fatani, A.J.; Alosaimi, M.; Jhugroo, P. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Month 2020, 66, 100971. [Google Scholar] [CrossRef]
- Watkins, R.R.; Bonomo, R.A. Overview: Global and Local Impact of Antibiotic Resistance. Infect. Dis. Clin. N. Am. 2016, 30, 313–322. [Google Scholar] [CrossRef]
- Donadio, S.; Maffioli, S.; Monciardini, P.; Sosio, M.; Jabes, D. Antibiotic discovery in the twenty-first century: Current trends and future perspectives. J. Antibiot. 2010, 63, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Ardal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.E.; Sloan, G.F.; Lawhern, G.V.; Volk, G.E.; Shumate, J.T.; Wolfe, A.L. Advances in antibiotic drug discovery: Reducing the barriers for antibiotic development. Future Med. Chem. 2020, 12, 2067–2087. [Google Scholar] [CrossRef]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S.A. Antibiotics--past, present and future. Praxis 1963, 51, 330–333. [Google Scholar]
- Kathrin, I. Mohr, History of Antibiotics Research. Curr. Top. Microb. Immunol. 2016, 398, 237–272. [Google Scholar]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Asgarpanah, J.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharm. 2012, 6, 1573–1580. [Google Scholar] [CrossRef]
- Fortunato, J.J.; Reus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Stertz, L.; Kapczinski, F.; Pinto, J.P.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; et al. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog. Neuro-Psychopharmacol. 2009, 33, 1425–1430. [Google Scholar] [CrossRef]
- Javeed, M.; Rasul, A.; Hussain, G.; Jabeen, F.; Rasool, B.; Shafiq, N.; Riaz, A.; Kaukab, G.; Ali, M. Harmine and its derivatives: Biological activities and therapeutic potential in human diseases. Bangl. J. Pharmacol. 2018, 13, 203–213. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Yu, S. Pharmacological effects of harmine and its derivatives: A review. Arch. Pharm. Res. 2020, 43, 1259–1275. [Google Scholar] [CrossRef]
- Darabpour, E.; Bavi, A.P.; Motamedi, H.; Nejad, S.M.S. Antibacterial Activity of Different Parts of Peganum Harmala L. Growing in Iran against Multi-Drug Resistant Bacteria. EXCLI J. 2011, 10, 252–263. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Dan, W.; Ren, S.; Shang, C.; Wang, J. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 2018, 160, 23–36. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Dan, W.; Jian, L.; Wang, J. Synthesis and Antimicrobial Activities of 3-Methyl-β-Carboline Derivatives. Nat. Prod. Commun. 2015, 10, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, S.; Sova, M.; Ergün, S. Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Parle, A.; Arora, T. Synthesis and antibacterial screening of some novel cinnamic acid derivatives. Int. J. Chem. Stud. 2017, 5, 643–647. [Google Scholar]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Yingyongnarongkul, B.E.; Apiratikul, N.; Aroonrerk, N.; Suksamrarn, A. Solid-phase synthesis and antibacterial activity of hydroxycinnamic acid amides and analogues against methicillin-resistant Staphylococcus aureus and vancomycin-resistant S. aureus. Bioorg. Med. Chem. Lett. 2006, 16, 5870–5873. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, B.; Yazdiniapour, Z.; Sadeghi, M.; Akbari, M.; Lanzotti, V. Cinnamic acid derivatives from welsh onion (Allium fistulosum) and their antibacterial and cytotoxic activities. Phytochem. Anal. 2020, 84, 90. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, D.; Wang, Q.; Huang, S.; Li, C. Semi-synthesis, antibacterial activity, and molecular docking study of novel pleuromutilin derivatives bearing cinnamic acids moieties. Arch. Pharm. 2019, 352, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, amides and substituted derivatives of cinnamic acid: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2004, 39, 827–834. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Dong, P. Synthesis and Biological Activity Evaluation of Novel Heterocyclic Pleuromutilin Derivatives. Molecules 2017, 22, 996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvers, M.A.; Robertson, G.T.; Taylor, C.M.; Waldrop, G.L. Design, synthesis, and antibacterial properties of dual-ligand inhibitors of acetyl-CoA carboxylase. J. Med. Chem. 2014, 57, 8947–8959. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, Y.; Deng, G.; Cao, N. Potential Pharmacokinetic Drug–Drug Interaction Between Harmine, a Cholinesterase Inhibitor, and Memantine, a Non-Competitive N-Methyl-d-Aspartate Receptor Antagonist. Molecules 2019, 24, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, Z.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).