Neuroprotective Potential of Limonene and Limonene Containing Natural Products
Abstract
1. Introduction
2. Neuroprotective Effects and Mechanisms of Limonene
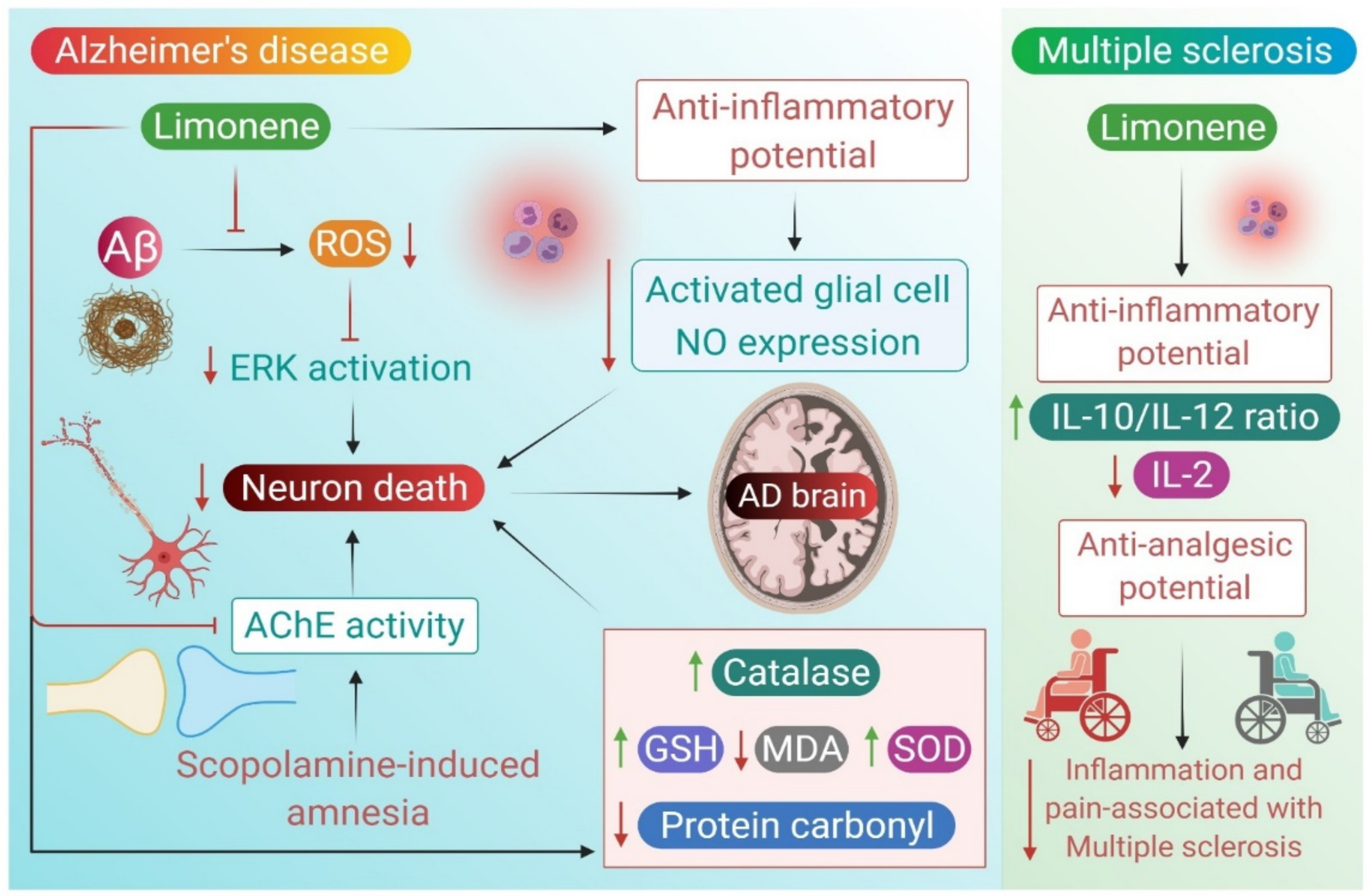
2.1. Alzheimer’s Disease
2.2. Multiple Sclerosis
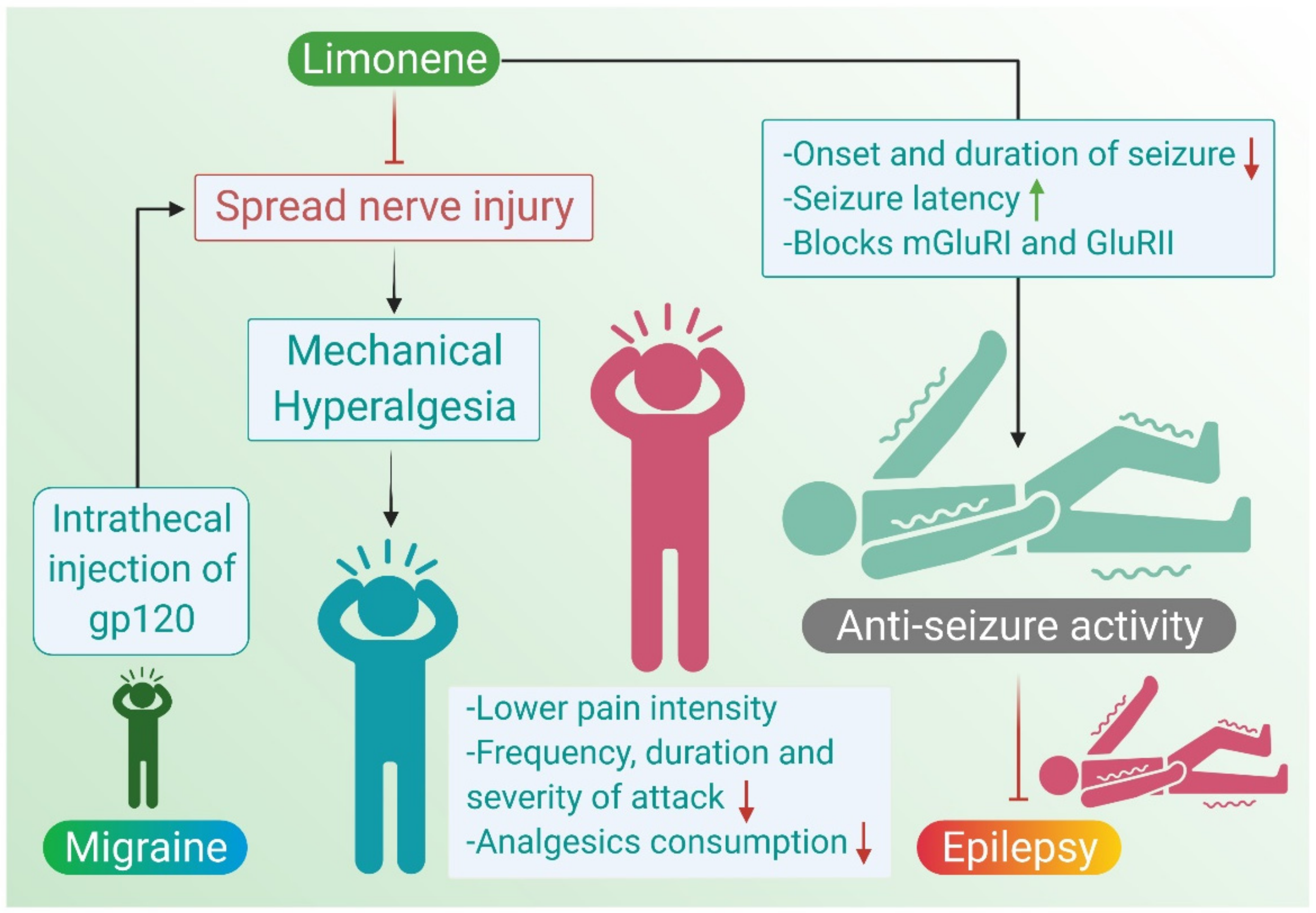
2.3. Migraine
2.4. Epilepsy
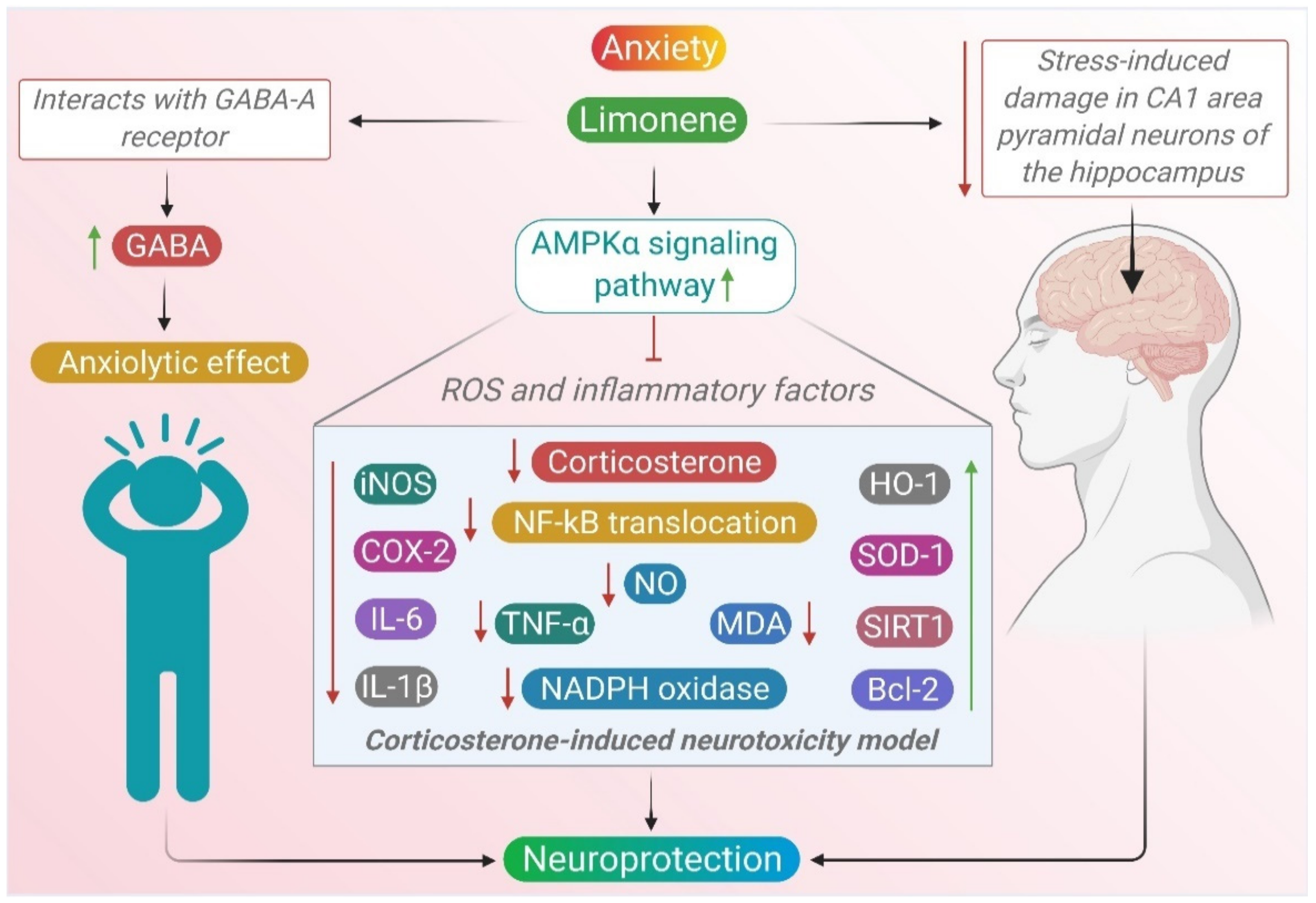
2.5. Anxiety
| In Vitro | ||||
|---|---|---|---|---|
| Alzheimer’s Disease | ||||
| Phytochemicals | Dose | Model of Study | Mechanisms | References |
| Citrus medica L. cv. (Diamante citron) | 164 μg/mL | In vitro based study | ↓ DPPH ⇥ AchE | [33] |
| Aloysia citrodora Palau | 0.001 and 0.01 mg/mL (w/v) | H2O2 (250, 200, 150, 100 and 50 μM) and β-Amyloid (2.5–25 μM)-induced neurotoxicity in CAD cells | ↑ DPPH radical-scavenging Showed neuroprotection | [37] |
| Black pepper | 4 mg/ml | β-amyloid (25 μM) in THP-1 cells | ⇥ AChE ⇥ β-Amyloid aggregation ↓ COX-2 | [38] |
| Multiple Sclerosis | ||||
| Cakile maritima Scop | 10–80 μg/ml | Bacterial cultures of Proteus mirabilis, Klebsiella pneumonia, Proteus vulgaris and Pseudomonas aeruginosa | ↓ DPPH radicals showed antimicrobial activity | [40] |
| Terminalia sericea | 200 mg/ml | Bacterial cultures of Acinetobacter baylyi and Pseudomonas aeruginosa | (-)Acinetobacter baylyi and Pseudomonas aeruginosa | [41] |
| D-limonene | 0.5–500 μM | Splenocytes culture | ↑ IL-10/IL-2 | [45] |
| Anxiety | ||||
| D-limonene | 0.2, 0.4, 0.6, 0.8 and 1.2 μL/ml | Corticosterone (20, 50, 100, 200 and 400 μM) in PC12 cells | ↑ phospho-AMPKα1/2 ↓ NF-κB nuclear translocation ↓ MDA and NO ↓ NADPH oxidase ↑SOD1 and HO-1 ↓ iNOS, COX-2, IL-6, IL-1β, and TNF-α, ↑ SIRT1 ↓ Bax and cleaved caspase-3, ↑ Bcl-2 | [97] |
| D-limonene epoxide | 0.9, 1.8, 3.6, 5.4 and 7.2 μg/ml | Griess method | ↓ NO2−, ↓ OH• ↓ TBARS | [101] |
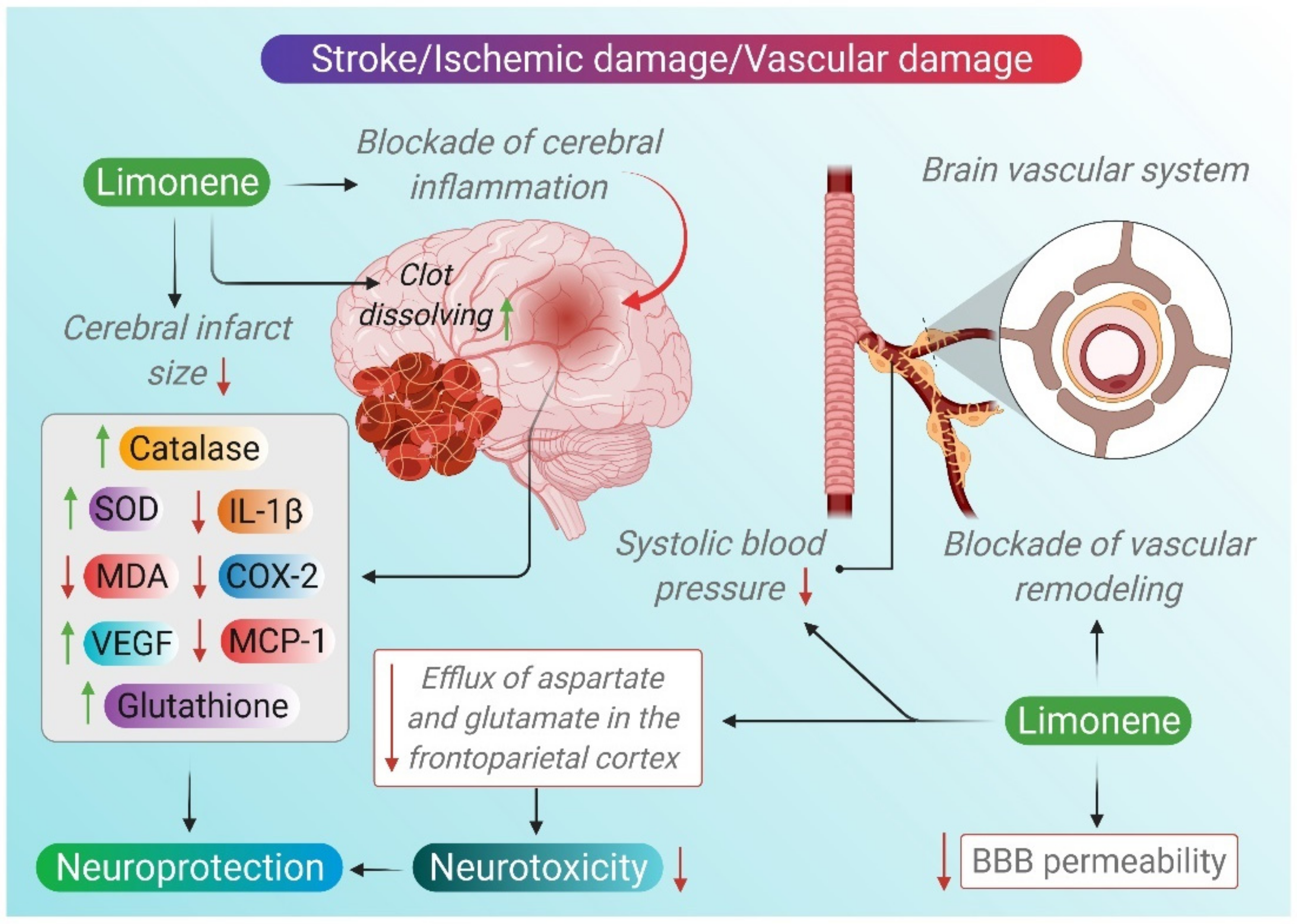
2.6. Stroke
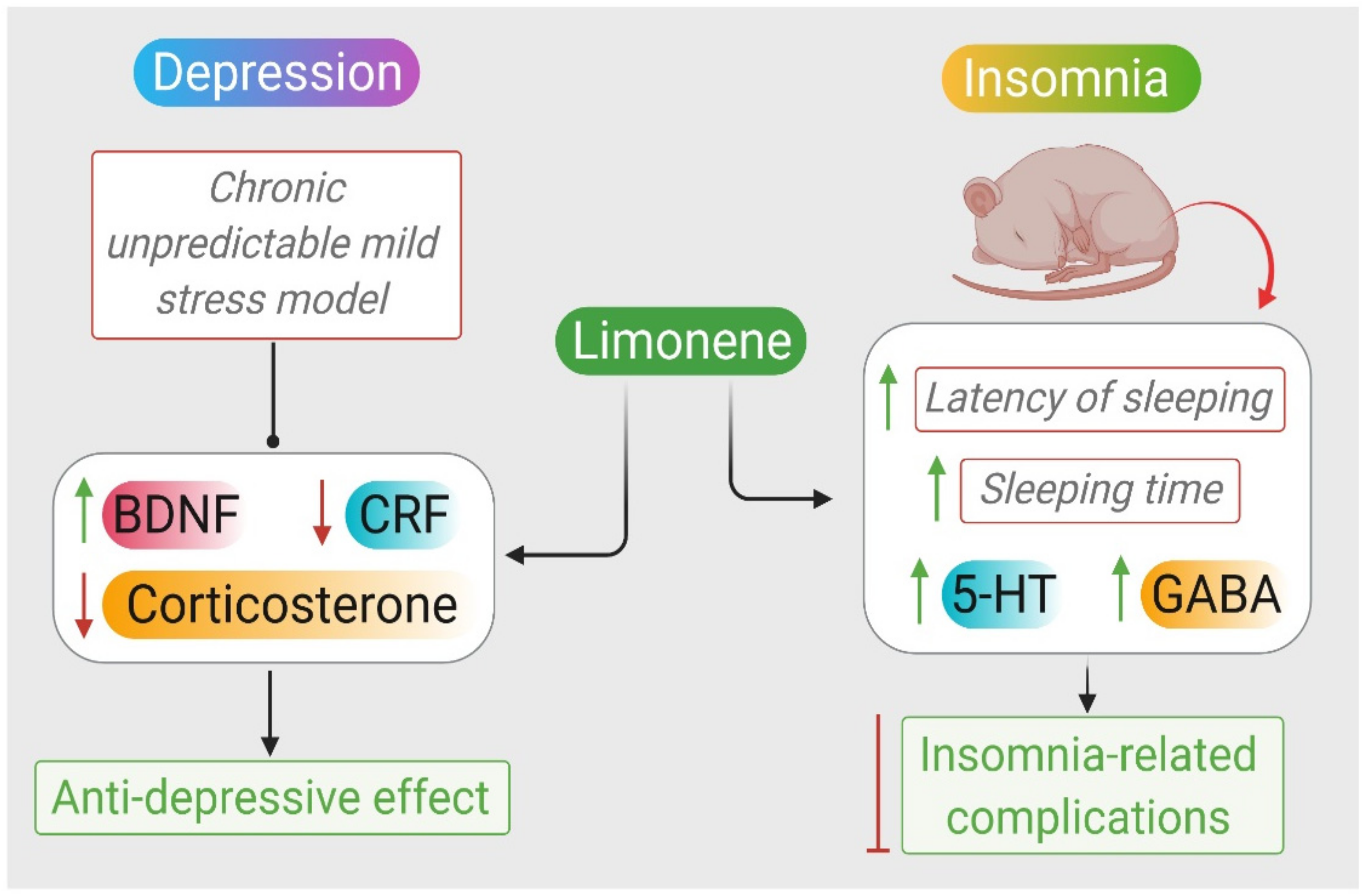
2.7. Depression
2.8. Insomnia
| In Vivo | ||||
|---|---|---|---|---|
| Alzheimer’s Disease | ||||
| Phytochemical | Dose | Model of study | Mechanisms | Reference |
| Limonene | 50 μg/mL or 200 μg/mL | Drosophila model | ↓ rough eye phenotype ↑ survival rate, ↓ ROS ↓ ERK phosphorylation ↓ glial cells ↓ NO, ↓ Drs-GFP | [31] |
| Essential oil mixure | 200 μL of 1% or 3% | Scopolamine (0.7 mg/kg b.w., i.p)-induced amnesia in rats | ⇥ AChE and BChE ↑ GSH, ↓ SOD, CAT and MDA ↑ short-term memory | [34] |
| Limonene | 100 mg/kg | Scopolamine (1 mg/kg)-induced dementia in rats | ↑ memory ↓ DOPAC/dopamine ⇥ AChE | [35] |
| Tetraclinis articulata | 200 μL of 1% and 3% | Aβ1-42 (4 μL i.c.v.)-induced Alzheimer’s disease amyloidosis | ↓ DPPH and ABTS radicals ↑ alternation behavior in Y-maze ↓ memory errors ⇥ AChE ↑ SOD, CAT, GPX and GSH, ↓ protein carbonyl and MDA | [36] |
| Multiple Sclerosis | ||||
| Limonene epoxide | 25, 50, 75 mg/kg | Carrageenan (500 μg/paw)-induced edema, acetic acid (10 mL/kg, i.p.) in Swiss mice | ↓ paw edema ↓ leukocytes and neutrophils, MPO, IL-1β and TNF-α, ↓ Contortions number | [46] |
| Migraine | ||||
| Limonene | 10 mg/kg orally | gp120-induced mechanical hyperalgesia in mice | ↓ mechanical sensitivity ↓ IL-1β and IL-10 ↓ hyperalgesia ↑ SOD | [17] |
| Epilepsy | ||||
| Artemisia dracunculus L. | 0.1, 0.15, 0.2, 0.4, 0.8 and 1.0 mL/kg | Electrical stimulus (50 mA), PTZ-induced seizures (60 mg/kg) in mice | (-)seizures | [54] |
| Citrus aurantium | 5, 10, 20, and 40 mg/kg, i.p | PTZ (85 mg/kg)-induced convulsion in mice | (-)seizures ↓ mortality | [55] |
| Citrus aurantium | 75, 150, 300 and 600 mg/kg, i.p | PTZ (90 mg/kg)-induced convulsion in rats | ↓ onset of seizures ↓ duration of seizure ↓ mortality | [56] |
| Citrus aurantium | 28 mg/ml | PTZ (3 mg/mL)-induced seizures in zebrafish | ↑ latency of seizures | [57] |
| Limonene | 200 mg/kg i.p | PTZ (80 mg/kg)-induced seizures in mice | ↑ latency of seizures ↑ survival rate | [58] |
| Cinnamosma madagascariensis Danguy | 0.4 and 0.8 mL/kg s.c | PTZ (60 mg/kg)-induced seizures in rats | (-)convulsions | [59] |
| Piper guineense | 50–200 mg/kg i.p | PTZ (85 mg/kg)-induced seizures in mice | (-)convulsions | [60] |
| Angelica archangelica Linn. | 50, 100, 200, 400, 500 mg/kg i.p | PTZ (80 mg/kg) and MES (50 mA)-induced seizures in mice | (-)clonic seizures | [61] |
| Anxiety | ||||
| Citrus aurantium L. | 0.5%, 2.5%, and 5%, i.p | EPM test on mice | ↑ time in open arms ↑ entries to open arms | [70] |
| Citrus aurantium L. | 0.5%, 2.5%, and 5%, i.p. for 5 days | EPM test on mice | ↑ time in open arms | [71] |
| Citrus aurantium L. | 0.5 or 1.0 g/kg orally | Light–dark box test on mice | ↑ time in light chamber | [72] |
| Citrus aurantium L. | 0.5 or 1.0 g/kg orally | EPM on mice | ↑ time in open arms | [74] |
| Citrus aurantium L. | 1, 5 or 10 mg/kg/day orally | Light–dark box test on mice | ↑ time in light chamber | [75] |
| Citrus latifolia and Citrus reticulate | 0.5 to 2 g/kg orally | Light–dark box and marble burying tests on mice | ↑ time in light chamber ↓ buried marbles | [76] |
| Citrus limon | 50, 100 and 150 mg/kg orally | EPM test on mice | ↑ time in open arms | [77] |
| Citrus sinensis | 100, 200 or 400 μL for 5 min inhaled | EPM test on rats | ↑ time in open arms | [78] |
| Foeniculum vulgare | 50, 100, 200, 400 mg/kg orally | EPM, staircase and OFT on mice | ↑ time in open arms ↓ rearing ↑ crossed squares | [79] |
| Bergamot | 1.0%, 2.5% and 5.0% w/w inhaled | EPM and hole-board tests on mice | ↑ entries to and time in open arms ↑ head dips ↓ corticosterone | [80] |
| Bergamot | 100 μL/kg i.p | Measured on rats | ↑ aspartate, glycine, taurine glutamate and GABA | [81] |
| (S)-Limonene | 0,5,25,50 mg/kg orally | Foot shock (5 mA) induced stress in rats | ↑ GABA ↓ glutamate ↓ corticosterone | [83] |
| Bergamot | 100, 250, or 500 μL/kg i.p | OFT, EPM and FST on rats | ↓ rearing, crossing and grooming ↑ entries to and time in open arms ↑ immobility and drowning recovery | [84] |
| D-Limonene | 10 mg/kg orally | Functional observational battery on rats | ↓ irritability and fear | [94] |
| D-Limonene | 0.5%, 1.0% and 2.5%, inhaled | EPM on mice | ↑ entries to and time in open arms | [98] |
| D-Limonene | 10 mg/kg, orally | Plastic rodent restrainer for six hours induced stress on rats | ↑ entries to and time in open arms | [99] |
| D-limonene epoxide | 25, 50 and 75 mg/kg, i.p | OFT and EPM on mice | ↓ crossing, rearing and grooming ↑ entries to and time in open arms | [100] |
| D-limonene epoxide | 25, 50 and 75 mg/kg, orally | Marble burying test on mice | ↓ buried glass beads ↓ lipid peroxidation ↓ NO2− ↓ catalase, SOD | [101] |
| Alpinia zerumbet | 8.7 ppm inhaled | LD, OF and EPM on mice | ↑ entries to and time in open arms | [102] |
| Alpinia zerumbet | 0.087, 0.87 or 8.7 ppm inhaled | Behavioral observation and EPM on mice | ↑ locomotor activity ↑ entries to and time in open arms | [103] |
| Stroke | ||||
| D-limonene | 20 mg/kg i.p | Transient middle cerebral artery occlusion for 60 min in rats | ↓ BP ↓ escape latency time ↑ time in target quadrant ↑ capacity to distinguish novel objects ↑ grip strength ↓ sensory neglect ↓ IL-1β, MCP-1 and COX-2 ↑ VEGF ↑ SOD and CAT and GSH ↓ MDA and DHE staining | [109] |
| Lavender oil | 100, 200 and 400 mg/kg i.p | Focal cerebral ischemia induced by transient occlusion of middle cerebral artery for 1 h in rats | ↑ cerebral blood flow ↓ brain water content ↑ SOD, GSH-Px ↓ MDA ↑ TAC marker ↑ VEGF | [111] |
| Nigella sativa oil (NSO) and thymoquinone (TQ) | TQ: 2.5, 5 and 10 mg/kg NSO: 0.048, 0.192 and 0.384 mg/kg i.p | Transient global cerebral ischemia induced by four-vessel-occlusion for 20 min in rate | ↓ MDA | [114] |
| Lavender | 100 and 200 mg/kg | Middle cerebral artery occlusion in rats | ↓ permeability of BBB ↓ MDA | [116] |
| Citrus aurantium | 50 and 75 mg/kg i.p | Carotid arteries occlusion induced ischemia for 30 min in rats | ↓ latency of finding the platform ↑ duration of swimming ↑ anti-oxidant capacity ↓MDA | [117] |
| Bergamot | 0.05, 0.1, 0.5, and 1.0 mL/kg i.p | Middle cerebral artery occlusion induced ischemia in rats | ↓infarct size ↓ aspartate and glutamate ↑p-Akt ↑p-GSK-3β | [120] |
| Depression | ||||
| Citrus sinensis L. Osbeck and D-limonene | 1 mL Citrus sinensis. 50 μL of D-limonene, sniffed | Chronic unpredictable mild stress in mice | ↑ weight, sucrose preference ↓ immobility ↓ TG, LDL-C and TC ↑ 5-HT, DA, and NE ↓ CRF and corticosterone ↑ BDNF and Tr-κB | [124] |
| D-limonene | 10 mg/kg orally | Spared nerve injury in rats | ↓ immobility | [125] |
| Insomnia | ||||
| Anshen | 1, 2, 4 × 10−3 inhaled | PCPA (300 mg/kg)-induced insomnia in mice | ↓ latency of sleeping ↑sleeping time ↑ 5-HT and GABA | [130] |
| Citrus limon | 50, 100 and 150 mg/kg orally | Pentobarbital-induced sleeping time on mice | ↑ sleeping time | [77] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Kava, V.; Latorre-García, L.; da Silva, G.J., Jr.; Pereira, R.G.; Glienke, C.; Ferreira-Maba, L.S.; Vicent, A.; Shimada, T.; Peña, L. Engineering d-limonene synthase down-regulation in orange fruit induces resistance against the fungus phyllosticta citricarpa through enhanced accumulation of monoterpene alcohols and activation of defence. Mol. Plant Pathol. 2018, 19, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Razavi, B.M.; Arasteh, E.; Imenshahidi, M.; Iranshahi, M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus Citrus, upon chronic administration. Iran. J. Basic Med. Sci. 2015, 18, 153–158. [Google Scholar]
- de Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Kazyoba, P.; Viljoen, A. Limonene—A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Malko, M.; Wróblewska, A. The importance of R-(+)-limonene as the raw material for organic syntheses and for organic industry. Chemik 2016, 70, 198–202. [Google Scholar]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of anti-inflammatory activity of Citrus latifolia tanaka essential oil and limonene in experimental mouse models. Evid. Based Complement. Altern. Med. 2013, 2013, 859083. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of peel essential oil composition of four tunisian citrus during fruit maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Romanucci, V.; Zarrelli, A.; Monaco, I.; Lolicato, F.; Spinella, N.; Galati, C.; Grasso, G.; D’Urso, L.; Romeo, M.; et al. Inhibition of Aβ Amyloid Growth and Toxicity by Silybins: The Crucial Role of Stereochemistry. ACS Chem. Neurosci. 2017, 8, 1767–1778. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Locatelli, M.; Navarra, M.; Ventura, C.A.; Wolfram, J.; Carafa, M.; Morittu, V.M.; Britti, D.; Di Marzio, L.; et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf. B Biointerfaces 2013, 112, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.C.; Morato, P.N.; Dos Santos Barbosa, M.; Croda, J.; Sampson, J.; Kong, X.; Konkiewitz, E.C.; Ziff, E.B.; Amaya-Farfan, J.; Kassuya, C.A. Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1 β and protein expression in spinal cord of mice. Life Sci. 2017, 174, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharm. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- d’Alessio, P.A.; Ostan, R.; Bisson, J.F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef]
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Velmurugan, B.; Baskaran, R.; Bharathi Priya, L.; Rajan, V.; Weng, C. Neuroprotective role of phytochemicals. Molecules 2018, 23, 2485. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 1282–1292. [Google Scholar] [CrossRef]
- Available online: https://www.x-mol.com/paper/1337142821747023872 (accessed on 7 July 2021).
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and phenylpropanoids as acetyl- and butyrylcholinesterase inhibitors: A comparative study. Curr. Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Triani, F.; Di Domenico, F.; Barone, E.; Cai, J.; Klein, J.B.; Perluigi, M.; Butterfield, D.A. Poly-ubiquitin profile in Alzheimer disease brain. Neurobiol. Dis. 2018, 118, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lenz, S.; Karsten, P.; Schulz, J.B.; Voigt, A. Drosophila as a screening tool to study human neurodegenerative diseases. J. Neurochem. 2013, 127, 453–460. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.S.; Koo, B.S.; et al. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a drosophila model of Alzheimer’s disease. Biol. Pharm Bull 2020, 43, 409–417. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In vitro neuroprotective potential of terpenes from industrial orange juice by-products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.A.; Tundis, R.; Loizzo, M.R.; Menichini, F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother. Res. 2007, 21, 427–433. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Erdogan Orhan, I.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef]
- Zhou, W.; Fukumoto, S.; Yokogoshi, H. Components of lemon essential oil attenuate dementia induced by scopolamine. Nutr. Neurosci. 2009, 12, 57–64. [Google Scholar] [CrossRef]
- Sadiki, F.Z.; Idrissi, M.E.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L.; Postu, P.A. Tetraclinis articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer’s disease amyloidosis model. Phytomedicine 2019, 56, 57–63. [Google Scholar] [CrossRef]
- Abuhamdah, S.; Abuhamdah, R.; Howes, M.J.; Al-Olimat, S.; Ennaceur, A.; Chazot, P.L. Pharmacological and neuroprotective profile of an essential oil derived from leaves of Aloysia citrodora Palau. J. Pharm Pharm. 2015, 67, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Lomarat, P.; Sripha, K.; Phanthong, P.; Kitphati, W.; Thirapanmethee, K.; Bunyapraphatsara, N. In vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J. Pharm. Sci. (TJPS) 2015, 39, 94–101. [Google Scholar]
- Zéphir, H. Progress in understanding the pathophysiology of multiple sclerosis. Rev. Neurol. 2018, 174, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A. Cakile maritima Scop. extracts inhibit the growth of some bacterial triggers of autoimmune diseases: GC-MS analysis of an inhibitory extract. Pharmacogn. J. 2016, 8, 361–374. [Google Scholar]
- Nel, A.L.; Murhekar, S.; Matthews, B.; White, A.; Cock, I.E. The interactive antimicrobial activity of Terminalia sericea Burch ex DC. leaf extracts and conventional antibiotics against bacterial triggers of selected autoimmune inflammatory diseases. S. Afr. J. Bot. 2020, 133, 17–29. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165771. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharm. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Leussink, V.I.; Husseini, L.; Warnke, C.; Broussalis, E.; Hartung, H.P.; Kieseier, B.C. Symptomatic therapy in multiple sclerosis: The role of cannabinoids in treating spasticity. Ther. Adv. Neurol Disord. 2012, 5, 255–266. [Google Scholar] [CrossRef]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- de Almeida, A.A.C.; Silva, R.O.; Nicolau, L.A.D.; de Brito, T.V.; de Sousa, D.P.; Barbosa, A.L.d.R.; de Freitas, R.M.; Lopes, L.d.S.; Medeiros, J.-V.R.; Ferreira, P.M.P. Physio-pharmacological investigations about the anti-inflammatory and antinociceptive efficacy of (+)-limonene epoxide. Inflammation 2017, 40, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Moskowitz, M. Pathophysiology of migraine. Annu. Rev. Physiol. 2012, 75. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Jaberi, A.R.; Sobhani, Z.; Mosaffa-Jahromi, M.; Iraji, A.; Moayedfard, A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilot randomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 236, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Conde, R.; Corrêa, V.S.; Carmona, F.; Contini, S.H.; Pereira, A.M. Chemical composition and therapeutic effects of Lippia alba (Mill.) N. E. Brown leaves hydro-alcoholic extract in patients with migraine. Phytomedicine 2011, 18, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P.; Lucas, P.; Eades, J.; Hogue, O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J. Headache Pain 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Giourou, E.; Stavropoulou-Deli, A.; Giannakopouou, A.; Kostopoulos, G.; Koutroumanidis, M. Introduction to Epilepsy and Related Brain Disorders. In Cyberphysical Systems for Epilepsy and Related Brain Disorders; Springer: Cham, Switzerland, 2015; pp. 11–38. [Google Scholar] [CrossRef]
- Falco-Walter, J.J.; Scheffer, I.E.; Fisher, R.S. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018, 139, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Johannessen Landmark, C.; Patsalos, P.N. Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev. Neurother. 2010, 10, 119–140. [Google Scholar] [CrossRef]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef]
- Azanchi, T.; Shafaroodi, H.; Asgarpanah, J. Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): Involvment of the GABAergic system. Nat. Prod. Commun. 2014, 9, 1615–1618. [Google Scholar] [PubMed]
- Abbasnejad, M.; Keramat, B.; Esmaeili Mahani, S.; Rezaeezade-Roukerd, M. Effect of hydro-methanolic extract of sour orange flowers, citrus aurantium, on pentylentetrazole induced seizure in male rats. J. Babol Univ. Med. Sci. 2012, 14, 20–28. [Google Scholar]
- Rosa-Falero, C.; Torres-Rodríguez, S.; Jordán, C.; Licier, R.; Santiago, Y.; Toledo, Z.; Santiago, M.; Serrano, K.; Sosa, J.; Ortiz, J.G. Citrus aurantium increases seizure latency to PTZ induced seizures in zebrafish thru NMDA and mGluR’s I and II. Front. Pharmacol. 2015, 5, 284. [Google Scholar] [CrossRef]
- Viana, G.S.; do Vale, T.G.; Silva, C.M.; Matos, F.J. Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (Mill.) N.E. Brown. Biol. Pharm. Bull. 2000, 23, 1314–1317. [Google Scholar] [CrossRef]
- Rakotosaona, R.; Randrianarivo, E.; Rasoanaivo, P.; Nicoletti, M.; Benelli, G.; Maggi, F. Effect of the Leaf Essential Oil from Cinnamosma madagascariensis Danguy on Pentylenetetrazol-induced Seizure in Rats. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Oyemitan, I.A.; Olayera, O.A.; Alabi, A.; Abass, L.A.; Elusiyan, C.A.; Oyedeji, A.O.; Akanmu, M.A. Psychoneuropharmacological activities and chemical composition of essential oil of fresh fruits of Piper guineense (Piperaceae) in mice. J. Ethnopharmacol. 2015, 166, 240–249. [Google Scholar] [CrossRef]
- Pathak, S.; Wanjari, M.M.; Jain, S.K.; Tripathi, M. Evaluation of Antiseizure Activity of Essential Oil from Roots of Angelica archangelica Linn. in Mice. Indian J. Pharm. Sci. 2010, 72, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Alam, O.; Mullick, P.; Verma, S.P.; Gilani, S.J.; Khan, S.A.; Siddiqui, N.; Ahsan, W. Synthesis, anticonvulsant and toxicity screening of newer pyrimidine semicarbazone derivatives. Eur. J. Med. Chem. 2010, 45, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Rajak, H.; Singh Thakur, B.; Singh, A.; Raghuvanshi, K.; Sah, A.K.; Veerasamy, R.; Sharma, P.C.; Singh Pawar, R.; Kharya, M.D. Novel limonene and citral based 2,5-disubstituted-1,3,4-oxadiazoles: A natural product coupled approach to semicarbazones for antiepileptic activity. Bioorg. Med. Chem. Lett. 2013, 23, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, P.A.; Rauch, S.L. Neural circuitry of anxiety: Evidence from structural and functional neuroimaging studies. Psychopharmacol. Bull. 2003, 37, 8–25. [Google Scholar] [PubMed]
- Adwas, A.; Jbireal, J.; Azab, A. Anxiety: Insights into signs, symptoms, etiology, pathophysiology, and treatment. S. Afr. J. Med. Sci. 2019, 2, 80–91. [Google Scholar]
- Nemeroff, C.B. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol. Bull. 2003, 37, 133–146. [Google Scholar]
- Ressler, K.J.; Nemeroff, C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety 2000, 12, 2–19. [Google Scholar] [CrossRef]
- Nash, J.R.; Nutt, D.J. Pharmacotherapy of anxiety. Handb. Exp. Pharm. 2005. [Google Scholar] [CrossRef]
- Lydiard, R.B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 2003, 64 (Suppl. 3), 21–27. [Google Scholar] [PubMed]
- Khakpour, S.; Khosravi, M.; Mashayekhipour, Z.; Hadipour Jahromy, M. Effect of Citrus aurantium L. essential oil and haloperidol on anxiety in male mice. World J. Neurosci. 2014, 04, 427–433. [Google Scholar] [CrossRef]
- Khosravi, M.; Khakpour, S.; Adibi, L.; Hadipour Jahromy, M. A Study of the effect of Citrus aurantium L. essential oil on anxiety and its interaction with gabaergic pathways in male mice. J. Behav. Brain Sci. 2014, 4, 470–476. [Google Scholar] [CrossRef]
- Pultrini Ade, M.; Galindo, L.A.; Costa, M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. 2006, 78, 1720–1725. [Google Scholar] [CrossRef]
- Silva Brum, L.F.; Emanuelli, T.; Souza, D.O.; Elisabetsky, E. Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochem. Res. 2001, 26, 191–194. [Google Scholar] [CrossRef]
- Carvalho-Freitas, M.I.; Costa, M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol. Pharm. Bull. 2002, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Cury, T.; Cassettari, B.; Takahira, R.; Flório, J.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT1A-receptors and reduces cholesterol after repeated oral treatment. BMC Complement. Altern. Med. 2013, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Gargano, A. Essential oils from citrus latifolia and citrus reticulata reduce anxiety and prolong ether sleeping time in mice. Tree For. Sci. Biotechnol. 2008, 2 (Suppl. 1), 121–124. [Google Scholar]
- Lopes Campêlo, L.M.; Gonçalves e Sá, C.; de Almeida, A.A.; da Costa, J.P.; Marques, T.H.; Feitosa, C.M.; Saldanha, G.B.; de Freitas, R.M. Sedative, anxiolytic and antidepressant activities of Citrus limon (Burn) essential oil in mice. Pharmazie 2011, 66, 623–627. [Google Scholar]
- Faturi, C.B.; Leite, J.R.; Alves, P.B.; Canton, A.C.; Teixeira-Silva, F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, M.; Asres, K.; Shibeshi, W. Evaluation of anxiolytic activity of the essential oil of the aerial part of Foeniculum vulgare Miller in mice. BMC Complement. Altern. Med. 2014, 14, 310. [Google Scholar] [CrossRef]
- Saiyudthong, S.; Marsden, C.A. Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother. Res. 2011, 25, 858–862. [Google Scholar] [CrossRef]
- Morrone, L.A.; Rombolà, L.; Pelle, C.; Corasaniti, M.T.; Zappettini, S.; Paudice, P.; Bonanno, G.; Bagetta, G. The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: Implication of monoterpene hydrocarbons. Pharm. Res. 2007, 55, 255–262. [Google Scholar] [CrossRef]
- Bartanusz, V.; Muller, D.; Gaillard, R.C.; Streit, P.; Vutskits, L.; Kiss, J.Z. Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur. J. Neurosci. 2004, 19, 777–782. [Google Scholar] [CrossRef]
- Zhou, W.; Yoshioka, M.; Yokogoshi, H. Sub-Chronic Effects of s-Limonene on Brain Neurotransmitter Levels and Behavior of Rats. J. Nutr. Sci. Vitaminol. 2009, 55, 367–373. [Google Scholar] [CrossRef]
- Rombolà, L.; Tridico, L.; Scuteri, D.; Sakurada, T.; Sakurada, S.; Mizoguchi, H.; Avato, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Bergamot Essential Oil Attenuates Anxiety-Like Behaviour in Rats. Molecules 2017, 22, 614. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Arman, S.; Pour, F.F. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: A randomized controlled clinical trial. Adv. Biomed. Res. 2013, 2. [Google Scholar] [CrossRef]
- Lehrner, J.; Eckersberger, C.; Walla, P.; Pötsch, G.; Deecke, L. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol. Behav. 2000, 71, 83–86. [Google Scholar] [CrossRef]
- Lehrner, J.; Marwinski, G.; Lehr, S.; Johren, P.; Deecke, L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol. Behav. 2005, 86, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.; Coneglian, F.S.; Batista, P.A.; Aragon, D.C.; Angelucci, M.A.; Martinez, E.Z.; Pereira, A.M.S. Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) powdered leaves are effective in treating anxiety symptoms: A phase-2, randomized, placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 242, 112060. [Google Scholar] [CrossRef] [PubMed]
- Soto Vásquez, M.R.; Alvarado García, P. Anxiolytic-like effect of Lippia alba essential oil: A randomized, placebo-controlled trial. J. Complementary Med. Res. 2018. [Google Scholar] [CrossRef]
- Goes, T.C.; Antunes, F.D.; Alves, P.B.; Teixeira-Silva, F. Effect of sweet orange aroma on experimental anxiety in humans. J. Altern. Complement. Med. 2012, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Bakhsha, F.; Mazandarani, M.; Aryaei, M.; Jafari, S.; Bayate, H. Phytochemical and anti-oxidant activity of lavandula angustifolia mill. essential oil on preoperative anxiety in patients undergoing diagnostic curettage. Int. J. Women’s Health Reprod. Sci. 2014, 2, 268–271. [Google Scholar] [CrossRef]
- Braden, R.; Reichow, S.; Halm, M.A. The use of the essential oil lavandin to reduce preoperative anxiety in surgical patients. J. Perianesthesia Nurs. 2009, 24, 348–355. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- d’Alessio, P.A.; Bisson, J.F.; Béné, M.C. Anti-stress effects of d-limonene and its metabolite perillyl alcohol. Rejuvenation Res. 2014, 17, 145–149. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Noort, A.R.; van Zoest, K.P.M.; Weijers, E.M.; Koolwijk, P.; Maracle, C.X.; Novack, D.V.; Siemerink, M.J.; Schlingemann, R.O.; Tak, P.P.; Tas, S.W. NF-κB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. J. Pathol. 2014, 234, 375–385. [Google Scholar] [CrossRef]
- Tang, X.P.; Guo, X.H.; Geng, D.; Weng, L.J. d-Limonene protects PC12 cells against corticosterone-induced neurotoxicity by activating the AMPK pathway. Environ. Toxicol. Pharm. 2019, 70, 103192. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.G.; De Sousa, D.P.; Pimenta, F.C.; Alves, M.F.; De Souza, F.S.; Macedo, R.O.; Cardoso, R.B.; de Morais, L.C.; Melo Diniz Mde, F.; de Almeida, R.N. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharm. Biochem. Behav. 2013, 103, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, Y.; Asle-Rousta, M.; Rahnema, M. Effects of limonene on chronic restraint stress-induced memory impairment and anxiety in male rats. Neurophysiology 2019, 51, 107–113. [Google Scholar] [CrossRef]
- de Almeida, A.A.; Costa, J.P.; de Carvalho, R.B.; de Sousa, D.P.; de Freitas, R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012, 1448, 56–62. [Google Scholar] [CrossRef]
- de Almeida, A.A.C.; de Carvalho, R.B.F.; Silva, O.A.; de Sousa, D.P.; de Freitas, R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014, 118, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Murakami, S.; Matsuura, M.; Hayashi, S.; Koike, K. Anxiolytic effect and tissue distribution of inhaled Alpinia zerumbet essential oil in mice. Nat. Prod. Commun. 2010, 5, 143–146. [Google Scholar] [CrossRef]
- Murakami, S.; Matsuura, M.; Satou, T.; Hayashi, S.; Koike, K. Effects of the essential oil from leaves of Alpinia zerumbet on behavioral alterations in mice. Nat. Prod. Commun. 2009, 4, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Chugh, C. Acute Ischemic Stroke: Management Approach. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2019, 23, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Shalak, L.; Perlman, J.M. Hypoxic–ischemic brain injury in the term infant-current concepts. Early Hum. Dev. 2004, 80, 125–141. [Google Scholar] [CrossRef]
- Hill, M.D. What kind of stroke is it? Clin. Chem. 2008, 54, 1943–1944. [Google Scholar] [CrossRef]
- Muir, K. Medical management of Stroke. J. Neurol. Neurosurg. Psychiatry 2001, 70 (Suppl. 1), I12–I16. [Google Scholar] [CrossRef][Green Version]
- Bayazit, V. Assessment of effects of nanomaterials in fennel (Foeniculum vulgare miller) seed on the cloth dissolution after spontaneously stroke of male mole rat (Spalax leucodon) in Muş, Turkey. Dig. J. Nanomater. Biostruct. 2010, 5, 503–510. [Google Scholar]
- Wang, X.; Li, G.; Shen, W. Protective effects of D-Limonene against transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Exp. Ther. Med. 2018, 15, 699–706. [Google Scholar] [CrossRef]
- Rosand, J.; Schwamm, L.H. Management of Brain Edema Complicating Stroke. J. Intensive Care Med. 2001, 16, 128–141. [Google Scholar] [CrossRef]
- Vakili, A.; Sharifat, S.; Akhavan, M.M.; Bandegi, A.R. Effect of lavender oil (Lavandula angustifolia) on cerebral edema and its possible mechanisms in an experimental model of stroke. Brain Res. 2014, 1548, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Bigdeli, M.R.; Rasoulian, B.; Ghassempour, A.; Mirzajani, F. The neuroprotection effect of pretreatment with olive leaf extract on brain lipidomics in rat stroke model. Phytomed. Int. J. Phytother. Phytopharm. 2012, 19, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Parvardeh, S.; Asl, M.N.; Sadeghnia, H.R.; Ziaee, T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 2007, 14, 621–627. [Google Scholar] [CrossRef]
- Gasche, Y.; Copin, J.C.; Sugawara, T.; Fujimura, M.; Chan, P.H. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 1393–1400. [Google Scholar] [CrossRef]
- Rabiei, Z.; Rafieian-Kopaei, M. Neuroprotective effect of pretreatment with Lavandula officinalis ethanolic extract on blood-brain barrier permeability in a rat stroke model. Asian Pac. J. Trop. Med. 2014, 7s1, S421–S426. [Google Scholar] [CrossRef]
- Sadeghimanesh, A.; Khalaji-Pirbalouty, V.; Lorigooini, Z.; Rafieian-kopaei, M.; Torki, A.; Rabiei, Z. Phytochemical and neuroprotective evaluation of Citrus aurantium essential oil on cerebral ischemia and reperfusion. Bangladesh J. Pharmacol. 2018, 13, 353–361. [Google Scholar] [CrossRef]
- Zhao, H.; Sapolsky, R.M.; Steinberg, G.K. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol. Neurobiol. 2006, 34, 249–270. [Google Scholar] [CrossRef]
- Corasaniti, M.T.; Maiuolo, J.; Maida, S.; Fratto, V.; Navarra, M.; Russo, R.; Amantea, D.; Morrone, L.A.; Bagetta, G. Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro. Br. J. Pharm. 2007, 151, 518–529. [Google Scholar] [CrossRef]
- Amantea, D.; Fratto, V.; Maida, S.; Rotiroti, D.; Ragusa, S.; Nappi, G.; Bagetta, G.; Corasaniti, M.T. Prevention of glutamate accumulation and upregulation of phospho-akt may account for neuroprotection afforded by bergamot essential oil against brain injury induced by focal cerebral ischemia in rat. Int. Rev. Neurobiol. 2009, 85, 389–405. [Google Scholar] [CrossRef]
- Vasilopoulou, C.; Bourtsi, E.; Giaple, S.; Koutelekos, I.; Theofilou, P.; Polikandrioti, M. The Impact of Anxiety and Depression on the Quality of Life of Hemodialysis Patients. Glob. J. Health Sci. 2015, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H.; Andersen, H.F.; Thase, M.E. Escitalopram in the treatment of major depressive disorder: A meta-analysis. Curr. Med. Res. Opin 2009, 25, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Hayakawa, M.; Kasuya, H.; Masuo, Y.; Koike, K. Mouse brain concentrations of α-pinene, limonene, linalool, and 1,8-cineole following inhalation. Flavour Fragr. J. 2017, 32, 36–39. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, Z.Y.; Fan, G.; Ren, J.N.; Yin, K.J.; Pan, S.Y. Antidepressant-like effect of Citrus sinensis (L.) osbeck essential oil and its main component limonene on mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef]
- Piccinelli, A.C.; Santos, J.A.; Konkiewitz, E.C.; Oesterreich, S.A.; Formagio, A.S.; Croda, J.; Ziff, E.B.; Kassuya, C.A. Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, α-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci. 2015, 18, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.S.; Wongwan, J.; Thiwan, K.; Rungseevijitprapa, W.; Klunklin, A.; Kunaviktikul, W. Effects of Litsea cubeba (Lour.) persoon essential oil aromatherapy on mood states and salivary cortisol levels in healthy volunteers. Evid. Based Complement. Altern. Med. 2020, 2020, 4389239. [Google Scholar] [CrossRef]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef]
- Krystal, A.D.; Prather, A.A.; Ashbrook, L.H. The assessment and management of insomnia: An update. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2019, 18, 337–352. [Google Scholar] [CrossRef]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Yang, M.; Ren, G.; Du, Q.; Luo, J.; Zhang, K.; Li, J.; et al. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabian, F.; Mirabzadeh Ardakani, M.; Rahmani, K.; Azadi, N.A.; Alemohammad, Z.B.; Bidaki, R.; Karimi, M.; Emtiazy, M.; Hashempur, M.H. Aloysia citriodora Palau (lemon verbena) for insomnia patients: A randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother. Res. 2019, 33, 350–359. [Google Scholar] [CrossRef] [PubMed]
| Plant | Limonene Abundance % |
|---|---|
| Citrus medica L. cv. Diamante peel | 15.20 |
| Piper nigrum | 25.41 |
| Aloysia citrodora Palau | 13.6–20.1 |
| Elettaria cardamomum Seeds | 4.35 |
| Tetraclinis articulata | 7.34 |
| Cakile maritima Scop | 0.7 |
| Lippia alba | 6.8 |
| Pinus roxburghii | 0.9 |
| Artemisia dracunculus L. | 12.4 |
| Citrus aurantium L. | 98.66 |
| Cinnamosma madagascariensis Danguy | 12 |
| Piper guineense | 5.8 |
| Terminalia sericea | 0.19 |
| Pimpinella anisum L. | 1.2 |
| Citrus latifolia | 58 |
| Citrus reticulate | 90 |
| Citrus limon | 52.7 |
| Citrus sinensis | 97 |
| Citrus bergamia | 39.6 |
| Lavandula angustifolia | 19 |
| Aloysia polystachya | 20.4 |
| Lavandula angustifolia Mill | 19.6 |
| Alpinia zerumbet | 8.7 |
| Nigella sativa | 4.3 |
| Citrus sinensis (L.) Osbeck | 92.81 |
| Schinus terebinthifolius | 5–24 |
| Compound Anshen oil * | 24.07 |
| Aloysia citriodora Palau | 6.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. https://doi.org/10.3390/molecules26154535
Eddin LB, Jha NK, Meeran MFN, Kesari KK, Beiram R, Ojha S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules. 2021; 26(15):4535. https://doi.org/10.3390/molecules26154535
Chicago/Turabian StyleEddin, Lujain Bader, Niraj Kumar Jha, M. F. Nagoor Meeran, Kavindra Kumar Kesari, Rami Beiram, and Shreesh Ojha. 2021. "Neuroprotective Potential of Limonene and Limonene Containing Natural Products" Molecules 26, no. 15: 4535. https://doi.org/10.3390/molecules26154535
APA StyleEddin, L. B., Jha, N. K., Meeran, M. F. N., Kesari, K. K., Beiram, R., & Ojha, S. (2021). Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules, 26(15), 4535. https://doi.org/10.3390/molecules26154535









