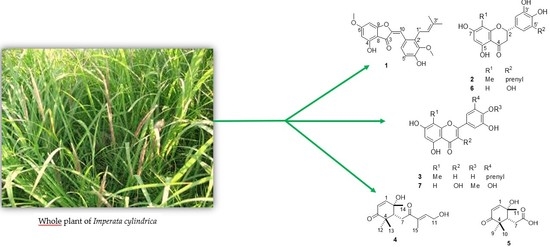
Prenylated Flavonoids and C-15 Isoprenoid Analogues with Antibacterial Properties from the Whole Plant of Imperata cylindrica (L.) Raeusch (Gramineae)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Purification
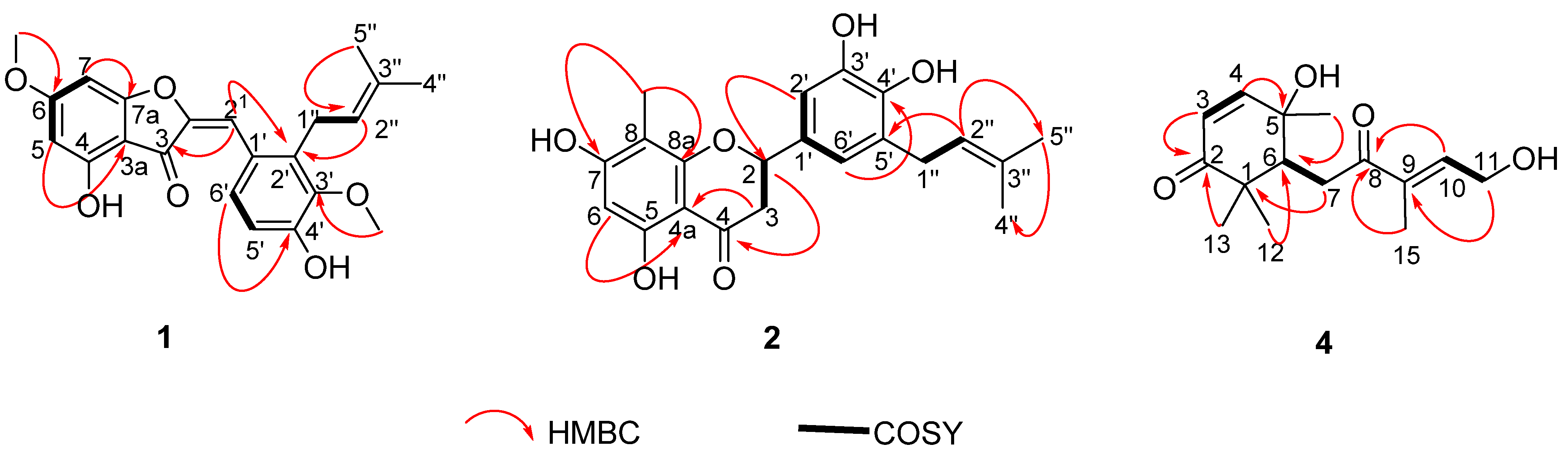
4.4. Cylindraucine (1)
4.5. Cylindricine A (2)
4.6. Cylindricine B (3)
4.7. Cylindracid A (4)
4.8. Cylindracid B (5)
4.9. Bioactivity: INT Colorimetric Assay for Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determinations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dye, C. Infectious diseases in a new era of health and development. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 1645. [Google Scholar] [CrossRef] [Green Version]
- Centres for Disease Control and Prevention (CDC). National Diabetes Statistics Report. 2017. Available online: www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report/ (accessed on 3 September 2020).
- Kuete, V.; Metuno, R.; Ngameni, B.; Tsafack, A.M.; Ngandeu, F.; Fotso, G.W.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial activity of the methanolic extracts and compounds from Treculia obovoidea (Moraceae). J. Ethnopharmacol. 2007, 112, 531–536. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fankam, A.G.; Kuiate, J.R.; Kuete, V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant Gram-negative phenotypes. BMC Complement. Altern. Med. 2014, 14, 241. [Google Scholar] [CrossRef] [Green Version]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC Complement. Altern. Med. 2016, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, A.M.; Suharti, A.; Sagala, P.S.; Hibani, H.; Van-Noordwijk, M. Fire management on Imperata grasslands as part of agroforestry development in Indonesia. Agrofor. Syst. 1997, 36, 203–217. [Google Scholar] [CrossRef]
- Peet, N.B.; Watkinson, A.R.; Bell, D.J.; Sharma, U.R. The conservation management of Imperata cylindrica grassland in Nepal with fire and cutting: An experimental approach. J. Appl. Ecol. 1999, 36, 374–387. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Han, K.-M.; Song, M.-C.; Lee, D.-G.; Rho, Y.-D.; Baek, N. A new lignan glycoside from the rhizomes of Imperata cylindrica. J. Asian Nat. Prod. Res. 2008, 10, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Labrada, R. Weed Management for Developing Countries. In Addendum 1. Plant Production and Protection Papers N°120; FAO: Rome, Italy, 2003. [Google Scholar]
- Kuusipalo, J.; Ådjers, G.; Jafarsidik, Y.; Otsamo, A.; Tuomela, K.; Vuokko, R. Restoration of natural vegetation in degraded Imperata cylindrica grassland: Understory development in forest plantations. J. Veget. Sci. 1995, 6, 205–210. [Google Scholar] [CrossRef]
- Macdicken, K.G.; Hairiah, K.; Otsamo, A.; Duguma, B.; Majid, N.M. Shade-based control of Imperata cylindrica: Tree fallows and cover crops. Agrofor. Syst. 1996, 36, 131–149. [Google Scholar] [CrossRef]
- Terry, P.J.; Adjers, G.; Akobundu, I.O.; Anoka, A.U.; Drilling, M.E.; Tjitrosemito, S. Herbicides and mechanical control of Imperata cylindrica as a first step in grassland rehabilitation. Agrofor. Syst. 1997, 36, 151–179. [Google Scholar] [CrossRef]
- Pinilla, V.; Luu, B. Isolation and partial characterization of immunostimulating polysaccharides from Imperata cylindrica. Planta Med. 1999, 65, 549–552. [Google Scholar] [CrossRef]
- Park, J.H. Medicinal Plants of Korea; Shinil Publishing Co.: Seoul, Korea, 2004; p. 101. [Google Scholar]
- Badawe, G.; Fankam, A.G.; Mbaveng, A.T.; Wamba, B.E.N.; Nayim, P.; Kuete, V. Cinnamomum zeylanicum, Dichrostachys glomerata and three other plants had anti-staphylococcal and antibioticmodifying activity against drug-resistant phenotypes. Investig. Med. Chem. Pharmacol. 2019, 2, 1–25. [Google Scholar]
- Nayim, P.; Mbaven, A.T.; Sanjukta, M.; Rikesh, J.; Kuete, V.; Sudhir, K. CD24 gene inhibition and TIMP-4 gene upregulation by Imperata cylindrica’s root extract prevents metastasis of CaSki cells via inhibiting PI3K/Akt/snail signaling pathway and blocking EMT. J. Ethnopharmacol. 2021, 275, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Nayim, P.; Mbaveng, A.T.; Ntyam, A.M.; Kuete, V. A botanical from the antiproliferative Cameroonian spice, Imperata cylindrica is safe at lower doses, as demonstrated by oral acute and sub-chronic toxicity screenings. BMC Complement. Med. Ther. 2020, 20, 273. [Google Scholar] [CrossRef]
- Liu, R.H.; Chen, S.S.; Ren, G.; Shao, F.; Huang, H.L. Phenolic compounds from roots of Imperata cylindrica var. major. Chin. Herb. Med. 2013, 5, 240–243. [Google Scholar]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J. Nat. Prod. 1994, 57, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J. Nat. Prod. 1995, 58, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Abdel-Lateff, A.; Fouad, M.A.; Ibrahim, S.R.; Elkhayat, E.S.; Okino, T. Chemical composition and hepato-protective activity of Imperata cylindrica Beauv. Pharmacogn. Mag. 2009, 5, 28–36. [Google Scholar]
- Mpetga, J.D.S.; He, H.-P.; Hao, X.-J.; Leng, Y.; Tane, P. Further cycloartane and friedelane triterpenoids from the leaves of Caloncoba glauca. Phytochem. Lett. 2014, 7, 52–56. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Fonkeng, L.S.; Kopa, T.K.; Tala, M.F.; Wabo, H.K.; Tume, C.B.; Tane, P.; Kuiate, J.-R. Antibacterial activity of ethanolic extract and compounds from fruits of Tectona grandis (Verbenaceae). BMC Altern. Complement. Med. 2015, 15, 265. [Google Scholar] [CrossRef] [Green Version]
- Atontsa, B.C.K.; Bitchagno, G.T.M.; Mpetga, J.D.S.; Wamba, B.E.N.; Nayim, P.; Tchuenguem, R.T.; Ndontsa, B.L.; Koagne, R.R.; Opatz, T.; Kuete, V.; et al. Caffeate and piperidine-3-ol derivatives from the stem bark of Cassia sieberiana. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Damen, F.; Demgne, O.M.F.; Bitchagno, G.T.M.; Celik, I.; Mpetga, J.D.S.; Tankeo, S.B.; Opatz, T.; Kuete, V.; Tane, P. A new polyketide from the bark of Hypericum roeperianum Schimp. (Hypericaceae). Nat. Prod. Res. 2019, 35, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Nganou, B.K.; Mbaveng, A.T.; Fobofou, S.A.T.; Fankam, A.G.; Bitchagno, G.T.M.; Mpetga, J.D.S.; Wessjohann, L.A.; Kuete, V.; Efferth, T.; Tane, P. Furoquinolines and dihydrooxazole alkaloids with cytotoxic activity from the stem bark of Araliopsis soyauxii. Fitoterapia 2019, 133, 193–199. [Google Scholar] [CrossRef]
- Mbaveng, T.A.; Chi, F.G.; Bonsou, I.N.; Ombito, J.O.; Yeboah, S.O.; Kuete, V.; Efferth, T. Cytotoxic phytochemicals from the crude extract of Tetrapleura tetraptera fruits towards multi-factorial drug resistant cancer cells. J. Ethnopharmacol. 2021, 267, 113–632. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Chari, V.M. Carbon-13 NMR spectroscopy of flavonoids. In The Flavonoids: Advances in Research; Harborne, J.B., Mabry, T.J., Eds.; Chapman and Hall: London, UK, 1982; pp. 19–134. [Google Scholar]
- Markham, K.R.; Geiger, H. 1H nuclear magnetic resonance spectroscopy of flavonoids and their glycosides in hexadeuterodimethylsulfoxide. In The Flavonoids Advances in Research Since 1986; Harborne, J.B., Ed.; Chapman and Hall: London, UK, 1994; pp. 441–497. [Google Scholar]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef]
- Fomum, Z.T.; Ayafor, J.F.; Mbafor, J.T.; Mbi, C.M. Erythrina Studies. Part 2.1 Structures of Three Novel Prenylated Antibacterial Flavanones, Sigmoidins A-C, from Erythrina sigmoidea Hua. J. Chem. Soc. Perkin Trans. 1986, 1, 33–37. [Google Scholar] [CrossRef]
- Lai, Y.; Zeng, H.; He, M.; Qian, H.; Wu, Z.; Luo, Z.; Xue, Y.; Yao, G.; Zhang, Y. 6,8-Di-C-methyl-flavonoids with neuroprotective activities from Rhododendron fortune. Fitoterapia 2016, 112, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bitchagno, G.T.M.; Tankeo, S.B.; Tsopmo, A.; Mpetga, J.D.S.; Tchinda, A.T.; Fobofou, S.A.T.; Nkuete, A.H.L.; Wessjohann, L.A.; Kuete, V.; Tane, P. Ericoside, a new antibacterial biflavoniod from Erica mannii (Ericaceae). Fitoterapia 2016, 109, 206–211. [Google Scholar] [CrossRef]
- Dawidar, A.M.; Jakupovic, J.; Abdel-Mogib, M.; Mashaly, I.A. Prenylstilbenes and prenylflavanones from Schoenus nigricans. Phytochemistry 1994, 36, 803–806. [Google Scholar] [CrossRef]
- Yan, W.; Zhao, S.; Gu, C.; Tian, K.; Wang, Z.; Liu, F.; Ye, Y. Antifungal meroterpenes and dioxolanone derivatives from plant-associated endophytic fungus Phyllosticta sp. WGHL2. Fitoterapia 2021, 148, 104778. [Google Scholar] [CrossRef] [PubMed]
- Milborrow, B.V. The conformation of abscisic acid by N.M.R. and a revision of the proposed mechanism for cyclization during its biosynthesis. Biochem. J. 1984, 220, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Que, S.; Yang, X.; Wang, B.; Qiao, L.; Zhao, Y. Isolation and identification of metabolites from dihydromyricetin. Magn. Reson. Chem. 2007, 45, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, A.O.; Oladosu, I.A.; Ali, M.S.; Ahmed, Z. Flavonoids from the roots of Dioclea reflexa (Hook f.). Bull. Chem. Soc. Ethiop. 2015, 29, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, J.Z.; Chan, Y.W.; Ho, W.S. Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica: Aerial Part Ethyl Acetate Extract. Molecules 2018, 23, 1807. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.; Braga, D.C.P.; Macedo, M.; Fátima, M.; da Silva, G.; Ferreira, A.; Fernandes, J.; Vieira, P. Phytochemistry of Trattinnickia burserifolia, T. rhoifolia, and Dacryodes hopkinsii: Chemosystematic implications. J. Braz. Chem. Soc. 2004, 15, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Sakai, Y.; Shinozaki, J.; Takano, A.; Masuda, K.; Nakane, T. Three novel 14-epiarborane triterpenoids from Imperata cylindrica Beauv. var. major. Phytochem. Lett. 2018, 26, 74–77. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Mic. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Nguemeving, J.R.; Azebaze, A.G.; Kuete, V.; Eric Carly, N.N.; Beng, V.P.; Meyer, M.; Blond, A.; Bodo, B.; Nkengfack, A.E. Laurentixanthones A and B, antimicrobial xanthones from Vismia laurentii. Phytochemistry 2006, 67, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 | 4 | 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δC | δH | HMBC | δC | δH | HMBC | δC | δH | HMBC | |
| 1 | 46.8 | - | - | 46.6 | - | - | |||
| 2 | 152.9 | - | - | 205.8 | - | - | 205.7 | - | - |
| 21 | 109.7 | 6.90 s | 3, 3′, 2′, 6′, 1′ | ||||||
| 3 | 182.5 | - | - | 124.1 | 5.82 d (10.3) | 1, 6, 4, 14 | 125.4 | 5.84 d (10.3) | 6, 4 |
| 3a | 105.5 | - | - | ||||||
| 4 | 170.4 | - | - | 156.2 | 6.78 d (10.3) | 3, 5, 7, 14 | 157.4 | 6.80 d (10.3) | 3, 5 |
| 5 | 97.7 | 6.13 d (2.1) | 4, 7, 8 | 71.9 | - | - | 71.4 | - | - |
| 6 | 169.2 | - | - | 49.6 | 2.86 m | 8, 6, 4 | 51.3 | 2.87 m | 6, 4, 10 |
| 7 | 90.2 | 6.38 d (2.1) | 5, 6, 9 | 33.3 | 2.99 dd (15.9, 6.1) 2.88 dd (15.9, 6.1) | 8, 6, 5, 4 | 31.5 | 2.63 dd (15.7, 6.3) 2.42 dd (15.7, 6.3) | 8, 6, 5, 4 8, 6, 5, 4 |
| 7a | 159.3 | - | - | ||||||
| 8 | 203.4 | - | - | 177.5 | - | - | |||
| 9 | 137.5 | - | - | 24.7 | 1.11 s | 3, 5, 4 | |||
| 10 | 140.9 | 6.80 d (3.4) | 8, 9, 15 | 21.7 | 1.05s | 3, 5, 4, 9 | |||
| 11 | 58.9 | 4.36 d (5.7) | 8, 10, 9,15 | 23.1 | 1.32 s | 1, 6, 5 | |||
| 12 | 25.0 | 1.01 s | 3, 5, 4, 13 | ||||||
| 13 | 21.8 | 1.07 s | 3, 5, 4, 12 | ||||||
| 14 | 23.0 | 1.34 s | 1, 6, 5 | ||||||
| 15 | 10.9 | 1.75 s | 8, 10, 9 | ||||||
| 1′ | 124.0 | - | - | ||||||
| 2′ | 138.3 | - | - | ||||||
| 3′ | 148.1 | - | - | ||||||
| 4′ | 147.0 | - | - | ||||||
| 5′ | 115.5 | 6.83 d (8.2) | 2, 4′, 2′, 1′ | ||||||
| 6′ | 129.5 | 7.93 d (8.2) | 2, 4′, 2′, 10 | ||||||
| 1″ | 26.3 | 3.55 d (6.7) | 4″, 5″, 4′, 2′ | ||||||
| 2″ | 124.7 | 5.09 m | 4″, 5″ | ||||||
| 3″ | 132.9 | - | - | ||||||
| 4″ | 25.9 | 1.70 s | 3″, 2″, 5″ | ||||||
| 5″ | 18.2 | 1.90 s | 3″, 2″, 4″ | ||||||
| 3ʹ-OMe | 61.2 | 3.78 s | 3′ | ||||||
| 6-OMe | 56.5 | 3.90 s | 6 | ||||||
| Position | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| δC | δH | HMBC | δC | δH | HMBC | |
| 2 | 80.5 | 5.23 dd (12.5, 3.1) | 4, 8a, 1′, 6′, 2′ | 146.1 | - | - |
| 3 | 44.1 | 3.04 dd (17.0, 12.5) 2.70 dd (17.1, 3.1) | 4, 1′, 2, 4, 1′, 5a | 113.3 | 6.55 s | 4, 2, 5′ |
| 4 | 197.7 | - | - | 183.9 | - | - |
| 4a | 103.0 | - | - | 104.4 | - | - |
| 5 | 162.4 | - | - | 166.5 | - | - |
| 6 | 95.2 | 5.95 s | 4, 5, 7, 8, 5a | 91.5 | 6. 28 s | 8, 5a, 5, 7 |
| 7 | 166.0 | - | - | 167.7 | - | - |
| 8 | 105.2 | - | - | 107.3 | - | - |
| 8a | 162.1 | - | - | 156.6 | - | - |
| 1′ | 130.9 | - | - | 124.7 | - | - |
| 2′ | 111.7 | 6.78 d (2.2) | 3′, 4′, 6′, 2, 1″ | 116.1 | 7.33 d (2.2) | 3′ |
| 3′ | 145.8 | - | - | 147.0 | - | - |
| 4′ | 144.4 | - | - | 146.7 | - | - |
| 5′ | 129.5 | - | - | 129.8 | - | - |
| 6′ | 119.7 | 6.69 d (2.2) | 4′, 2′, 2, 1″ | 126.3 | 7.13 d (2.2) | 1″, 2, 2′, 3′ |
| 1″ | 29.3 | 3.32 m | 4′, 3″, 5′, 2″, 6′ | 29.9 | 3.34 m | 3″, 5′, 6′, 3′ |
| 2″ | 123.7 | 5.33 m | 4″, 3″ | 123.6 | 5.39 d (2.2) | 5″, 4″, 1″ |
| 3″ | 132.8 | - | - | 133.4 | - | - |
| 4″ | 25.6 | 1.73 s | 3″, 2″ | 26.1 | 1.80 s | 3″, 2″, 5″ |
| 5″ | 17.2 | 1.74 s | 3″, 2″ | 17.9 | 1.78 s | 3″, 2″, 4″ |
| 1‴ | 6.9 | 1.96 s | 7, 8a, 8 | 7.4 | 2.3 s | 7, 8a, 15 |
| Bacterial Strains | Tested Samples | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Extract | 1 | 2 | 3 | 4 | 5 | 6 | CHL | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. coli | ||||||||||||||||
| ATCC 8739 | 512 | - | 128 | - | 128 | - | 128 | - | 1024 | - | 512 | - | 128 | - | 64 | 512 |
| AG102 | 1024 | - | 1024 | - | 128 | - | 256 | - | 512 | - | 256 | - | 64 | - | 64 | 512 |
| E. aerogenes | ||||||||||||||||
| ATCC 13048 | 256 | - | 1024 | - | 128 | - | 64 | - | 512 | - | 64 | - | 128 | - | 128 | 128 |
| EA 27 | 512 | - | 512 | - | 128 | - | 128 | - | 512 | - | 128 | - | 128 | - | 64 | 256 |
| S. aureus | ||||||||||||||||
| ATCC 25923 | 128 | - | 1024 | - | 32 | - | 128 | - | 512 | - | 256 | - | 32 | - | 32 | - |
| MRSA 1 | 256 | - | 512 | - | 16 | - | 64 | - | 512 | - | 128 | - | 128 | - | 64 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nago, R.D.T.; Nayim, P.; Mbaveng, A.T.; Mpetga, J.D.S.; Bitchagno, G.T.M.; Garandi, B.; Tane, P.; Lenta, B.N.; Sewald, N.; Tene, M.; et al. Prenylated Flavonoids and C-15 Isoprenoid Analogues with Antibacterial Properties from the Whole Plant of Imperata cylindrica (L.) Raeusch (Gramineae). Molecules 2021, 26, 4717. https://doi.org/10.3390/molecules26164717
Nago RDT, Nayim P, Mbaveng AT, Mpetga JDS, Bitchagno GTM, Garandi B, Tane P, Lenta BN, Sewald N, Tene M, et al. Prenylated Flavonoids and C-15 Isoprenoid Analogues with Antibacterial Properties from the Whole Plant of Imperata cylindrica (L.) Raeusch (Gramineae). Molecules. 2021; 26(16):4717. https://doi.org/10.3390/molecules26164717
Chicago/Turabian StyleNago, Romeo D. Tadjouate, Paul Nayim, Armelle T. Mbaveng, James D. Simo Mpetga, Gabin T. Mbahbou Bitchagno, Badawe Garandi, Pierre Tane, Bruno N. Lenta, Norbert Sewald, Mathieu Tene, and et al. 2021. "Prenylated Flavonoids and C-15 Isoprenoid Analogues with Antibacterial Properties from the Whole Plant of Imperata cylindrica (L.) Raeusch (Gramineae)" Molecules 26, no. 16: 4717. https://doi.org/10.3390/molecules26164717
APA StyleNago, R. D. T., Nayim, P., Mbaveng, A. T., Mpetga, J. D. S., Bitchagno, G. T. M., Garandi, B., Tane, P., Lenta, B. N., Sewald, N., Tene, M., Kuete, V., & Ngouela, A. S. (2021). Prenylated Flavonoids and C-15 Isoprenoid Analogues with Antibacterial Properties from the Whole Plant of Imperata cylindrica (L.) Raeusch (Gramineae). Molecules, 26(16), 4717. https://doi.org/10.3390/molecules26164717