Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Propolis Samples
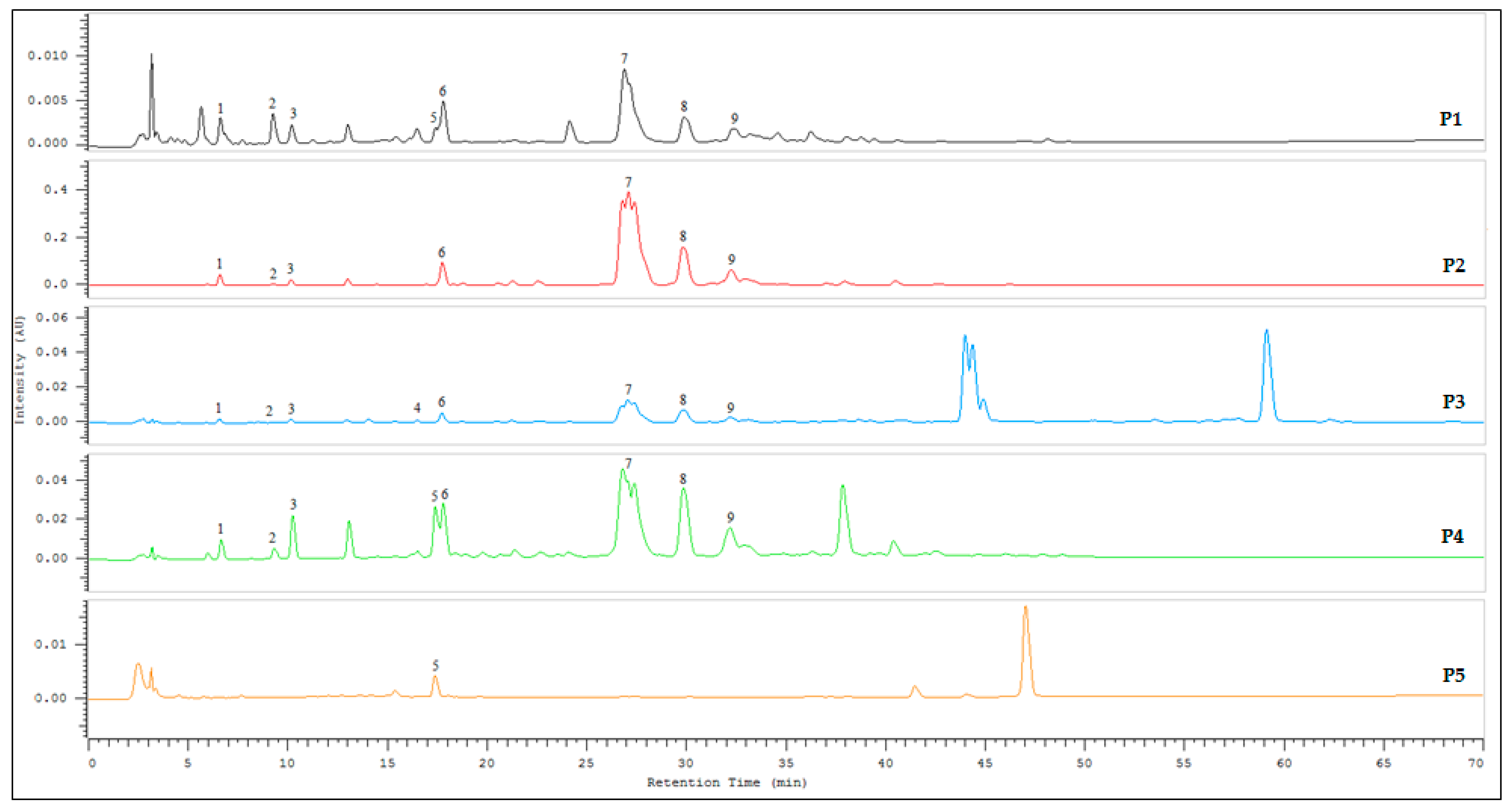
2.2. HPLC Analysis
2.2.1. Method Development and Calibration Curves
2.2.2. Method Validation
2.2.3. Estimation of Marker Compounds
2.3. Bioactive Compounds and Antioxidant Activity
2.4. Antibacterial Activity of the Propolis Sample
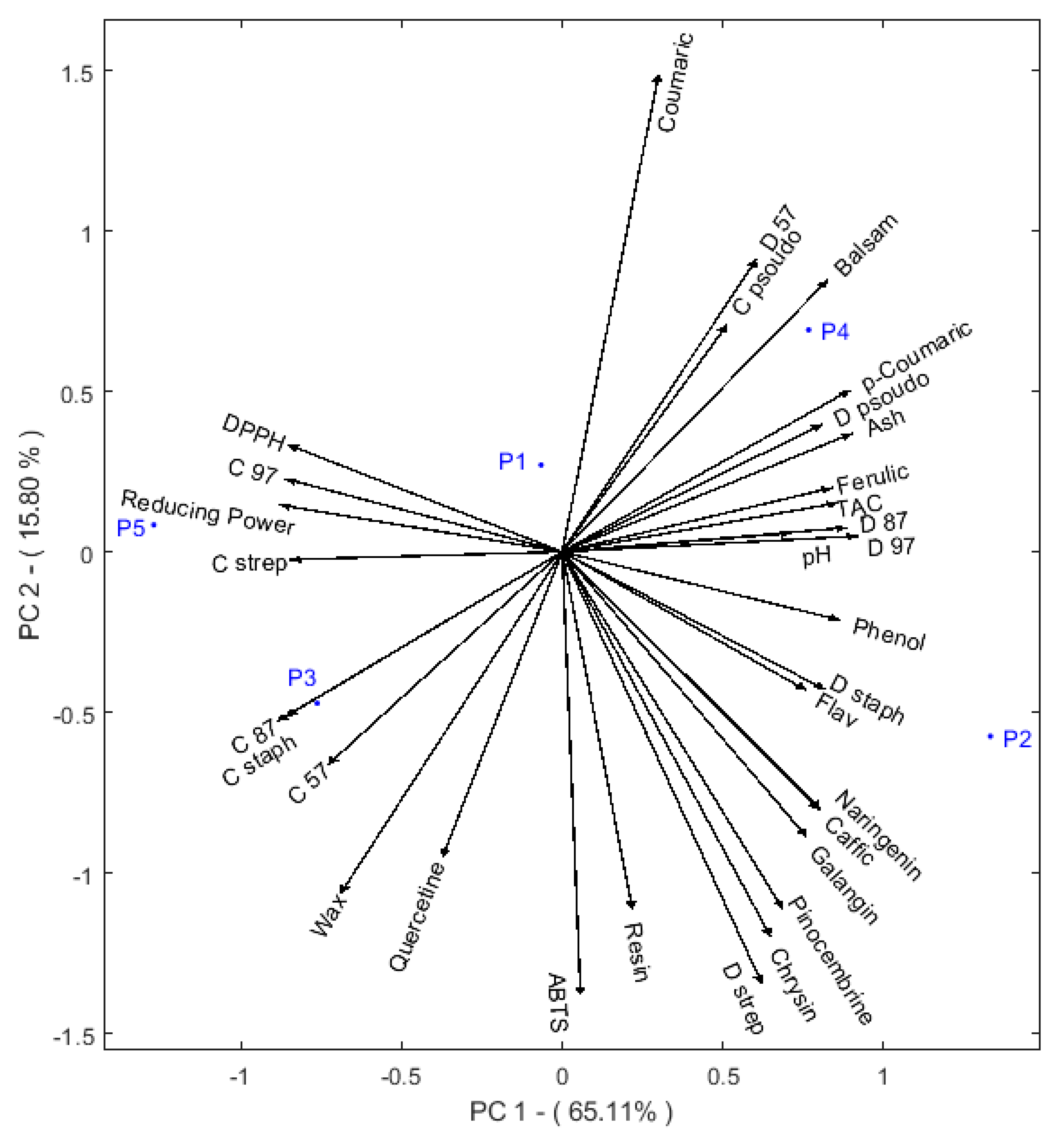
2.5. Multivariable Analysis
3. Materials and Methods
3.1. Source of Propolis
3.2. Physicochemical Characterization of Propolis
3.3. Antioxidant Activities of Propolis Samples
3.4. Antioxidant Potential of Propolis Samples
3.5. HPLC Analysis
3.5.1. Sample Preparation
3.5.2. Method Development
3.5.3. Calibration Curve of Standards
3.5.4. Method Validation
3.6. Bacterial Strain and Inoculum Standardization
3.7. Agar Well Diffusion (AWD) Assay
3.8. Determination of the Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Kamaly, O.M.A.; Jawhari, F.Z.; Imtara, H.; Grafov, A.; Bousta, D. The Potential of Parsley Polyphenols and Their Antioxidant Capacity to Help in the Treatment of Depression and Anxiety: An In Vivo Subacute Study. Molecules 2021, 26, 2009. [Google Scholar] [CrossRef]
- Tabti, L.; Dib, M.E.A.; Gaouar, N.; Samira, B.; Tabti, B. Antioxidant and Antifungal Activity of Extracts of the Aerial Parts of Thymus Capitatus (L.) Hoffmanns Against Four Phytopathogenic Fungi of Citrus Sinensis. Jundishapur. J. Nat. Pharm. Prod. 2014, 9, 49–54. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum Vulgare L. Essential Oil. Evid. Based Complementary Altern. Med. 2018, 2018, e7842583. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Daraghmeh, J.; Imtara, H. In Vitro Evaluation of Palestinian Propolis as a Natural Product with Antioxidant Properties and Antimicrobial Activity against Multidrug-Resistant Clinical Isolates. J. Food Qual. 2020, 2020, e8861395. [Google Scholar] [CrossRef]
- Imtara, H.; Kmail, A.; Touzani, S.; Khader, M.; Hamarshi, H.; Saad, B.; Lyoussi, B. Chemical Analysis and Cytotoxic and Cytostatic Effects of Twelve Honey Samples Collected from Different Regions in Morocco and Palestine. Evid. Based Complementary Altern. Med. 2019, 2019, e8768210. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid. Based Complementary Altern. Med. 2013, 2013, e697390. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-Type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015, 10, 1934578X1501001117. [Google Scholar] [CrossRef]
- Paulino, N.; Teixeira, C.; Martins, R.; Scremin, A.; Dirsch, V.M.; Vollmar, A.M.; Abreu, S.R.; de Castro, S.L.; Marcucci, M.C. Evaluation of the Analgesic and Anti-Inflammatory Effects of a Brazilian Green Propolis. Planta Med. 2006, 72, 899–906. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent Progress in Pharmacological Research of Propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, Antifungal and Antiviral Activity of Propolis of Different Geographic Origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-Acetylcholinesterase, Antidiabetic, Anti-Inflammatory, Antityrosinase and Antixanthine Oxidase Activities of Moroccan Propolis. Int. J. Food Sci. Technol. 2016, 51, 1762–1773. [Google Scholar] [CrossRef]
- Seo, K.W.; Park, M.; Song, Y.J.; Kim, S.-J.; Yoon, K.R. The Protective Effects of Propolis on Hepatic Injury and Its Mechanism. Phytother. Res. 2003, 17, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Al-Hariri, M.T. Propolis and Its Direct and Indirect Hypoglycemic Effect. J. Family Community Med. 2011, 18, 152–154. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Effects of Brazilian Green Propolis on Proteinuria and Renal Function in Patients with Chronic Kidney Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Nephrol. 2019, 20, 140. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive Properties of Honey with Propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Ministry of Health. National Health Surveillance Agency (ANVISA). Resolution n° 899, of May 29, 2003: Guide to the Validation of Analytical and Bioanalytical Methods. Available online: http://portal.anvisa.gov.br/wps/wcm/connect/4983b0004745975da005f43fbc4c6735/RE_899_2003_Determina+a+publica%C3%A7%C3%A3o+do+Guia+para+valida%C3%A7%C3%A3o+de+m%C3%A9todos+anal%C3%ADticos+e+bioanal%C3%ADticos.pdf?MOD=AJPERES (accessed on 10 July 2021).
- Osés, S.M.; Melgosa, L.; Pascual-Maté, A.; Fernández-Muiño, M.A.; Sancho, M.T. Design of a Food Product Composed of Honey and Propolis. J. Apic. Res. 2015, 54, 461–467. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef]
- Popova, M.; Lyoussi, B.; Aazza, S.; Antunes, D.; Bankova, V.; Miguel, G. Antioxidant and α-Glucosidase Inhibitory Properties and Chemical Profiles of Moroccan Propolis. Nat. Prod. Commun. 2015, 10, 1934578X1501001139. [Google Scholar] [CrossRef]
- Touzani, S.; Embaslat, W.; Imtara, H.; Kmail, A.; Kadan, S.; Zaid, H.; ElArabi, I.; Badiaa, L.; Saad, B. In Vitro Evaluation of the Potential Use of Propolis as a Multitarget Therapeutic Product: Physicochemical Properties, Chemical Composition, and Immunomodulatory, Antibacterial, and Anticancer Properties. BioMed Res. Int. 2019, 2019, e4836378. [Google Scholar] [CrossRef]
- Cunha, I.B.S.; Sawaya, A.C.H.F.; Caetano, F.M.; Shimizu, M.T.; Marcucci, M.C.; Drezza, F.T.; Povia, G.S.; Carvalho, P.D.O. Factors That Influence the Yield and Composition of Brazilian Propolis Extracts. J. Braz. Chem. Soc. 2004, 15, 964–970. [Google Scholar] [CrossRef]
- Touzani, S.; Al-Waili, N.; Menyiy, N.E.; Filipic, B.; Pereyra, A.; Arabi, I.E.; Al-Waili, W.; Lyoussi, B. Chemical Analysis and Antioxidant Content of Various Propolis Samples Collected from Different Regions and Their Impact on Antimicrobial Activities. Asian Pac. J. Trop. Med. 2018, 11, 436. [Google Scholar] [CrossRef]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.d.A.; Silva, J.R.; da Silva, D.F.; da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical Characterization and Biological Activity of Six Different Extracts of Propolis through Conventional Methods and Supercritical Extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Watson, D.G.; Peyfoon, E.; Zheng, L.; Lu, D.; Seidel, V.; Johnston, B.; Parkinson, J.A.; Fearnley, J. Application of Principal Components Analysis to 1H-NMR Data Obtained from Propolis Samples of Different Geographical Origin. Phytochem. Anal. 2006, 17, 323–331. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Adgaba, N.; Bayaqoob, N.I.M.; Al-Khazim, A.; Simoneit, B.R.T.; El-Mubarak, A.H.; Al-Mutlaq, K.F. Characteristics and Chemical Compositions of Propolis from Ethiopia. SpringerPlus 2014, 3, 253. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran Endod. J. 2018, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Bankova, V.; Popova, M.; Neto, L.; Faleiro, M.L.; Miguel, M.d.G. Moroccan Propolis: A Natural Antioxidant, Antibacterial, and Antibiofilm against Staphylococcus aureus with No Induction of Resistance after Continuous Exposure. Evid. Based Complementary Altern. Med. 2018, 2018, e9759240. [Google Scholar] [CrossRef] [PubMed]
- Asem, N.; Gapar, N.A.A.; Hapit, N.H.A.; Omar, E.A. Correlation between Total Phenolic and Flavonoid Contents with Antioxidant Activity of Malaysian Stingless Bee Propolis Extract. J. Apic. Res. 2020, 59, 437–442. [Google Scholar] [CrossRef]
- Wang, X.; Sankarapandian, K.; Cheng, Y.; Woo, S.O.; Kwon, H.W.; Perumalsamy, H.; Ahn, Y.-J. Relationship between Total Phenolic Contents and Biological Properties of Propolis from 20 Different Regions in South Korea. BMC Complement Altern. Med. 2016, 16, 65. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; dos Santos Arruda, R.E.; da Cruz Almeida, E.T.; dos Santos Oliveira, J.M.; Basílio-Júnior, I.D.; Celerino de Moraes Porto, I.C.; Rodrigues Sabino, A.; Tonholo, J.; Gray, A.; Ebel, R.E.; et al. Comprehensive Multivariate Correlations between Climatic Effect, Metabolite-Profile, Antioxidant Capacity and Antibacterial Activity of Brazilian Red Propolis Metabolites during Seasonal Study. Sci. Rep. 2019, 9, 18293. [Google Scholar] [CrossRef]
- Priya, S.H.; Prakasan, N.; Purushothaman, J. Antioxidant Activity, Phenolic-Flavonoid Content and High-Performance Liquid Chromatography Profiling of Three Different Variants of Syzygium Cumini Seeds: A Comparative Study. J. Intercult. Ethnopharmacol. 2017, 6, 107–114. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is There a Potential for the Development of New Drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Nadia, B.H.; Wided, K.; Kheira, B.; Hassiba, R.; Lamia, B.; Rhouati, S.; Alyane, M.; Zellagui, A.; Lahouel, M. Disruption of Mitochondrial Membrane Potential by Ferulenol and Restoration by Propolis Extract: Antiapoptotic Role of Propolis. Acta Biol. Hung. 2009, 60, 385–398. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Svabić-Vlahović, M. In Vitro Antimicrobial Activity of Propolis and Synergism between Propolis and Antimicrobial Drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug. Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Du Toit, K.; Buthelezi, S.; Bodenstein, J. Anti-Inflammatory and Antibacterial Profiles of Selected Compounds Found in South African Propolis. S. Afr. J. Sci. 2009, 105, 470–472. [Google Scholar] [CrossRef][Green Version]
- Rodríguez Vaquero, M.J.; Alberto, M.R.; de Nadra, M.C.M. Influence of Phenolic Compounds from Wines on the Growth of Listeria Monocytogenes. Food Control 2007, 18, 587–593. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.L.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E.; et al. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef]
- Dias, L.G.; Pereira, A.P.; Estevinho, L.M. Comparative Study of Different Portuguese Samples of Propolis: Pollinic, Sensorial, Physicochemical, Microbiological Characterization and Antibacterial Activity. Food Chem. Toxicol. 2012, 50, 4246–4253. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miguel, M.G.; Doughmi, O.; S, A.; Antunes, M.D.; Lyoussi, B. Antioxidant, Anti-Inflammatory and Acetylcholinesterase Inhibitory Activities of Propolis from Different Regions of Morocco. Food Sci. Biotechnol. 2014. [Google Scholar] [CrossRef]
- Oyaizu, M.; Ogihara, H.; Fujimoto, Y. Antioxidative Activity of Extracts from Propolis. J. Jpn. Oil Chem. Soc. 1999, 48, 135–138. [Google Scholar] [CrossRef][Green Version]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology; European Medicines Agency: London, UK, 1995; Volume CPMP/ICH/3.
- Joshi, R.; Sharma, R. Development and Validation of RP-HPLC Method for Simultaneous Estimation of Three-Component Tablet Formulation Containing Acetaminophen, Chlorzoxazone, and Aceclofenac. Anal. Lett. 2008, 41, 3297–3308. [Google Scholar] [CrossRef]
- Shinde, P.B.; Katekhaye, S.D.; Mulik, M.B.; Laddha, K.S. Rapid Simultaneous Determination of Marmelosin, Umbelliferone and Scopoletin from Aegle Marmelos Fruit by RP-HPLC. J. Food Sci. Technol. 2014, 51, 2251–2255. [Google Scholar] [CrossRef]
- Chew, Y.L.; Chan, E.W.L.; Tan, P.L.; Lim, Y.Y.; Stanslas, J.; Goh, J.K. Assessment of Phytochemical Content, Polyphenolic Composition, Antioxidant and Antibacterial Activities of Leguminosae Medicinal Plants in Peninsular Malaysia. BMC Complement Altern. Med. 2011, 11, 12. [Google Scholar] [CrossRef]
- Wicks, J.H.; Nelson, R.L.; Krejcarek, G.E. Rapid Inoculum Standardization System: A Novel Device for Standardization of Inocula in Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 1983, 17, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Hacek, D.M.; Dressel, D.C.; Peterson, L.R. Highly Reproducible Bactericidal Activity Test Results by Using a Modified National Committee for Clinical Laboratory Standards Broth Macrodilution Technique. J. Clin. Microbiol. 1999, 37, 1881–1884. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Abdulamir, A.S.; Vern, L.S.; Abu Bakar, F.; Abas, F.; Jahanshiri, F.; Sekawi, Z. Inhibition of Growth of Highly Resistant Bacterial and Fungal Pathogens by a Natural Product. Open Microbiol. J. 2011, 5, 96–106. [Google Scholar] [CrossRef]
| Scheme | Wax (%) | Resin (%) | Balsam (%) | Moisture (%) | Ash (%) | pH |
|---|---|---|---|---|---|---|
| P1 | 26.09 ± 1.92 c | 56.33 ± 1.03 c | 1.02 ± 0.02 b | 2.05 ± 0.01 b | 3.42 ± 0.01 b | 5.2 ± 0.01 a |
| P2 | 20.31 ± 1.03 d | 59.01 ± 0.12 a | 1.11 ± 0.01 ab | 1.01 ± 0.01 c | 4.83 ± 0.01 a | 5.1 ± 0.11 a |
| P3 | 43.12 ± 1.23 a | 54.14 ± 0.19 d | 0.76 ± 0.01 c | 2.07 ± 0.02 b | 2.53 ± 0.02 c | 4.5 ± 0.2 bc |
| P4 | 13.39 ± 1.08 e | 57.76 ± 0.08 b | 1.3 ± 0.03 a | 1.03 ± 0.01 c | 5.11 ± 0.01 a | 4.8 ± 0.2 ab |
| P5 | 29.86 ± 1.02 b | 45.25 ± 0.13 e | 0.69 ± 0.01 c | 2.79 ± 0.09 a | 1.67 ± 0.02 d | 4.2 ± 0.1 c |
| Analyte | Equation of Calibration Curve | Determination Coefficient (R2) | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) | Precision RSD (%), n = 3 | Stability RSD (%) | Repeatability RSD (%) | Accuracy | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day AUC | Inter-Day AUC | Mean % Recovery | Mean RSD (%) | ||||||||
| Caffeic acid | y = 577,684x + 504.35 | 0.9801 | 0.05–0.50 | 0.017 | 0.05 | 1.74 | 1.58 | 1.1 | 1.3 | 100.48 | 1.25 |
| p-Coumaric acid | y = 2 × 106x + 98.8 | 0.9999 | 0.01–0.05 | 0.003 | 0.01 | 1.92 | 1.85 | 1.5 | 0.5 | 100.37 | 1.69 |
| Ferulic acid | y = 730,425x + 8016.8 | 0.9811 | 0.001–0.05 | 0.0003 | 0.001 | 2.23 | 2.10 | 0.8 | 1.0 | 100.57 | 1.97 |
| Quercetin | y = 1 × 106x − 14,970 | 0.9651 | 0.01–0.30 | 0.003 | 0.01 | 1.66 | 1.52 | 0.9 | 1.6 | 100.25 | 1.01 |
| Cinnamic acid | y = 1 × 106x − 176.2 | 0.9997 | 0.01–0.30 | 0.003 | 0.01 | 4.31 | 4.19 | 0.8 | 0.7 | 100.60 | 1.20 |
| Naringenin | y = 9 × 106x − 12,307 | 0.9996 | 0.01–0.10 | 0.003 | 0.01 | 6.50 | 6.37 | 1.0 | 0.8 | 100.39 | 1.10 |
| Pinocembrine | y = 1 × 107x − 1492.3 | 0.9998 | 0.01–1.00 | 0.003 | 0.01 | 5.19 | 4.99 | 1.2 | 1.3 | 100.51 | 1.65 |
| Chrysin | y = 8 × 106x − 1982.3 | 0.9999 | 0.005–0.05 | 0.0016 | 0.005 | 3.83 | 3.72 | 0.7 | 1.9 | 100.59 | 1.43 |
| Galangin | y = 4 × 106x + 6009.3 | 0.9947 | 0.01–0.30 | 0.003 | 0.01 | 4.55 | 4.47 | 1.2 | 1.4 | 100.44 | 1.23 |
| Amounts of Marker Compounds (µg/g) | |||||
|---|---|---|---|---|---|
| Standard | P1 | P2 | P3 | P4 | P5 |
| Caffeic acid | 431 | 2152 | 108 | 572 | nd |
| p-Coumaric acid | 1110 | 2258 | 157 | 2479 | nd |
| Ferulic acid | 75.04 | 1829 | 89.18 | 1867 | nd |
| Quercetin | nd | nd | 1591 | nd | nd |
| Cinnamic acid | 820 | nd | nd | 21,513 | 2901 |
| Naringenin | 599 | 8988 | 583 | 2805 | nd |
| Pinocembrine | 8721 | 352,001 | 12,423 | 25,445 | nd |
| Chrysin | 542 | 30,062 | 1384 | 598 | nd |
| Galangin | 234 | 25,014 | 643 | 5856 | nd |
| Samples | Total Phenolic Content (mg GAE/g) | Flavone and Flavonol Content (mg QE/g) | TAC (mg AAE/g) | DPPH EC50 (mg/mL) | ABTS EC50 (mg/mL) | Reducing Power EC50 (mg/mL) |
|---|---|---|---|---|---|---|
| P1 | 122 ± 0.81 c | 76.52 ± 0.41 d | 83.34 ± 1.20 a | 0.08 ± 0.02 b | 0.02 ± 0.001 d | 0.06 ± 0.002 c |
| P2 | 148 ± 1.31 a | 118 ± 1.92 a | 90.87 ± 2.91 a | 0.02 ± 0.02 c | 0.05 ± 0.001 a | 0.04 ± 0.001 e |
| P3 | 115 ± 1.42 c | 98.21 ± 0. 41 c | 67.82 ± 3.46 b | 0.07 ± 0.01 bc | 0.04 ± 0.01 b | 0.07 ± 0.006 b |
| P4 | 136 ± 1.73 b | 107 ± 0.98 b | 87.67 ± 1.92 a | 0.04 ± 0.001 bc | 0.03 ± 0.02 c | 0.05 ± 0.006 d |
| P5 | 74.71 ± 0.89 d | 26.97 ± 2.44 e | 48.01 ± 0.51 c | 0.14 ± 0.01 a | 0.04 ± 0.01 b | 0.09 ± 0.003 a |
| Samples | Tests | E. coli BLSE (ATB:87) BGN | E. coli (ATB:57) B6N | E. coli (ATB:97) BGM | Pseudomonas aeruginosa | Streptococcus faecalis | Staphylococcus aureus |
|---|---|---|---|---|---|---|---|
| P1 | DI (mm) | 17.23 ± 1.21 ab | 13.6 ± 0.5 c | 16.3 ± 1.26 bc | 10.3 ± 0.6 bc | 21.33 ± 1.57 c | 23 ± 1.12 b |
| MIC (mg/mL) | 0.625 | 2.50 | 1.25 | 1.25 | 0.625 | 0.31 | |
| MBC (mg/mL) | 1.25 | >5 | 2.50 | >5 | 1.25 | 0.31 | |
| P2 | DI (mm) | 21.33 ± 1.52 a | 19.76 ± 0.40 b | 20.66 ± 1.57 a | 15.54 ± 1.1 ab | 32.5 ± 1.02 a | 31.2 ± 1.73 a |
| MIC (mg/mL) | 0.31 | 0.31 | 0.31 | 0.625 | 0.09 | 0.09 | |
| MBC (mg/mL) | 0.31 | 0.625 | 0.31 | 1.25 | 0.09 | 0.09 | |
| P3 | DI (mm) | 13.31 ± 1.57 b | - | 15.22 ± 0.56 c | 9.66 ± 0.6 c | 27 ± 1.18 b | 25 ± 0.57 b |
| MIC (mg/mL) | 1.25 | 5 | 1.25 | >5 | 0.625 | 1.25 | |
| MBC (mg/mL) | >5 | >5 | 2.50 | >5 | 0.625 | 1.25 | |
| P4 | DI (mm) | 18.71± 1.73 ab | 21.8 ± 0.35 a | 19.76 ± 0.40 ab | 20.33 ± 0.57 a | 22.8 ± 1.25 bc | 27.66 ± 0.57 ab |
| MIC (mg/mL) | 0.31 | 0.31 | 0.625 | 0.625 | 0.17 | 0.17 | |
| MBC (mg/mL) | 0.31 | 0.31 | 0.625 | 1.25 | 0.17 | 0.17 | |
| P5 | DI (mm) | 14.33 ± 1.15 b | 14.6 ± 0.5 c | 13.53 ± 0.89 c | - | 18 ± 0.57 c | 13 ± 1 c |
| MIC (mg/mL) | 1.25 | 2.50 | 2.50 | - | 0.625 | 1.25 | |
| MBC (mg/mL) | >5 | >5 | >5 | - | 0.625 | 2.50 | |
| Eth70% | DI (mm) | - | - | - | - | - | - |
| MIC (mg/mL) | |||||||
| MBC (mg/mL) |
| Bacterial Strains | Antibiotic Resistance |
|---|---|
| E. coli BLSE (ATB:87) BGN | CXM, CRO, CEC, AMX, CAZ, CTX, KF, and CIP |
| E. coli (ATB:57) B6N | CXM, AMX, CTX, KF, SXT, and CIP |
| E. coli (ATB:97) BGM | AMX |
| Pseudomonas aeruginosa | SXT and AMC |
| Streptococcus faecalis | SXT, TE, VA, E, P, and OX |
| Staphylococcus aureus | VA |
| Total Phenolic | Flavone and Flavonol | TAC | DPPH | ABTS | Reducing Power | DI E. coli 87 | DI E. coli 57 | DI E. coli 97 | DI P. aeruginosa | DI S. faecalis | DI S. aureus | MIC E. coli 87 | MIC E. coli 57 | MIC E. coli 97 | MIC P. aeruginosa | MIC S. faecalis | MIC S. aureus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total phenolic | 1 | 0.953 * | 0.968** | −0.984 ** | −0.068 | −0.994 ** | 0.793 | 0.342 | 0.923 * | 0.917 * | 0.763 | 0.982 ** | −0.841 | −0.507 | −0.995 ** | 0.543 | −0.762 | −0.827 |
| Flavone and flavonol | 0.953 * | 1 | 0.862 | −0.978 ** | 0.008 | −0.914 * | 0.609 | 0.121 | 0.839 | 0.897 * | 0.826 | 0.990 ** | −0.667 | −0.312 | −0.970 ** | 0.304 | −0.698 | −0.629 |
| TAC | 0.968 ** | 0.862 | 1 | −0.915 * | −0.257 | −0.984 ** | 0.825 | 0.448 | 0.900 * | 0.909 * | 0.596 | 0.908 * | −0.910 * | −0.575 | −0.947 * | 0.725 | −0.709 | −0.918 * |
| Caffeic acid | 0.753 | 0.644 | 0.687 | −0.760 | 0.480 | −0.776 | 0.887 * | 0.517 | 0.822 | 0.534 | 0.807 | 0.727 | −0.723 | −0.660 | −0.745 | 0.335 | −0.807 | −0.708 |
| p-Coumaric acid | 0.850 | 0.724 | 0.883 * | −0.836 | −0.085 | −0.870 | 0.921 * | 0.765 | 0.968 ** | 0.898 * | 0.457 | 0.778 | −0.978 ** | −0.863 | −0.853 | 0.560 | −0.915 * | −0.935* |
| Ferulic acid | 0.768 | 0.713 | 0.725 | −0.815 | 0.211 | −0.762 | 0.848 | 0.716 | 0.947 * | 0.834 | 0.551 | 0.746 | −0.850 | −0.839 | −0.801 | 0.230 | −0.992 ** | −0.761 |
| Codes | Region and Country | Predominant Vegetation | Year of Harvest |
|---|---|---|---|
| P1 | Fez Region, Morocco | Pistacia, Olea, Pinus, Quercus, Juniperus, Rosmarinus, Cistus and Lavandula | 2017 |
| P2 | Sefrou region, Morocco | Pistacia, Olea, Pinus, Quercus, Juniperus, Rosmarinus, Cistus and Lavandula | 2017 |
| P3 | Boulemane Region, Morocco | Ceratonia, Silybum, Thymus, Juniperus, Rosmarinus | 2017 |
| P4 | Jenin, Palestine | Olea, Citrus, Prunus | 2017 |
| P5 | Ramallah, Palestine | Olea, Citrus, Prunus | 2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules 2021, 26, 4589. https://doi.org/10.3390/molecules26154589
Touzani S, Imtara H, Katekhaye S, Mechchate H, Ouassou H, Alqahtani AS, Noman OM, Nasr FA, Fearnley H, Fearnley J, et al. Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules. 2021; 26(15):4589. https://doi.org/10.3390/molecules26154589
Chicago/Turabian StyleTouzani, Soumaya, Hamada Imtara, Shankar Katekhaye, Hamza Mechchate, Hayat Ouassou, Ali S. Alqahtani, Omar M. Noman, Fahd A. Nasr, Hugo Fearnley, James Fearnley, and et al. 2021. "Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities" Molecules 26, no. 15: 4589. https://doi.org/10.3390/molecules26154589
APA StyleTouzani, S., Imtara, H., Katekhaye, S., Mechchate, H., Ouassou, H., Alqahtani, A. S., Noman, O. M., Nasr, F. A., Fearnley, H., Fearnley, J., Paradkar, A., ElArabi, I., & Lyoussi, B. (2021). Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules, 26(15), 4589. https://doi.org/10.3390/molecules26154589







