Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats
Abstract
:1. Introduction
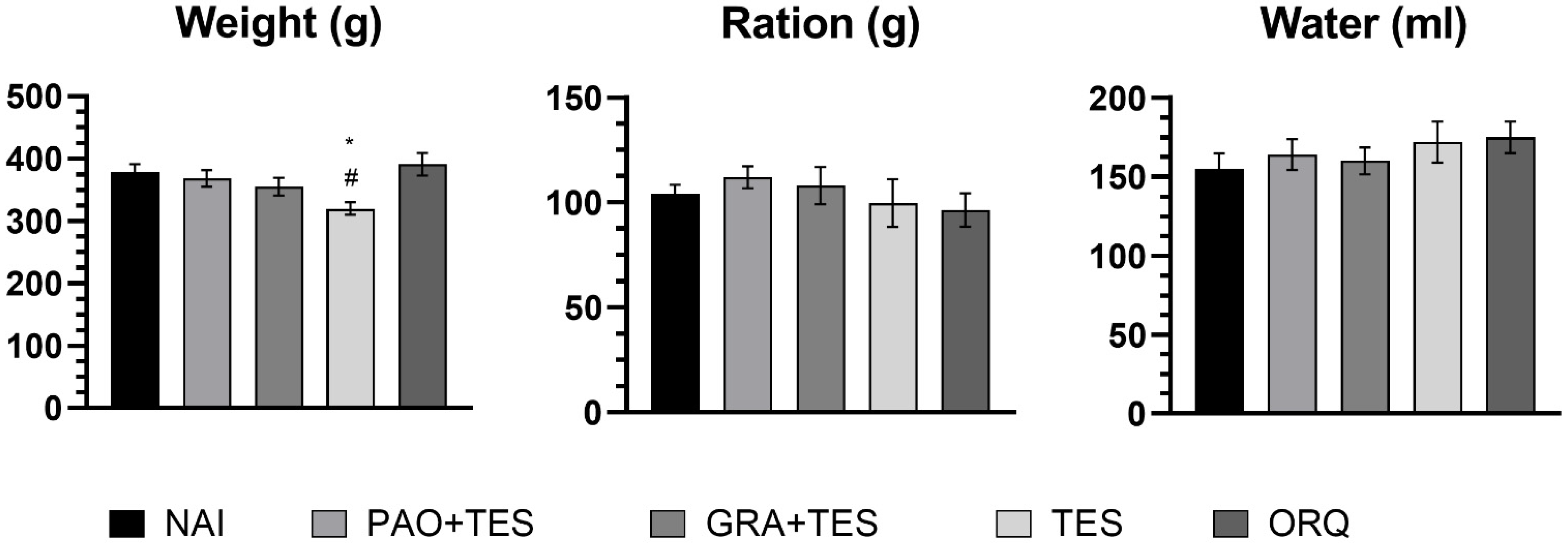
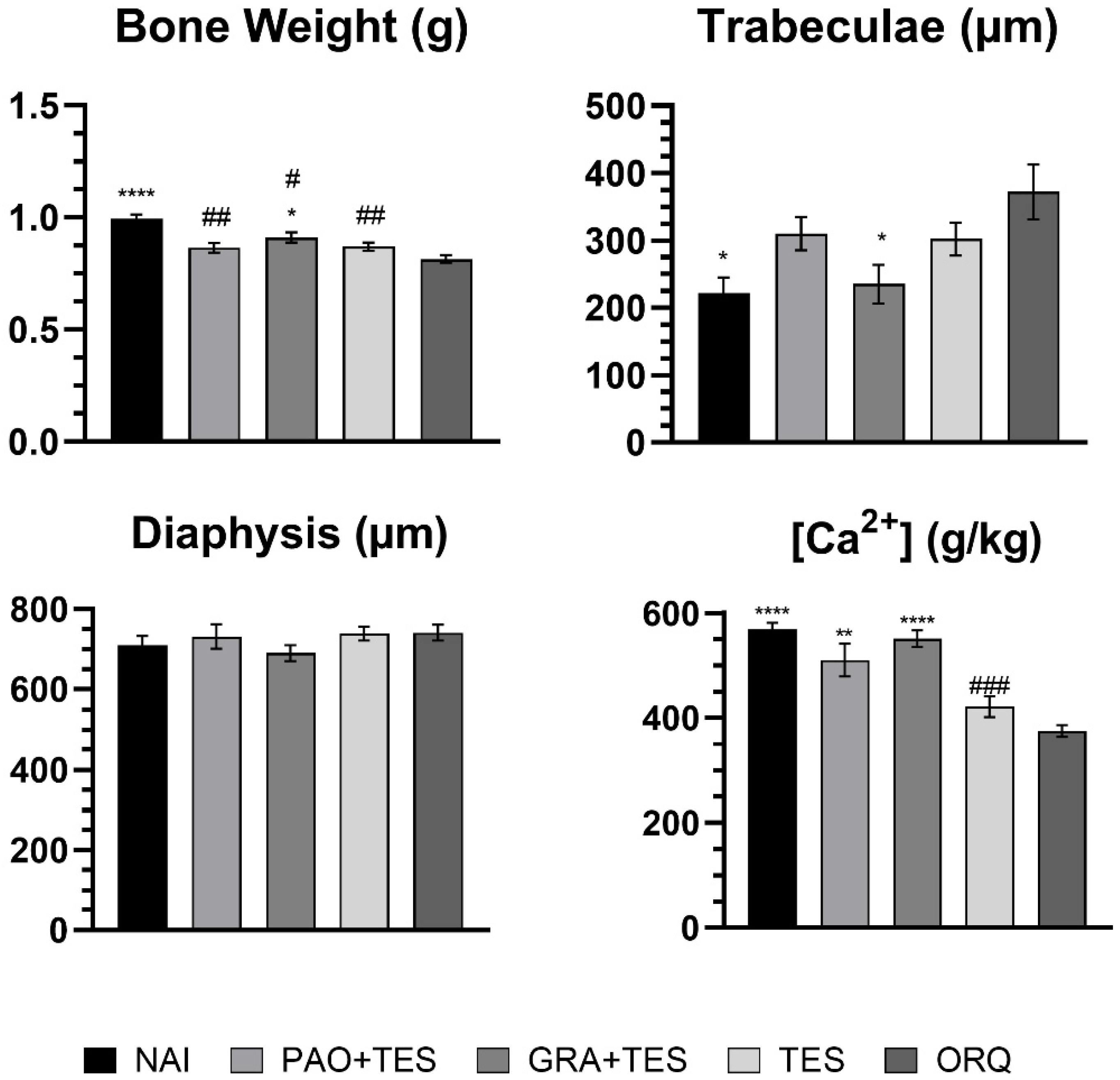
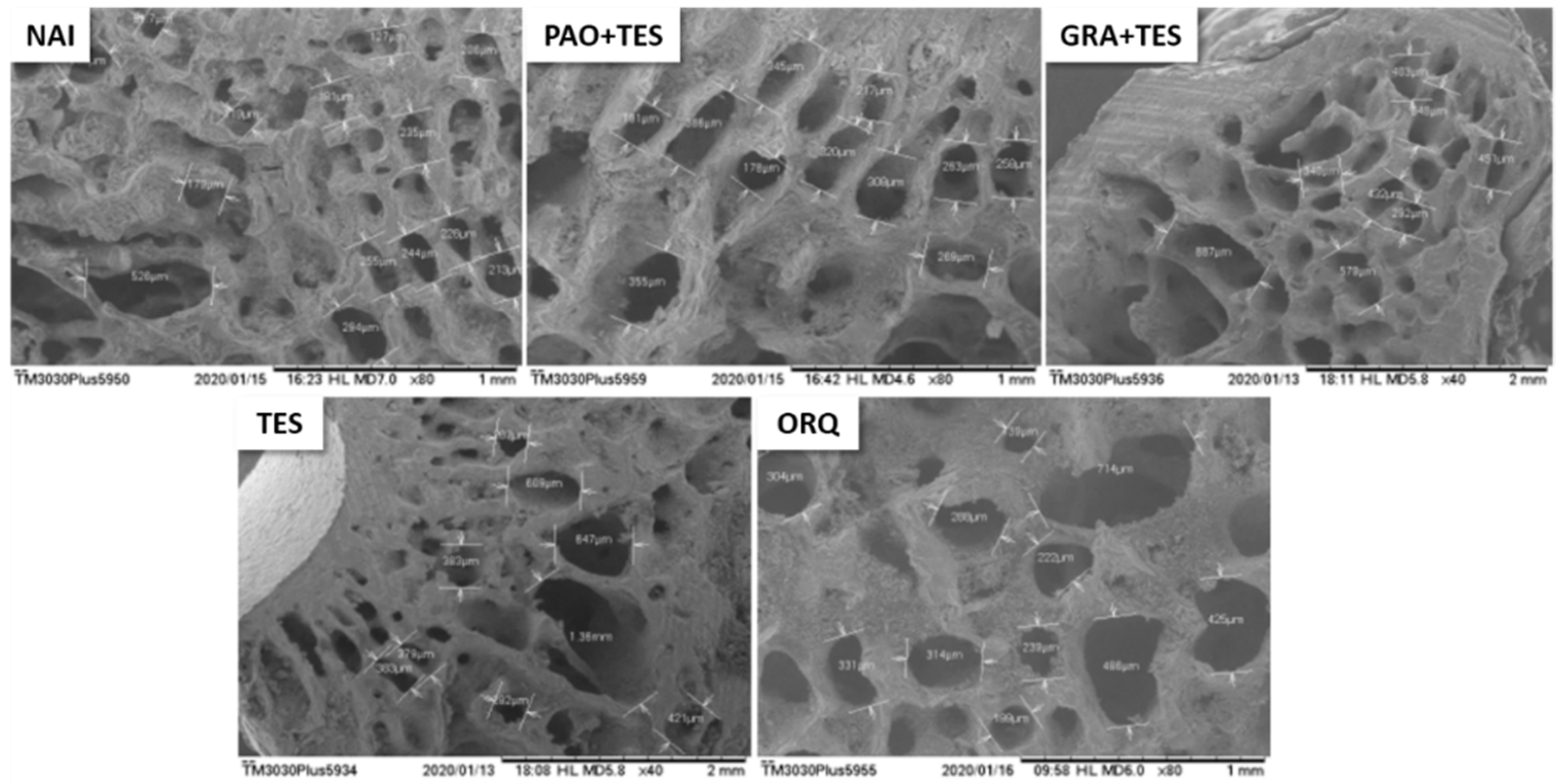
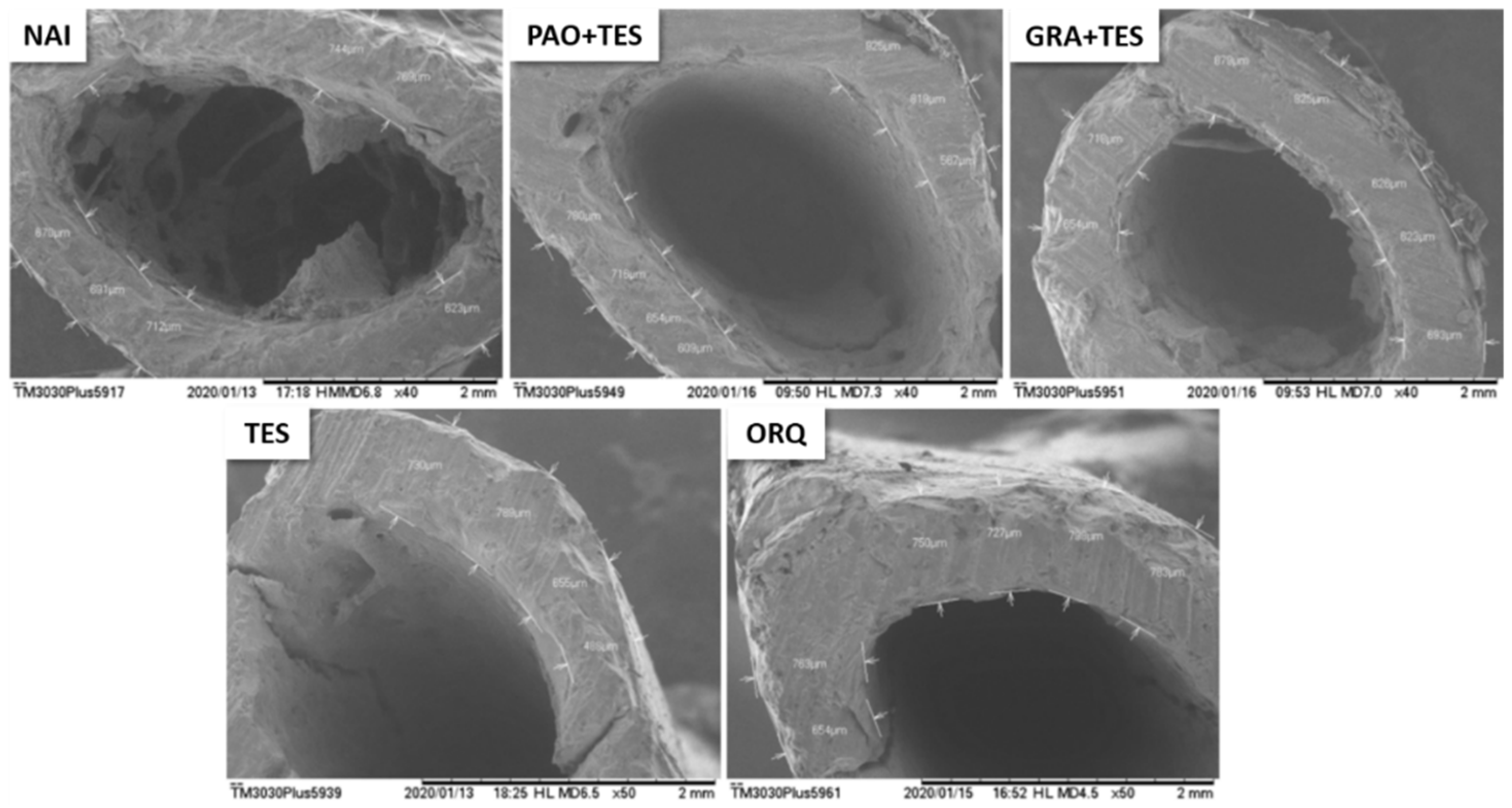
2. Results
3. Discussion
4. Materials and Methods
4.1. Test Material
4.2. Animals and Ethical Aspects
4.3. Orchiectomy and Experimental Design
- Naïve control group (NAI): non-orchiectomized animals that received only distilled water (0.5 mL/kg, p.o.);
- Experimental group (PAO + TES): orchiectomized animals treated with PAO (200 mg/kg, p.o.) + testosterone (7 mg/kg, IM);
- Experimental group (GRA + TES): orchiectomized animals treated with GRA (200 mg/kg, p.o.) + testosterone (7 mg/kg, IM);
- Positive control group (TES): orchiectomized animals treated with only testosterone (7 mg/kg, IM);
- Negative control group (ORQ): orchiectomized animals that received only distilled water (0.5 mL/kg, p.o.).
4.4. Biochemical Analyzes
4.5. Femur Scanning Electron Microscopy
4.6. Bone Matrix Calcium Quantification
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Armas, L.A.G.; Recker, R.R. Pathophysiology of Osteoporosis. New Mechanistic Insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.K.; Vandenput, L.; Börjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Riggs, B.L.; Khosla, S.; Melton, L.J. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef]
- Compston, J.E. Sex Steroids and Bone. Physiol. Rev. 2001, 81, 419–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orel-lana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Chin, K.Y.; Gengatharan, D.; Nasru, F.S.M.; Khairussam, R.A.; Ern, S.L.H.; Aminuddin, S.A.W.; Ima-Nirwana, S. The effects of annatto tocotrienol on bone biomechanical strength and bone calcium content in an animal model of osteopo-rosis due to testosterone deficiency. Nutrients 2016, 8, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testos-terone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Khan, D.A. Pharmacokinetics and Bioavailability of Annatto δ-tocotrienol in Healthy Fed Subjects. J. Clin. Exp. Cardiolog. 2015, 6, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Vilar, D.D.A.; Vilar, M.S.D.A.; Moura, T.F.A.D.L.E.; Raffin, F.N.; De Oliveira, M.R.; Franco, C.F.D.O.; De Athayde-Filho, P.F.; Diniz, M.D.F.F.M.; Barbosa-Filho, J.M. Traditional Uses, chemical constituents, and biological activities of Bixa Orellana L.: A review. Sci. World J. 2014, 2014, 857292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.J.; Shirakawa, H.; Giriwono, P.E.; Ito, A.; Komai, M. A novel function of geranylgeraniol in regulating testosterone production. Biosci. Biotechnol. Biochem. 2018, 82, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Ding, Y.; Peng, Y.; Wu, Y.; Fan, J.; Li, W.; Yang, R.; Yang, M.; Fu, Q. γ-Tocotrienol protects against ovariecto-my-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone 2014, 67, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The role of tocotrienol in preventing male osteoporosis—A review of current evidence. Int. J. Mol. Sci. 2019, 20, 1355. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Devel. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawacka, M.; Murawska, D.; Gesek, M. The effect of age and castration on the growth rate, blood lipid profile, liver histology and feed conversion in Green-legged Partridge cockerels and capons. Animal 2017, 11, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, N.; Ivanova, Z.; Bjorndal, B.; Vachkova, E.; Penchev, G.; Berge, R.; Ribarski, S.; Georgieva, T.M.; Yonkova, P.; Georgiev, I.P. Effect of fish oil supplementation and restricted feeding on body fat distribution and blood lipid profile in a rabbit model of castration-induced obesity. Res. Vet. Sci. 2019, 124, 99–105. [Google Scholar] [CrossRef]
- Nagarajan, P.; Arindkar, S.; Singh, S.; Majumdar, S.S. Effect of long-term castration on serum biochemistry in rhesus monkeys. J. Med. Primatol. 2013, 42, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Storer, T.W.; Berman, N.; Szeszycki, E.E. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J. Parenter. Enter. Nutr. 1997, 21, 241–242. [Google Scholar] [CrossRef]
- Traish, A.M. Testosterone and weight loss: The evidence. Curr. Opin. Endocrinol. Diabetes. Obes. 2014, 21, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Zgliczynski, S.; Ossowski, M.; Slowinska-Srzednicka, J.; Brzezinska, A.; Zgliczynski, W.; Soszynski, P.; Chotkowska, E.; Srzednicki, M.; Sadowski, Z. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis 1996, 121, 35–43. [Google Scholar] [CrossRef]
- Sever, N.; Song, B.L.; Yabe, D.; Goldstein, J.L.; Brown, M.S.; DeBose-Boydb, R.A. Insig-dependent Ubiquitination and Degradation of Mammalian 3-Hydroxy-3-methylglutaryl-CoA Reductase Stimulated by Sterols and Geranylgeraniol. J. Biol. Chem. 2003, 278, 52479–52490. [Google Scholar] [CrossRef] [Green Version]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.J.K. Hypocholesterolemic Activity of Synthetic and Natural Tocotrienols. J. Med. Chem. 1992, 35, 3595–3606. [Google Scholar] [CrossRef]
- Qureshi, A.; Khan, D.; Mahjabeen, W.; Qureshi, N. Dose-dependent Modulation of Lipid Parameters, Cytokines and RNA by δ-tocotrienol in Hypercholesterolemic Subjects Restricted to AHA Step-1 Diet. Br. J. Med. Med. Res. 2015, 6, 351–366. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kimura, J. Mechanism of statin-induced rhabdomyolysis. J. Pharmacol. Sci. 2013, 123, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelger, R.C.M.; Lycklama, A. Nijeholt, G.A.B.; Zwinderman, A.H.; Papapoulos, S.E.; Hamdy, N.A.T. The flare in serum alkaline phosphatase activity after orchiectomy: A valuable negative prognostic index for progression-free survival in prostatic carcinoma. J. Urol. 1996, 156, 122–126. [Google Scholar] [CrossRef]
- Morote, J.; M’Hammed, Y.I.; Martinez, E.; Esquena, S.; Lorente, J.A.; Gelabert, A. Increase of bone alkaline phosphatase after androgen deprivation therapy in patients with prostate cancer. Urology 2002, 59, 277–280. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Bryson, C.F.; Schlegel, P.N.; Paduch, D.A. The effect of hypogonadism and testosterone-enhancing therapy on alkaline phosphatase and bone mineral density. BJU Int. 2015, 115, 480–485. [Google Scholar] [CrossRef]
- Gomes, N.A.; Guarenghi, G.G.; Valenga, H.M.; Warnavin, S. von S.C.; Chaves, J.D.P.; Cardoso, A.C.; Steffens, J.P. Man-dibular-related bone metabolism in orchiectomized rats treated with sex hormones. Arch. Oral Biol. 2021, 122, 105000–105007. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.J.; Shirakawa, H.; Yoshida, R.; Ito, A.; Maeda, M.; Goto, T.; Komai, M. Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci. Biotechnol. Biochem. 2016, 80, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A.; et al. Potential role of tocotrienols on non-communicable diseases: A review of current evi-dence. Nutrients 2020, 12, 259. [Google Scholar] [CrossRef] [Green Version]
- Ima-Nirwana, S.; Suhaniza, S. Effects of Tocopherols and Tocotrienols on Body Composition and Bone Calcium Content in Adrenalectomized Rats Replaced with Dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evidence-based Complement. Altern. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The effects of tocotrienol on bone peptides in a rat model of osteoporosis in-duced by metabolic syndrome: The possible communication between bone cells. Int. J. Environ. Res. Public Health 2019, 16, 3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, M.L.; Mo, H.; Ji, X.; Shen, C.-L. Tocotrienols in Bone Protection: Evidence from Preclinical Studies. eFood 2020, 1, 217. [Google Scholar] [CrossRef]
- Casati, L.; Pagani, F.; Limonta, P.; Vanetti, C.; Stancari, G.; Sibilia, V. Beneficial effects of δ-tocotrienol against oxidative stress in osteoblastic cells: Studies on the mechanisms of action. Eur. J. Nutr. 2020, 59, 1975–1987. [Google Scholar] [CrossRef] [Green Version]
- Hiruma, Y.; Nakahama, K.I.; Fujita, H.; Morita, I. Vitamin K2 and geranylgeraniol, its side chain component, inhibited osteoclast formation in a different manner. Biochem. Biophys. Res. Commun. 2004, 314, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, T.; Koch, F.; Klein, M.O.; Guth, J.; Adler, J.; Pabst, A.; Al-Nawas, B.; Walter, C. Geranylgeraniol—A new poten-tial therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011, 47, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef]
- INPI National Invention Application, Utility Model, Invention Addition Certificate and entry into the national phase of the PCT Process Number: BR 10 2020 015050 2. 2020. Available online: http://revistas.inpi.gov.br/rpi/ (accessed on 3 August 2021).
- Idris, A.I. Ovariectomy/orchidectomy in rodents. Methods Mol. Biol. 2012, 816, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Nazrun, A.S.; Luke, D.A.; Khalid, B.A.K.; Ima-Nirwana, S. Vitamin E protects from free-radical damage on femur of rats treated with ferric nitrilotriacetate. Curr. Top. Pharmacol. 2005, 9, 107–115. [Google Scholar]
- De Oliveira Carvalho, H.; Souza, B.S.F.; Dos Santos, I.V.F.; Resque, R.L.; Keita, H.; Fernandes, C.P.; Carvalho, J.C.T. Hypoglycemic effect of formulation containing hydroethanolic extract of Calophyllum brasiliense in diabetic rats in-duced by streptozotocin. Rev. Bras. Farmacogn. 2016, 26, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Palma, M.N.N. Evaluation of Acid Digestion Procedures to Estimate Mineral Contents in Materials from Animal Trials. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2015. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.C.M.; de Oliveira Carvalho, H.; Gonçalves, D.E.S.; Picanço, K.R.T.; de Lima Teixeira dos Santos, A.V.T.; da Silva, H.R.; Braga, F.S.; Bezerra, R.M.; de Sousa Nunes, A.; Nazima, M.T.S.T.; et al. Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats. Molecules 2021, 26, 4720. https://doi.org/10.3390/molecules26164720
Pereira ACM, de Oliveira Carvalho H, Gonçalves DES, Picanço KRT, de Lima Teixeira dos Santos AVT, da Silva HR, Braga FS, Bezerra RM, de Sousa Nunes A, Nazima MTST, et al. Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats. Molecules. 2021; 26(16):4720. https://doi.org/10.3390/molecules26164720
Chicago/Turabian StylePereira, Arlindo César Matias, Helison de Oliveira Carvalho, Danna Emanuelle Santos Gonçalves, Karyny Roberta Tavares Picanço, Abrahão Victor Tavares de Lima Teixeira dos Santos, Heitor Ribeiro da Silva, Francinaldo Sarges Braga, Roberto Messias Bezerra, Alessandro de Sousa Nunes, Maira Tiyomi Sacata Tongo Nazima, and et al. 2021. "Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats" Molecules 26, no. 16: 4720. https://doi.org/10.3390/molecules26164720
APA StylePereira, A. C. M., de Oliveira Carvalho, H., Gonçalves, D. E. S., Picanço, K. R. T., de Lima Teixeira dos Santos, A. V. T., da Silva, H. R., Braga, F. S., Bezerra, R. M., de Sousa Nunes, A., Nazima, M. T. S. T., Cerqueira, J. G., Taglialegna, T., Teixeira, J. M., & Carvalho, J. C. T. (2021). Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats. Molecules, 26(16), 4720. https://doi.org/10.3390/molecules26164720








