LC-ToF-ESI-MS Patterns of Hirsutinolide-like Sesquiterpenoids Present in the Elephantopus mollis Kunth Extract and Chemophenetic Significance of Its Chemical Constituents
Abstract
1. Introduction
2. Results
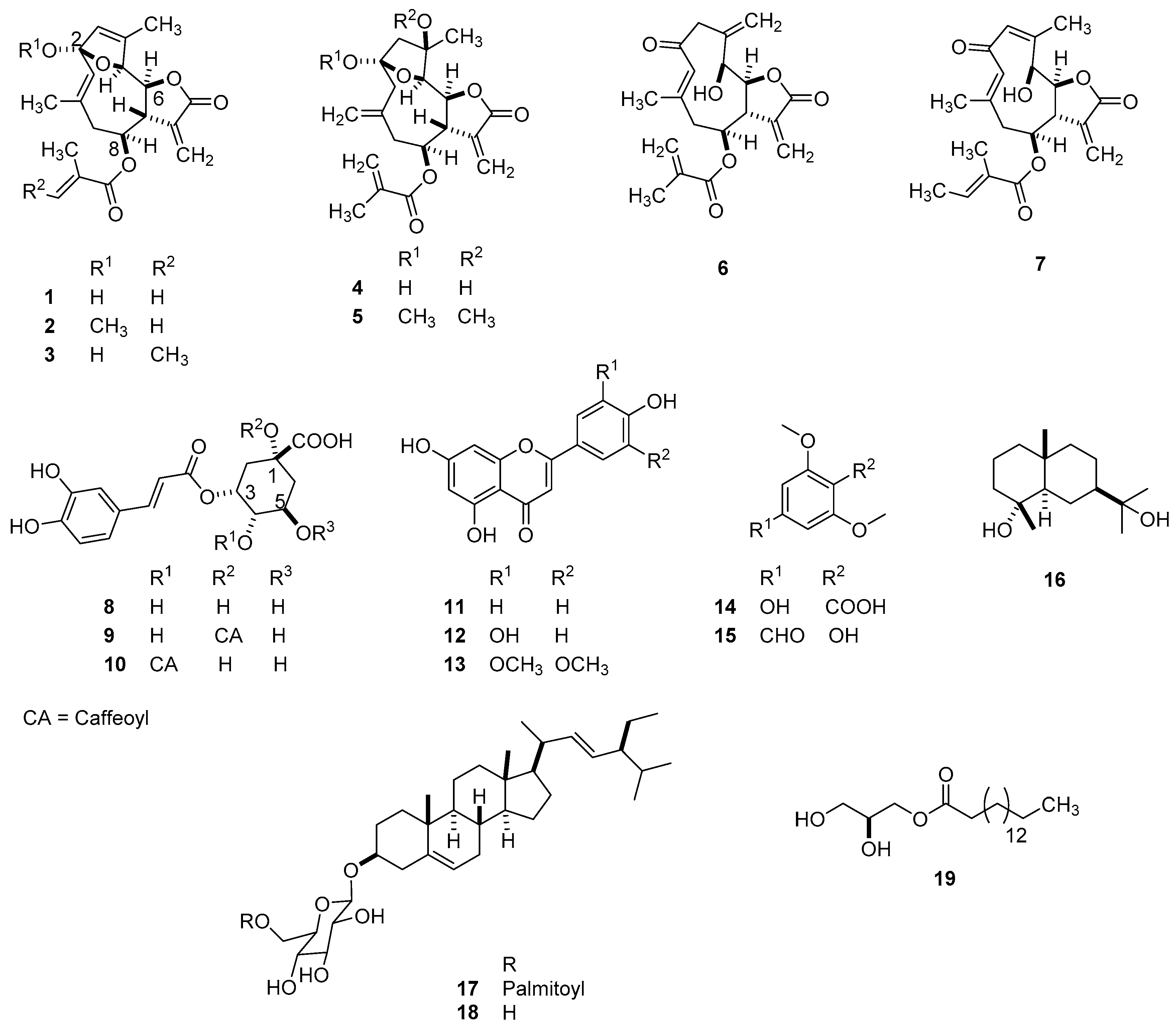
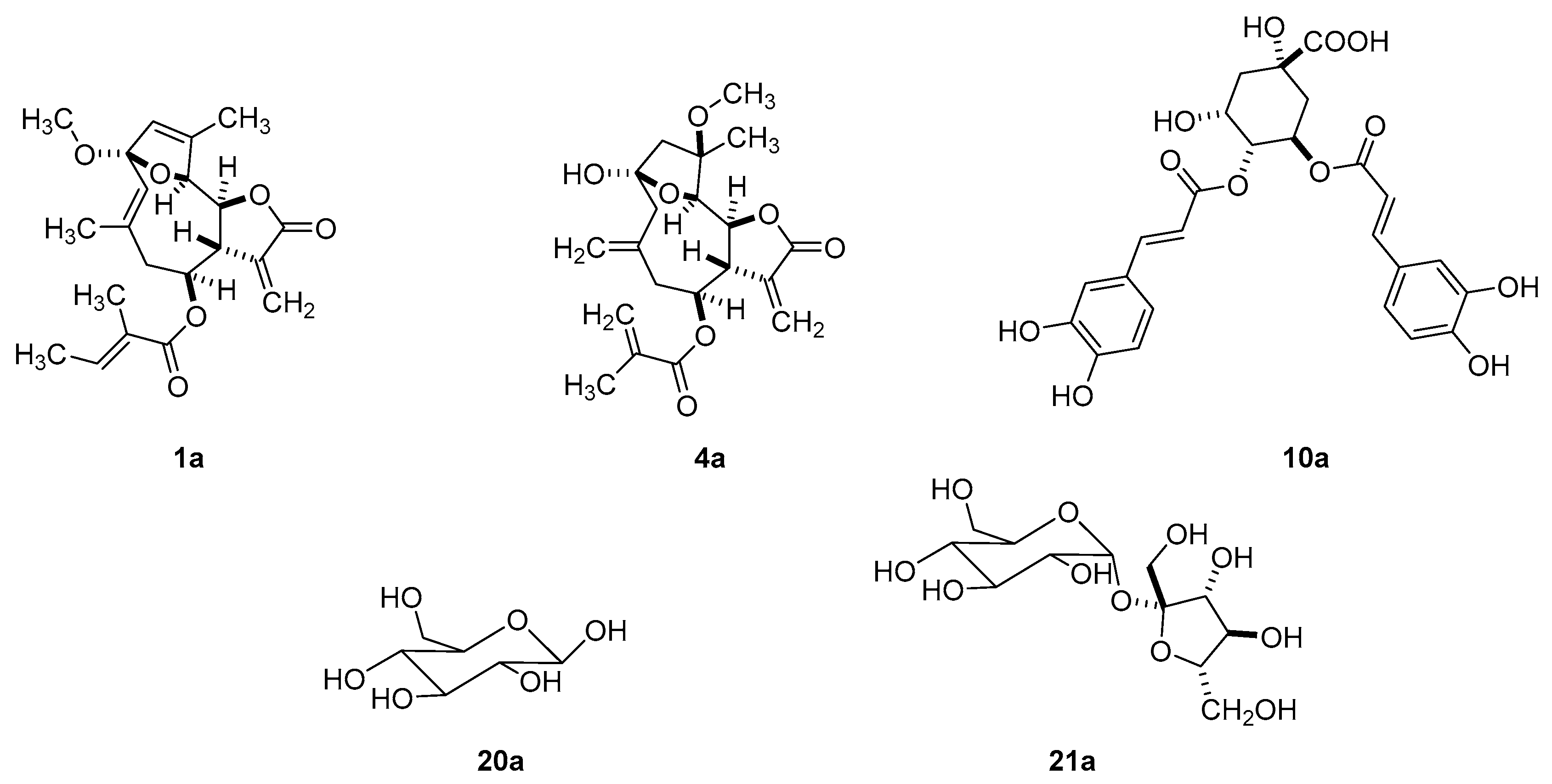
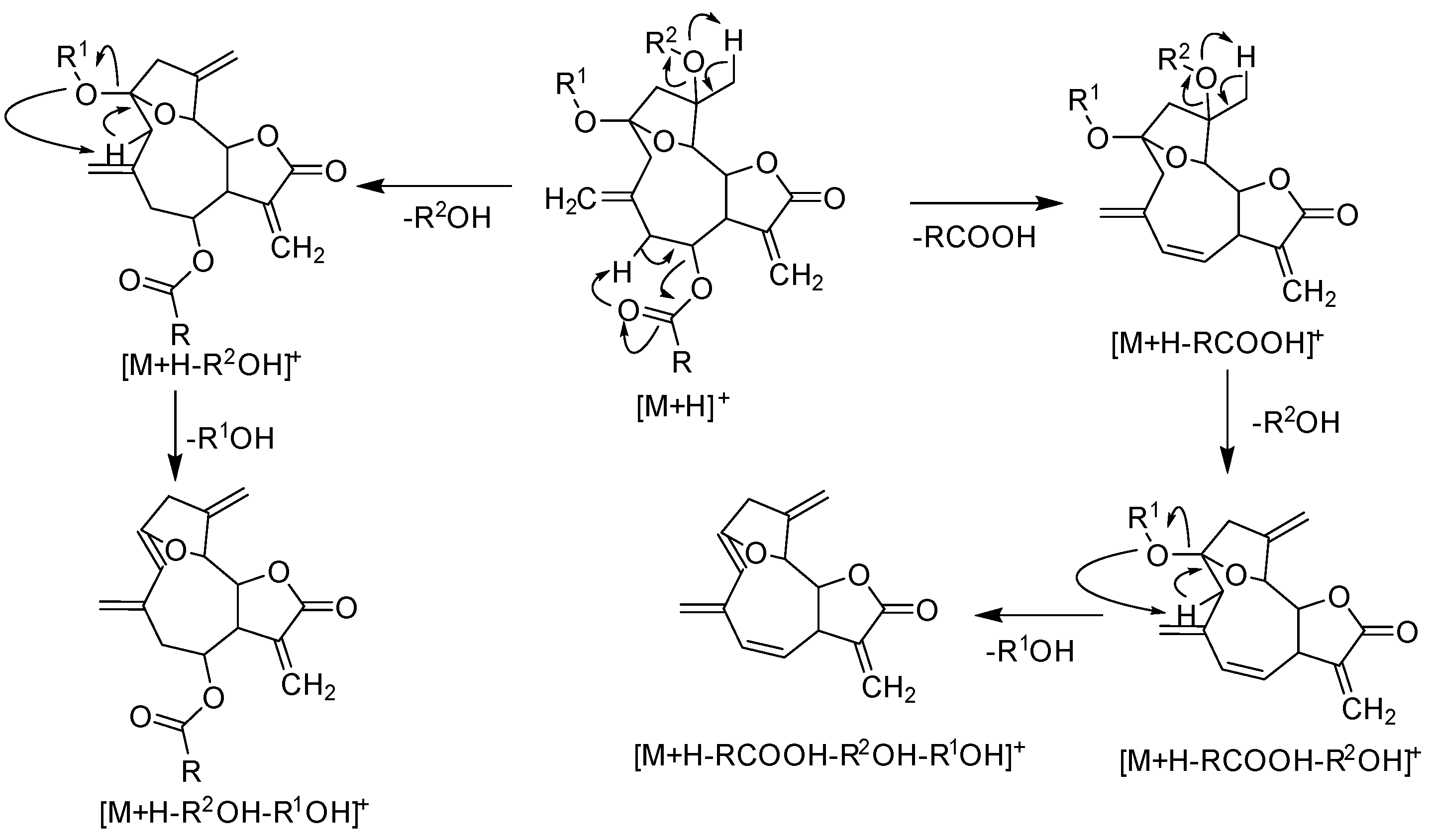
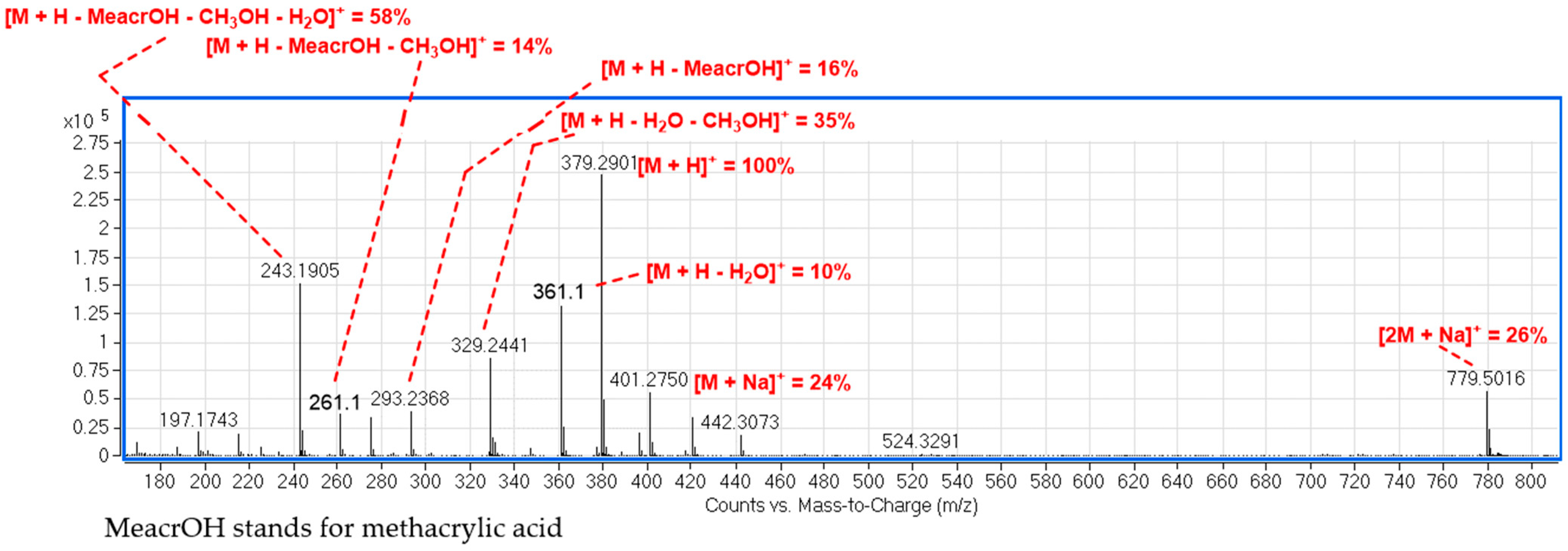
2.1. LC–MS Analysis of Fractions for Structure Identification
2.2. Biological Endpoints of Compounds and Fractions
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Purification
4.4. Bioactivity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jakupovic, J.; Jia, Y.; Zdero, C.; Warning, U.; Bohlmann, F.; Jones, S. Germacranolides from Elephantopus species. Phytochemistry 1987, 26, 1467–1469. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Huang, Z.; Huang, X.-H.; Zheng, W.-B.; Yin, X.-F.; Li, Y.-L.; Li, B.; He, Q.-Y. Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis. 2017, 8, e2876. [Google Scholar] [CrossRef]
- Ooi, K.L.; Muhammad, T.S.T.; Lam, L.Y.; Sulaiman, S.F. Cytotoxic and Apoptotic Effects of Ethyl Acetate Extract of Elephantopus mollis Kunth. in Human Liver Carcinoma HepG2 Cells Through Caspase-3 Activation. Integr. Cancer Ther. 2012, 13, NP1–NP9. [Google Scholar] [CrossRef] [PubMed]
- But, P.P.-H.; Hon, P.-M.; Cao, H.; Che, C.-T. A New Sesquiterpene Lactone fromElephantopus mollis. Planta Medica 1996, 62, 474–476. [Google Scholar] [CrossRef]
- Wu, Z.-N.; Zhang, Y.-B.; Chen, N.-H.; Li, M.-J.; Li, M.-M.; Tang, W.; Zhuang, L.; Li, Y.-L.; Wang, G.-C. Sesquiterpene lactones from Elephantopus mollis and their anti-inflammatory activities. Phytochemistry 2017, 137, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kabiru, A.; Por, L.Y. Elephantopus Species: Traditional Uses, Pharmacological Actions and Chemical Composition. Adv. Life Sci. Technol. 2013, 15, 6–13. [Google Scholar]
- Daisy, P.; Jasmine, R.; Ignacimuthu, S.; Murugan, E. A novel Steroid from Elephantopus scaber L. an Ethnomedicinal plant with antidiabetic activity. Phytomedicine 2009, 16, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Bitchagno, G.T.M.; Nchiozem-Ngnitedem, V.-A.; Wandji, N.T.; Noulala, G.C.T.; Fobofou, S.A.T.; Lenta, B.N. Plant-Derived Compounds against Microbial Infections and Cancers. In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health; IntechOpen: London, UK, 2021. [Google Scholar]
- Da Silva, L.A.; Sandjo, L.P.; Misturini, A.; Caramori, G.F.; Biavatti, M.W. ESI-QTof-MS characterization of hirsutinolide and glaucolide sesquiterpene lactones: Fragmentation mechanisms and differentiation based on Na+ /H+ adducts interactions in complex mixture. J. Mass Spectrom. 2019, 54, 915–932. [Google Scholar] [CrossRef]
- Mbougnia, J.F.T.; Bitchagno, G.T.M.; Wouamba, S.C.N.; Jouda, J.-B.; Awouafack, M.D.; Tene, M.; Lenta, B.N.; Kouam, S.F.; Tane, P.; Sewald, N. Two new triterpenoid fatty acid esters from Schefflera barteri Harms (Araliaceae). Nat. Prod. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Mbougnia, J.F.; Happi, G.M.; Bitchagno, G.T.; Awouafack, M.D.; Lenta, B.N.; Kouam, S.F.; Tane, P.; Sewald, N.; Tene, M. Chemical constituents from Ficus natalensis hochst (Moraceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 95, 104227. [Google Scholar] [CrossRef]
- Chouna, H.S.D.; Bankeu, J.J.K.; Fongang, Y.S.F.; Dize, D.; Ponou, B.K.; Bitchagno, G.T.M.; Awantu, A.F.; Lenta, B.N.; Fekam, F.B.; Ngouela, S.A.; et al. Constituents of Peperomia vulcanica Baker & C. H. Wright (Piperaceae) with antiparasitic activity. Phytochem. Lett. 2021, 41, 14–20. [Google Scholar] [CrossRef]
- Tabopda, T.K.; Ngoupayo, J.; Liu, J.; Ali, M.S.; Khan, S.N.; Ngadjui, B.T.; Luu, B. Further Cytotoxic Sesquiterpene Lactones from Elephantopus mollis KUNTH. Chem. Pharm. Bull. 2008, 56, 231–233. [Google Scholar] [CrossRef][Green Version]
- Pollora, G.C.; Bardon, A.; Catalan, C.A.; Griffin, C.L.; Herz, W. Elephantopus-type sesquiterpene lactones from a second Vernonanthura species, Vernonanthura lipeoensis. Biochem. Syst. Ecol. 2004, 32, 619–625. [Google Scholar] [CrossRef]
- Tabopda, T.K.; Liu, J.; Ngadjui, B.T.; Luu, B. Cytotoxic Triterpene and Sesquiterpene Lactones from Elephantopus mollis and Induction of Apoptosis in Neuroblastoma Cells. Planta Medica 2007, 73, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, L.; Gao, Y.; Zhao, B.; Wang, D.; Duan, W.; Yu, Z. Isolation of isochlorogenic acid isomers in flower buds of Lonicera japonica by high-speed counter-current chromatography and preparative high performance liquid chromatography. J. Chromatogr. B 2015, 981–982, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic Compounds from the Leaf Extract of Artichoke (Cynara scolymusL.) and Their Antimicrobial Activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, X.-Q.; Dong, J.-X. [Apigenin glycosides from Euphorbia humifusa wild]. Acta Pharm. Sin. 2009, 44, 496–499. [Google Scholar]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Medica 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Flavonoid patterns in leaves of the gramineae. Biochem. Syst. Ecol. 1976, 4, 267–280. [Google Scholar] [CrossRef]
- Stanikunaite, R.; Khan, S.I.; Trappe, J.M.; Ross, S.A. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffleElaphomyces granulatus. Phytotherapy Res. 2009, 23, 575–578. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, S.; Xuan, L. Oxanthrone C-glycosides and epoxynaphthoquinol from the roots of Rumex japonicus. Phytochemistry 2007, 68, 2444–2449. [Google Scholar] [CrossRef]
- Evans, F.E.; Miller, D.W.; Cairns, T.; Baddeley, G.V.; Wenkert, E. Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances. Part 74. Structure analysis of proximadiol (cryptomeridiol) by carbon-13 NMR spectroscopy. Phytochemistry 1982, 21, 937–938. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Schueffler, A.; Simo, I.K.; Krumb, M.; Tane, P.; Opatz, T. Neo-clerodane diterpenoids from Conyza pyrrhopappa Sch.Bip. ex A.Rich. Nat. Prod. Res. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.; Joutsamo, T.; Mattlla, S.; Kämäräinen, T.; Jalonen, J. Identification of Isomeric Dicaffeoylquinic Acids from Eleutherococcus senticosus using HPLC-ESI/ToF/MS and H-NMR Methods. Phytochem. Anal. 2002, 13, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Martínez; Taboada, J.; González-Diddi, M. Cytotoxicity of some sesquiterpene lactones “in vitro”. Arch. Invest. Med. (Mex.) 1980, 11, 435–443. [Google Scholar]
- Zidorn, C. Plant chemophenetics—A new term for plant chemosystematics/plant chemotaxonomy in the macro-molecular era. Phytochemistry 2019, 163, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.L.; Muhammad, T.S.T.; Tan, M.L.; Sulaiman, S.F. Cytotoxic, apoptotic and anti-α-glucosidase activities of 3,4-di-O-caffeoyl quinic acid, an antioxidant isolated from the polyphenolic-rich extract of Elephantopus mollis Kunth. J. Ethnopharmacol. 2011, 135, 685–695. [Google Scholar] [CrossRef]
- Kabeer, F.A.; Prathapan, R. Phytopharmacological Profile of Elephantopus scaber. Pharmacology 2014, 5, 272–285. [Google Scholar] [CrossRef]
- Kuete, V.; Fokou, F.W.; Karaosmanoğlu, O.; Beng, V.P.; Sivas, H. Cytotoxicity of the methanol extracts of Elephantopus mollis, Kalanchoe crenata and 4 other Cameroonian medicinal plants towards human carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Wang, W.; Shao, F.; Chen, W.; Xia, C.; Wang, S.; Li, Y.; Zhou, G.; Liu, Z. EM23, A natural sesquiterpene lactone from Elephantopus mollis, induces apoptosis in human myeloid leukemia cells through thioredoxin and reactive oxygen spe-cies-mediated signaling pathways. Front. Pharmacol. 2016, 7, 77. [Google Scholar] [CrossRef]
- Fuchino, H.; Koide, T.; Takahashi, M.; Sekita, S.; Satake, M. New Sesquiterpene Lactones from Elephantopus mollis and Their Leishmanicidal Activities. Planta Medica 2001, 67, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, W.; Wu, X.; Wang, G.; Wang, Y.; Zhang, X.; Zhang, D. Elephantopus Scaber Extract, Its Preparation Method and Application in Preparing Antiviral Medicine. China, CN 102219687A, 22 April 2011. [Google Scholar]
- Nguemo, R.T.; Mbouangouere, R.; Bitchagno, G.T.M.; Tchuenguem, R.; Temgoua, E.V.N.; Ndontsa, B.L.; Mpetga, J.S.; Opatz, T.; Ngouela, A.S.; Tane, P. A new ceramide from the leaves of Lannea schimperi (Hochst. ex A.Rich.). Engl. Nat. Prod. Res. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitchagno, G.T.M.; Koffi, J.G.; Simo, I.K.; Kagho, D.U.K.; Ngouela, A.S.; Lenta, B.N.; Sewald, N. LC-ToF-ESI-MS Patterns of Hirsutinolide-like Sesquiterpenoids Present in the Elephantopus mollis Kunth Extract and Chemophenetic Significance of Its Chemical Constituents. Molecules 2021, 26, 4810. https://doi.org/10.3390/molecules26164810
Bitchagno GTM, Koffi JG, Simo IK, Kagho DUK, Ngouela AS, Lenta BN, Sewald N. LC-ToF-ESI-MS Patterns of Hirsutinolide-like Sesquiterpenoids Present in the Elephantopus mollis Kunth Extract and Chemophenetic Significance of Its Chemical Constituents. Molecules. 2021; 26(16):4810. https://doi.org/10.3390/molecules26164810
Chicago/Turabian StyleBitchagno, Gabin Thierry M., Jean Garba Koffi, Ingrid Konga Simo, Donald Ulrich K. Kagho, Augustin Silvere Ngouela, Bruno Ndjakou Lenta, and Norbert Sewald. 2021. "LC-ToF-ESI-MS Patterns of Hirsutinolide-like Sesquiterpenoids Present in the Elephantopus mollis Kunth Extract and Chemophenetic Significance of Its Chemical Constituents" Molecules 26, no. 16: 4810. https://doi.org/10.3390/molecules26164810
APA StyleBitchagno, G. T. M., Koffi, J. G., Simo, I. K., Kagho, D. U. K., Ngouela, A. S., Lenta, B. N., & Sewald, N. (2021). LC-ToF-ESI-MS Patterns of Hirsutinolide-like Sesquiterpenoids Present in the Elephantopus mollis Kunth Extract and Chemophenetic Significance of Its Chemical Constituents. Molecules, 26(16), 4810. https://doi.org/10.3390/molecules26164810





