A Simple Method for Evaluating the Bioactive Phenolic Compounds’ Presence in Brazilian Craft Beers
Abstract
:1. Introduction
2. Results
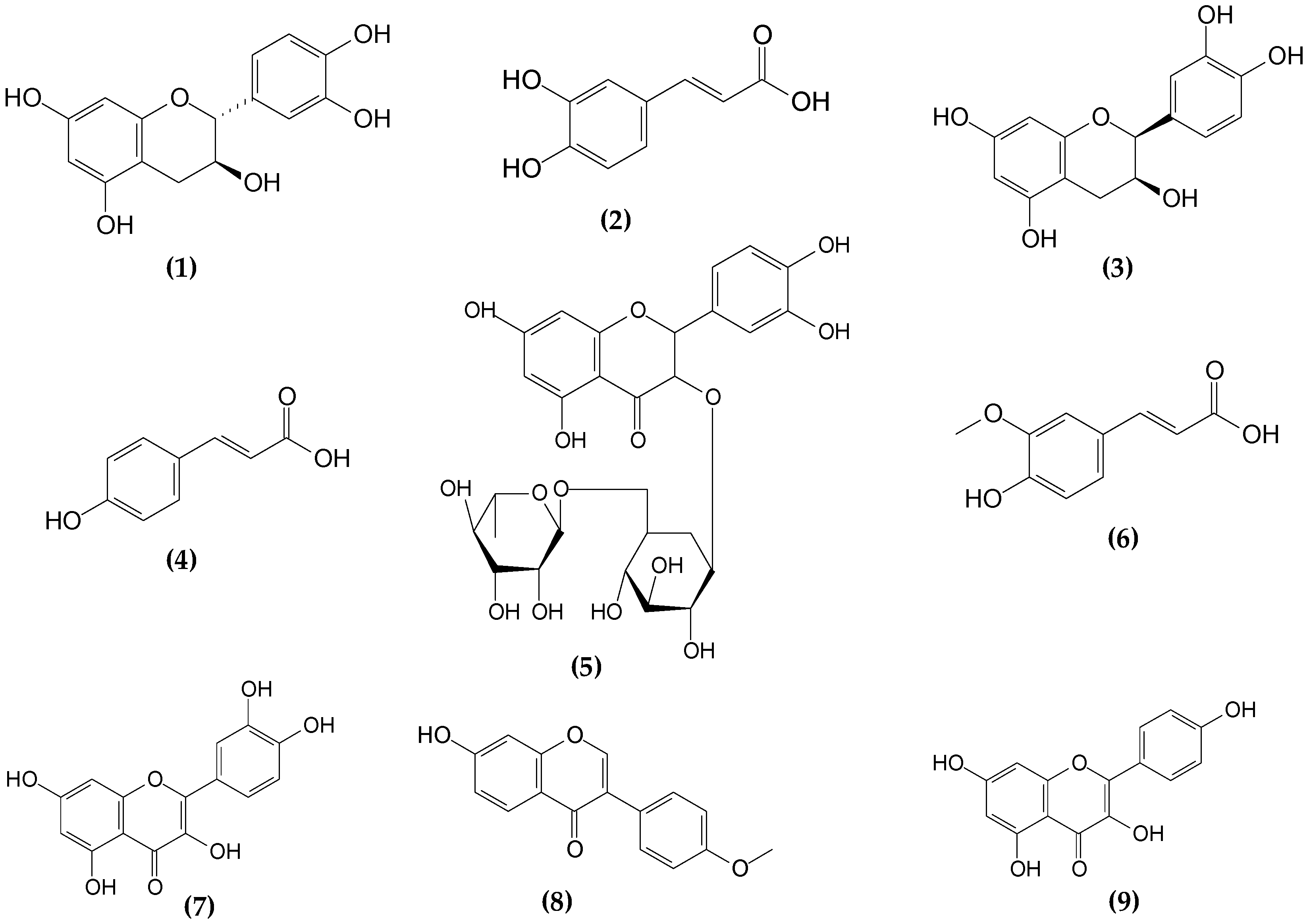
2.1. Chromatographic Profile of the Phenolic Compounds in Craft Beers
2.2. Validation of the Method
2.2.1. Selectivity
2.2.2. Linearity
2.2.3. Limits of Detection and Quantification
2.2.4. Precision
2.2.5. Accuracy
2.3. Application of the Method in Real Samples
3. Discussion
4. Materials and Methods
4.1. Standards and Samples
4.2. Analysis
Quality Assurance and Quality Control (QA/QC)
4.3. Optimization of Wavelengths for the Phenolic Compounds Detection
4.4. Validation of the Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Oliveira Neto, J.R.; de Oliveira, T.S.; Ghedini, P.C.; Vaz, B.G.; de Souza Gil, E. Antioxidant and vasodilatory activity of commercial beers. J. Funct. Foods 2017, 34, 130–138. [Google Scholar] [CrossRef]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef]
- Fagherazzi, M.M.; Rufato, L.; Oliveira, B.F.; Stockhausen, H.; Oliveira, E.R.; Fagherazzi, A.F.; Arruda, A.L.; Camargo, S.S. Cervejaria artesanal: Um mercado em expansão. Rev. UNIPLAC 2018, 6. Available online: http://revista.uniplac.net/ojs/index.php/uniplac/article/view/3347 (accessed on 28 January 2019).
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME-GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Brito, T.C.; Da Fonseca, N.D.; De Aguiar, P.F.; Monteiro, M.; Perrone, D.; Torres, A.G. Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem. 2016, 199, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, J.M.; Holbrook, C.; Sedin, D.; Heuberger, A.L. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2018, 113, 487–504. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Popłoński, J.; Bartmańska, A. Flavonoids as Phytoestrogenic Components of Hops and Beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef] [PubMed]
- Dvořáková, M.; Hulín, P.; Karabín, M.; Dostálek, P. Determination of polyphenols in beer by an effective method based on solid-phase extraction and high performance liquid chromatography with diode-array detection. Czech J. Food Sci. 2007, 25, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Lamuela-Raventós, R.M.; Estruch, R.; Sasot, G.; Doménech, M.; Tresserra-Rimbau, A. Beer Polyphenols and Menopause: Effects and Mechanisms—A Review of Current Knowledge. Oxid. Med. Cell. Longev. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H. Effects of Processing Stages on the Profile of Phenolic Compounds in Beer. In Processing and Impact on Active Components in Food; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 533–539. ISBN 9780124047099. [Google Scholar]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant activity and the most abundant phenolics in commercial dark beers. Int. J. Food Prop. 2017, 20, 595–609. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Gerhäuser, C. Phenolic beer compounds to prevent cancer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 669–684. ISBN 9780123738912. [Google Scholar]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, P.A.; Vidal, J.; Ávila, M.I.; Labbe, M.; Cohen, S.; Salazar, F.N. Effect of the Addition of Propolis Extract on Bioactive Compounds and Antioxidant Activity of Craft Beer. J. Chem. 2017, 2017, 6716053. [Google Scholar] [CrossRef] [Green Version]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.d.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [Green Version]
- Marques, D.R.; Cassis, M.A.; Quelhas, J.O.F.; Bertozzi, J.; Visentainer, J.V.; Oliveira, C.C.; Monteiro, A.R.G. Characterization of craft beers and their bioactive compounds. Chem. Eng. Trans. 2017, 57, 1747–1752. [Google Scholar] [CrossRef]
- Garavaglia, C.; Swinnen, J. The Craft Beer Revolution: An International Perspective. Agric. Appl. Econ. Assoc. 2017, 32, 1–8. [Google Scholar] [CrossRef]
- García-Moreno, A.T.; Lomares, F.S.; Taboada, J.M.V.; Majem, P.V. La cerveza Artesanal: Cómo Hacer Cerveza en Casa, 1st ed.; Sabadell: Cerveart, Spain, 2004. [Google Scholar]
- Nardini, M.; Foddai, M.S. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef]
- Jandera, P. Methods for the HPLC analysis of phenolic compounds and flavonoids in beer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1003–1014. ISBN 9780123738912. [Google Scholar]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; De la Torre, R. Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Kurowska-Susdorf, A.; Zwierżdżyński, M.; Bevanda, A.M.; Talić, S.; Ivanković, A.; Płotka-Wasylka, J. Green analytical chemistry: Social dimension and teaching. TrAC Trends Anal. Chem. 2019, 111, 185–196. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Corrêa, V.G.; Tureck, C.; Locateli, G.; Peralta, R.M.; Koehnlein, E.A. Estimate of consumption of phenolic compounds by Brazilian population. Rev. Nutr. 2015, 28, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Pai, T.V.; Sawant, S.Y.; Ghatak, A.A.; Chaturvedi, P.A.; Gupte, A.M.; Desai, N.S. Characterization of Indian beers: Chemical composition and antioxidant potential. J. Food Sci. Technol. 2015, 52, 1414–1423. [Google Scholar] [CrossRef] [Green Version]
- Brasil, V.C.B.; Guimarães, B.P.; Evaristo, R.B.W.; Carmo, T.S.; Ghesti, G.F. Buckwheat (Fagopyrum esculentum Moench) characterization as adjunct in beer brewing. Food Sci. Technol. 2021, 41, 265–272. [Google Scholar] [CrossRef]
- Deng, Y.; Lim, J.; Lee, G.-H.; Nguyen, T.T.H.; Xiao, Y.; Piao, M.; Kim, D. Brewing Rutin-Enriched Lager Beer with Buckwheat Malt as Adjuncts. J. Microbiol. Biotechnol. 2019, 29, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Starowicz, M.; Koutsidis, G.; Zieliński, H. Sensory analysis and aroma compounds of buckwheat containing products—A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1767–1779. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of phenolic compounds in honey using dispersive liquid-liquid microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J.Á. Vanilla (Vanilla planifolia Andr.), its residues and other industrial by-products for recovering high value flavor molecules: A review. J. Appl. Res. Med. Aromat. Plants 2017, 6, 1–9. [Google Scholar] [CrossRef]
- do Nascimento, K.S.; Gasparotto Sattler, J.A.; Lauer Macedo, L.F.; Serna González, C.V.; Pereira de Melo, I.L.; da Silva Araújo, E.; Granato, D.; Sattler, A.; de Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. LWT Food Sci. Technol. 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Wongsa, P.; Khampa, N.; Horadee, S.; Chaiwarith, J.; Rattanapanone, N. Quality and bioactive compounds of blends of Arabica and Robusta spray-dried coffee. Food Chem. 2019, 283, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile Compounds Content, Physicochemical Parameters, and Antioxidant Activity of Beers with Addition of Mango Fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Marova, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of Phenolic Compounds in Lager Beers of Different Origin: A Contribution to Potential Determination of the Authenticity of Czech Beer. Chromatographia 2011, 73, 83–95. [Google Scholar] [CrossRef]
- Cortese, M.; Gigliobianco, M.R.; Peregrina, D.V.; Sagratini, G.; Censi, R.; Di Martino, P. Quantification of phenolic compounds in different types of crafts beers, worts, starting and spent ingredients by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1612, 460622. [Google Scholar] [CrossRef] [PubMed]
- dos Anjos, J.P.; Cardoso, M.D.G.; Saczk, A.A.; Dórea, H.S.; Santiago, W.D.; Machado, A.M.R.; Zacaroni, L.M.; Nelson, D.L. Evolution of the concentration of phenolic compounds in cachaça during aging in an oak (Quercus sp.) barrel. J. Braz. Chem. Soc. 2011, 22, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Ribani, M.; Bottoli, C.B.G.; Collins, C.H.; Jardim, I.C.S.F.; Melo, L.F.C. Validação em métodos cromatográficos e eletroforéticos. Quim. Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]



| Phenolic Compounds | Linear Range (mg L−1) | R2 | LOD * (mg L−1) | LOQ * (mg L−1) |
|---|---|---|---|---|
| Catechin | 0.10–12.5 | 0.9999 | 0.08 | 0.28 |
| Caffeic acid | 0.25–12.5 | 0.9994 | 0.22 | 0.74 |
| Epicatechin | 0.50–12.5 | 0.9980 | 0.39 | 1.29 |
| p-Coumaric acid | 0.10–10.0 | 0.9999 | 0.08 | 0.27 |
| Hydrated rutin | 0.25–12.5 | 0.9999 | 0.10 | 0.35 |
| Trans-ferulic acid | 0.10–10.0 | 0.9998 | 0.08 | 0.28 |
| Quercetin | 0.50–12.5 | 0.9990 | 0.35 | 1.16 |
| Kaempferol | 1.0–12.5 | 0.9966 | 0.83 | 2.78 |
| Formononetin | 0.10–7.5 | 0.9997 | 0.09 | 0.32 |
| Phenolic Compounds | RSD (%) * | ||
|---|---|---|---|
| 0.5 mg L−1 | 7.5 mg L−1 | 12.5 mg L−1 | |
| Catechin | 4.20 | 5.70 | 2.77 |
| Caffeic acid | 4.51 | 4.50 | 3.08 |
| Epicatechin | 5.19 | 5.22 | 3.85 |
| p-Coumaric acid | 5.19 | 5.21 | 3.73 |
| Hydrated rutin | 14.5 | 11.5 | 16.2 |
| Trans-ferulic acid | 6.78 | 4.53 | 5.86 |
| Quercetin | 7.68 | 4.76 | 2.75 |
| Kaempferol | 5.72 | 3.38 | 2.59 |
| Formononetin | 4.68 | 5.30 | 3.15 |
| Phenolic Compounds | Concentration in the Sample * (mg L−1) | Added Concentration (mg L−1) | ||
|---|---|---|---|---|
| 0.5 | 7.5 | 12.5 | ||
| Accuracy (%) | ||||
| Catechin | ND | 105 | 103 | 95.9 |
| Caffeic acid | ND | 85.1 | 85.8 | 93.9 |
| Epicatechin | 1.43 | 88.6 | 94.3 | 99.3 |
| p-Coumaric acid | ND | 87.5 | 86.4 | 88.2 |
| Hydrated rutin | 0.61 | 112 | 88.3 | 91.4 |
| Trans-ferulic acid | <LOQ | 111 | 90.9 | 94.2 |
| Quercetin | <LOQ | 91.8 | 95.1 | 105 |
| Kaempferol | <LOQ | 69.8 | 98.2 | 99.2 |
| Formononetin | 0.70 | 68.6 | 101 | 105 |
| Phenolic Compounds | Concentration (mg L−1) * | |||||||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 | Sample 8 | |
| Catechin | ND | ND | ND | 16.8 ± 0.3 | 0.41 ± 0.02 | ND | 38.6 ± 0.08 | 65.2 ± 1.6 |
| Caffeic acid | ND | ND | ND | <LOQ | <LOQ | <LOQ | 8.13 ± 0.21 | ND |
| Epicatechin | 1.43 ± 0.07 | 2.50 ± 0.16 | 3.16 ± 0.22 | 3.55 ± 0.07 | 1.94 ± 0.08 | ND | 51.1 ± 0.0 | ND |
| p-Coumaric acid | ND | 0.97 ± 0.02 | 1.11 ± 0.04 | ND | 0.70 ± 0.01 | <LOQ | 0.47 ± 0.06 | ND |
| Hydrated rutin | 0.62 ± 0.02 | 0.92 ± 0.02 | 0.62 ± 0.02 | 1.07 ± 0.02 | 0.39 ± 0.02 | 0.93 ± 0.03 | 1.45 ± 0.04 | 1.13 ± 0.04 |
| Trans-ferulic acid | <LOQ | 2.10 ± 0.03 | 2.19 ± 0.01 | 1.74 ± 0.02 | 1.57 ± 0.04 | 1.42 ± 0.02 | 1.34 ± 0.11 | 1.59 ± 0.13 |
| Quercetin | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ND | <LOQ |
| Kaempferol | <LOQ | <LOQ | ND | ND | ND | ND | ND | ND |
| Formononetin | 0.71 ± 0.02 | 1.47 ± 0.01 | 1.55 ± 0.02 | 1.70 ± 0.02 | 1.88 ± 0.01 | 1.49 ± 0.00 | 2.40 ± 0.06 | 0.68 ± 0.04 |
| Total (Sum of the phenolic compounds) | 2.76 | 7.96 | 8.63 | 24.9 | 6.89 | 3.84 | 103.5 | 68.6 |
| Phenolic Compounds | Concentration (mg L−1) * | |||||||
| Sample 9 | Sample 10 | Sample 11 | Sample 12 | Sample 13 | Sample 14 | Sample 15 | ||
| Catechin | 37.4 ± 0.9 | 108.3 ± 1.7 | 124.8 ± 1.9 | ND | ND | ND | ND | |
| Caffeic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| Epicatechin | 6.73 ± 0.36 | ND | 9.12 ± 0.28 | ND | 2.46 ± 0.09 | 2.61 ± 0.06 | ND | |
| p-Coumaric acid | 4.17 ± 0.23 | <LOQ | ND | <LOQ | 0.66 ± 0.00 | <LOQ | <LOQ | |
| Hydrated rutin | 1.36 ± 0.02 | 0.79 ± 0.02 | 1.17 ± 0.09 | 2.94 ± 0.04 | 1.29 ± 0.02 | 0.63 ± 0.08 | 0.49 ± 0.02 | |
| Trans-ferulic acid | 1.26 ± 0.09 | 0.89 ± 0.02 | 1.31 ± 0.06 | <LOQ | <LOQ | <LOQ | 0.66 ± 0.01 | |
| Quercetin | 1.19 ± 0.02 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 1.45 ± 0.02 | |
| Kaempferol | ND | ND | ND | ND | ND | ND | <LOQ | |
| Formononetin | 0.52 ± 0.01 | 0.74 ± 0.01 | 1.67 ± 0.00 | 1.88 ± 0.01 | 1.35 ± 0.02 | 1.08 ± 0.02 | 1.67 ± 0.04 | |
| Total (Sum of the phenolic compounds) | 52.6 | 110.7 | 138.1 | 4.82 | 5.76 | 4.32 | 4.27 | |
| Samples | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Total phenolic compounds (mg per drink) a | 0.97 | 2.79 | 3.02 | 8.72 | 2.41 | 1.34 | 36.2 | 24.0 |
| Daily value (%) b | 0.2 | 0.6 | 0.7 | 1.9 | 0.5 | 0.3 | 7.9 | 5.2 |
| Samples | ||||||||
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| Total phenolic compounds (mg per drink) a | 18.4 | 38.7 | 48.3 | 1.69 | 2.02 | 1.51 | 1.49 | |
| Daily value (%) b | 4.0 | 8.4 | 11 | 0.4 | 0.4 | 0.3 | 0.3 | |
| Compounds Analyzed | Sample-Preparation Technique | Analysis Technique | LOD (mg L−1) | LOQ (mg L−1) | Concentration Range (mg L−1) | Ref. |
|---|---|---|---|---|---|---|
| Gallic acid; protocatechuic acid; gentisic acid; catechin; caffeic acid; epicatechin; p-coumaric acid; ferulic acid; salicylic acid; quercetin | Solid-phase extraction (SPE) | HPLC-DAD | 0.04–0.41 | 0.14–0.80 | 0.28–5.04 | [10] |
| Gallic acid; (-)-catechin; epicatechin; ferulic acid; chlorogenic acid; morin; rutin; quercetin; kaempferol; naringenin; luteolin | Liquid–liquid extraction (LLE) | LC-PDA LC-ESI-MS | 0.02–0.04 0.006–0.012 | 0.06–0.012 0.02–0.04 | 0.24–5.70 0.010–2.38 | [45] |
| Gallic acid; vanillic acid; p-coumaric acid; trans-ferulic acid; sinapic acid; caffeic acid; syringic acid; 4-hydroxibenzoic acid; (+)-catechin; hydrate quercetin; 3-caffeoylquinic acid; xanthohumol; isoxanthohumol; 8-prenylnarigenin; cohumulone, colupulone; lupulone; colupulone | Dilution Solid-phase extraction (SPE) Simplified liquid extraction (SLE) | HPLC-API-MS/MS | 0.001–0.150 | 0.004–0.500 | 0.1–34.4 (µg kg−1) | [46] |
| Catechin; caffeic acid; epicatechin; p-coumaric acid; hydrated rutin; trans-ferulic acid; quercetin; kaempferol and formononetin | Not applicable | HPLC-DAD | 0.08–0.83 | 0.27–2.78 | <0.27–124.8 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.C.; dos Anjos, J.P.; Guarieiro, L.L.N.; Machado, B.A.S. A Simple Method for Evaluating the Bioactive Phenolic Compounds’ Presence in Brazilian Craft Beers. Molecules 2021, 26, 4716. https://doi.org/10.3390/molecules26164716
Silva MC, dos Anjos JP, Guarieiro LLN, Machado BAS. A Simple Method for Evaluating the Bioactive Phenolic Compounds’ Presence in Brazilian Craft Beers. Molecules. 2021; 26(16):4716. https://doi.org/10.3390/molecules26164716
Chicago/Turabian StyleSilva, Marcelo Coelho, Jeancarlo Pereira dos Anjos, Lilian Lefol Nani Guarieiro, and Bruna A. Souza Machado. 2021. "A Simple Method for Evaluating the Bioactive Phenolic Compounds’ Presence in Brazilian Craft Beers" Molecules 26, no. 16: 4716. https://doi.org/10.3390/molecules26164716
APA StyleSilva, M. C., dos Anjos, J. P., Guarieiro, L. L. N., & Machado, B. A. S. (2021). A Simple Method for Evaluating the Bioactive Phenolic Compounds’ Presence in Brazilian Craft Beers. Molecules, 26(16), 4716. https://doi.org/10.3390/molecules26164716