Photometric Determination of Iron in Pharmaceutical Formulations Using Double-Beam Direct Injection Flow Detector
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Parameters
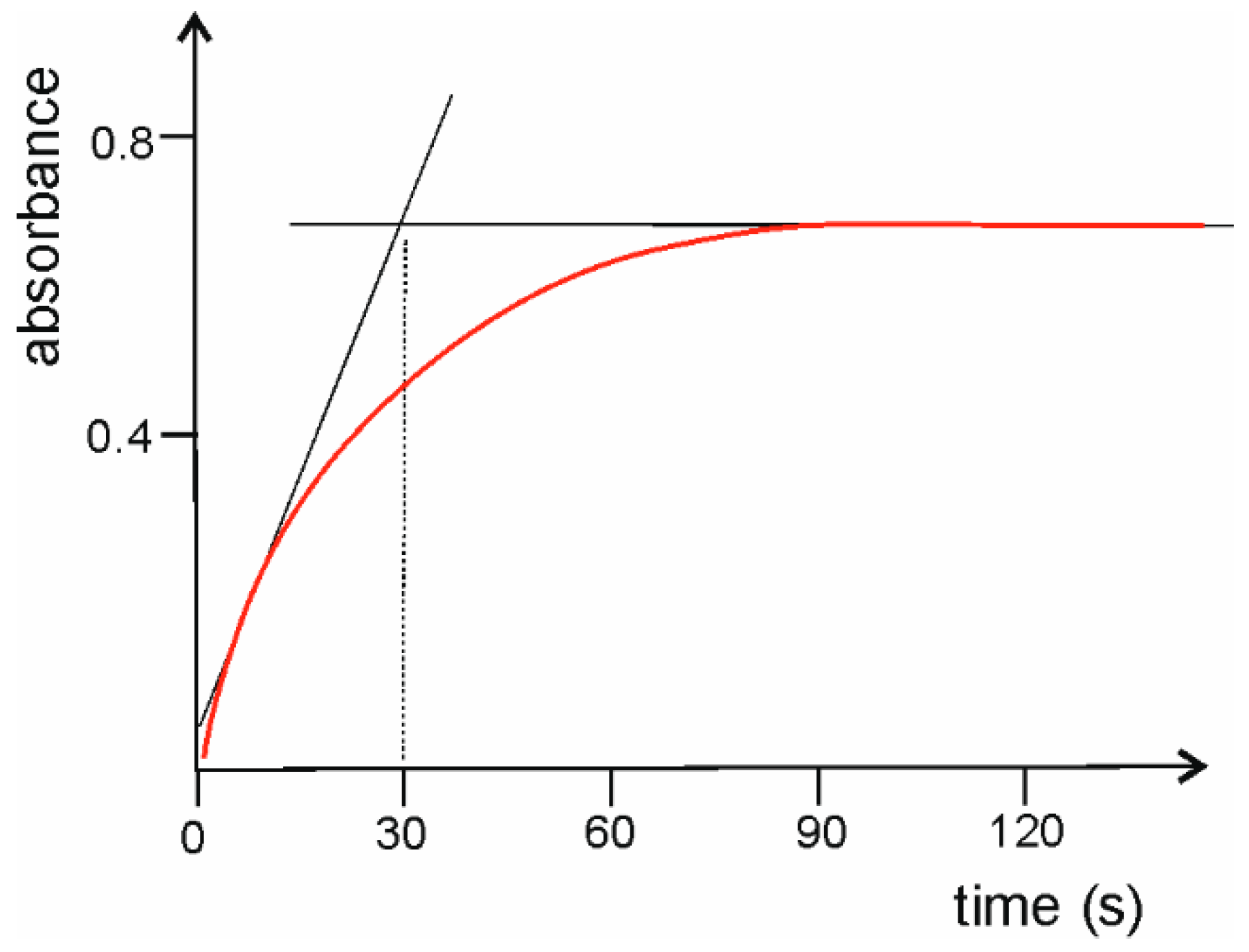
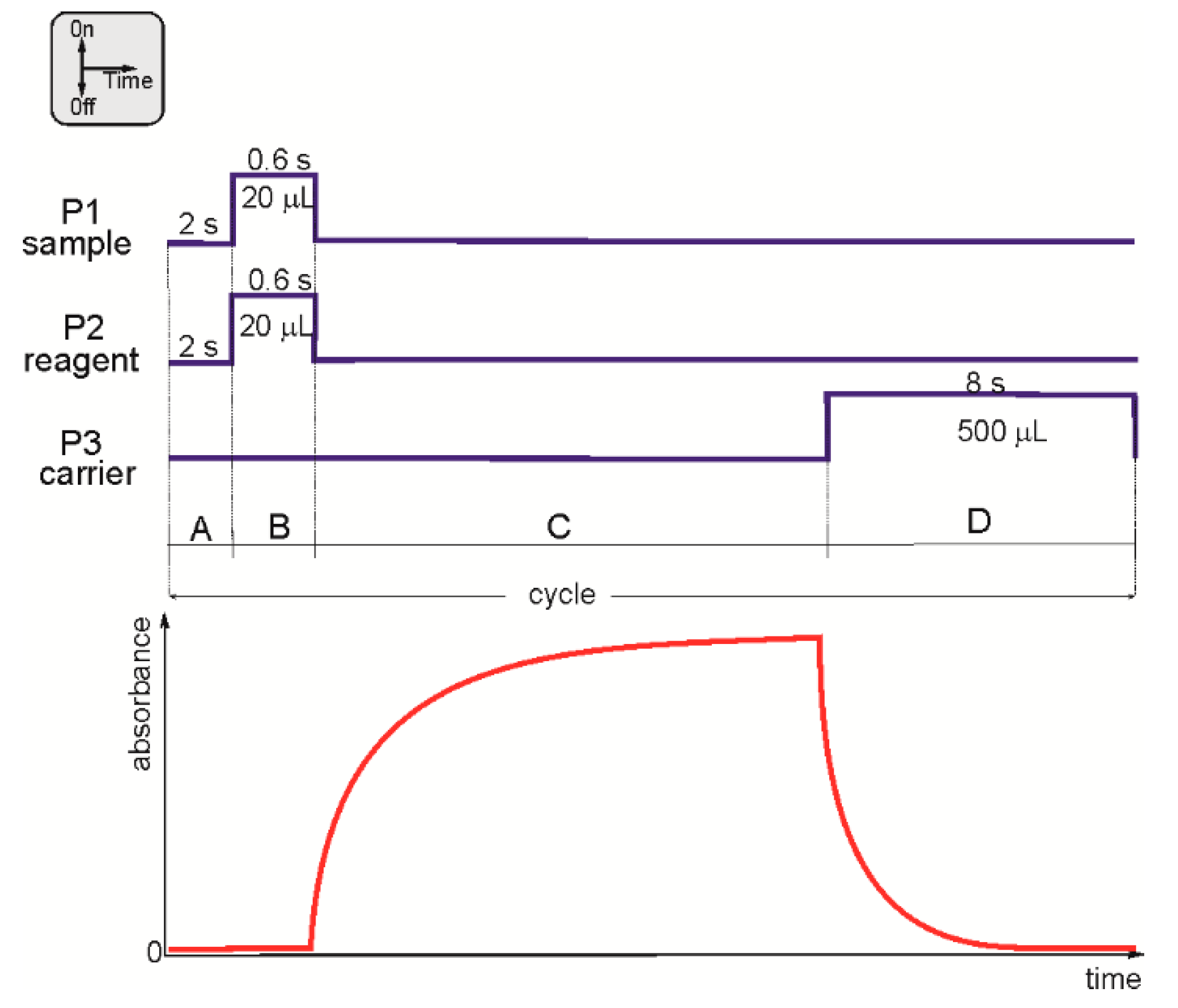
2.1.1. Stop-Flow Time
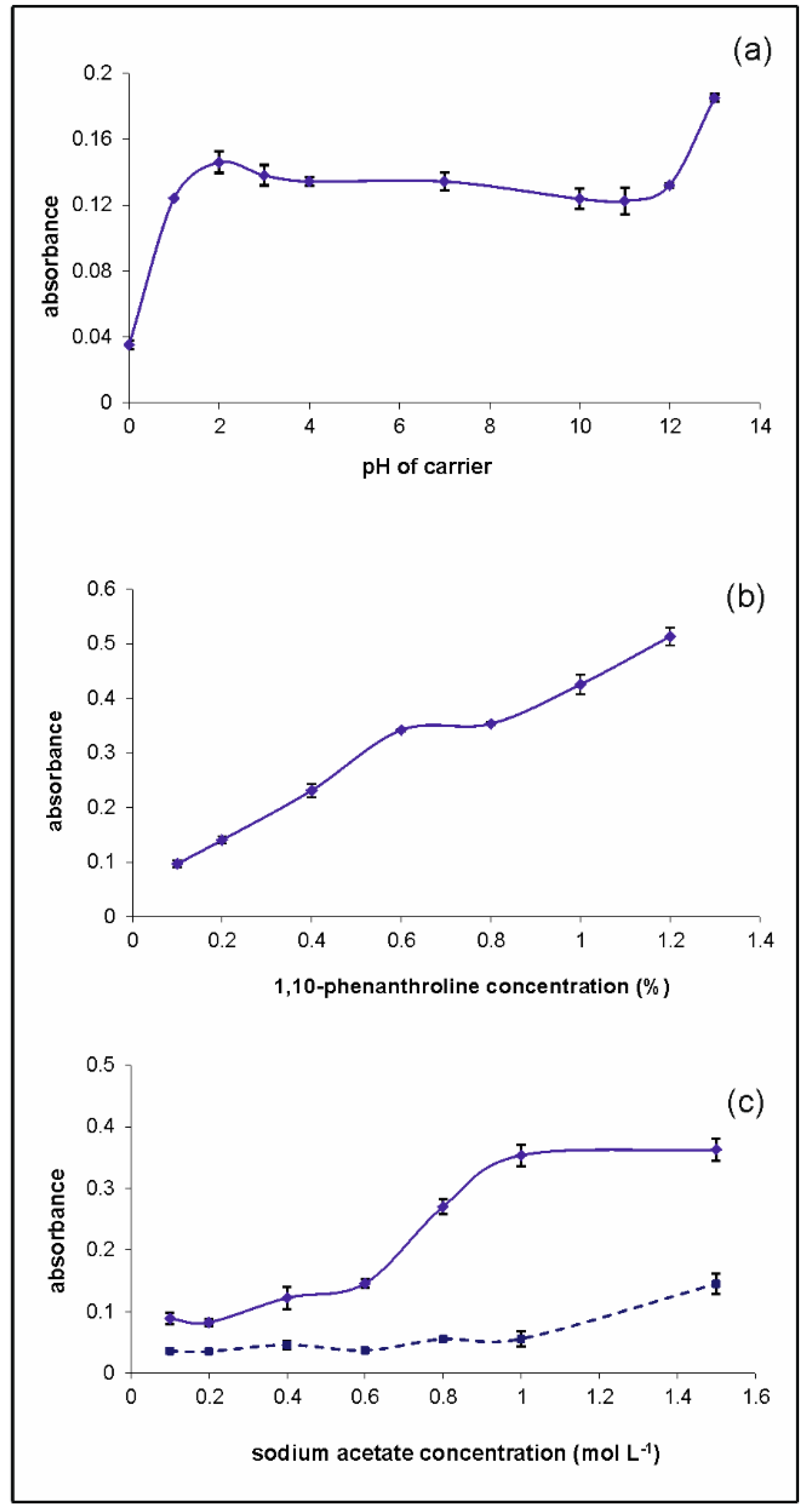
2.1.2. Chemical Parameters
2.2. Method Evaluation
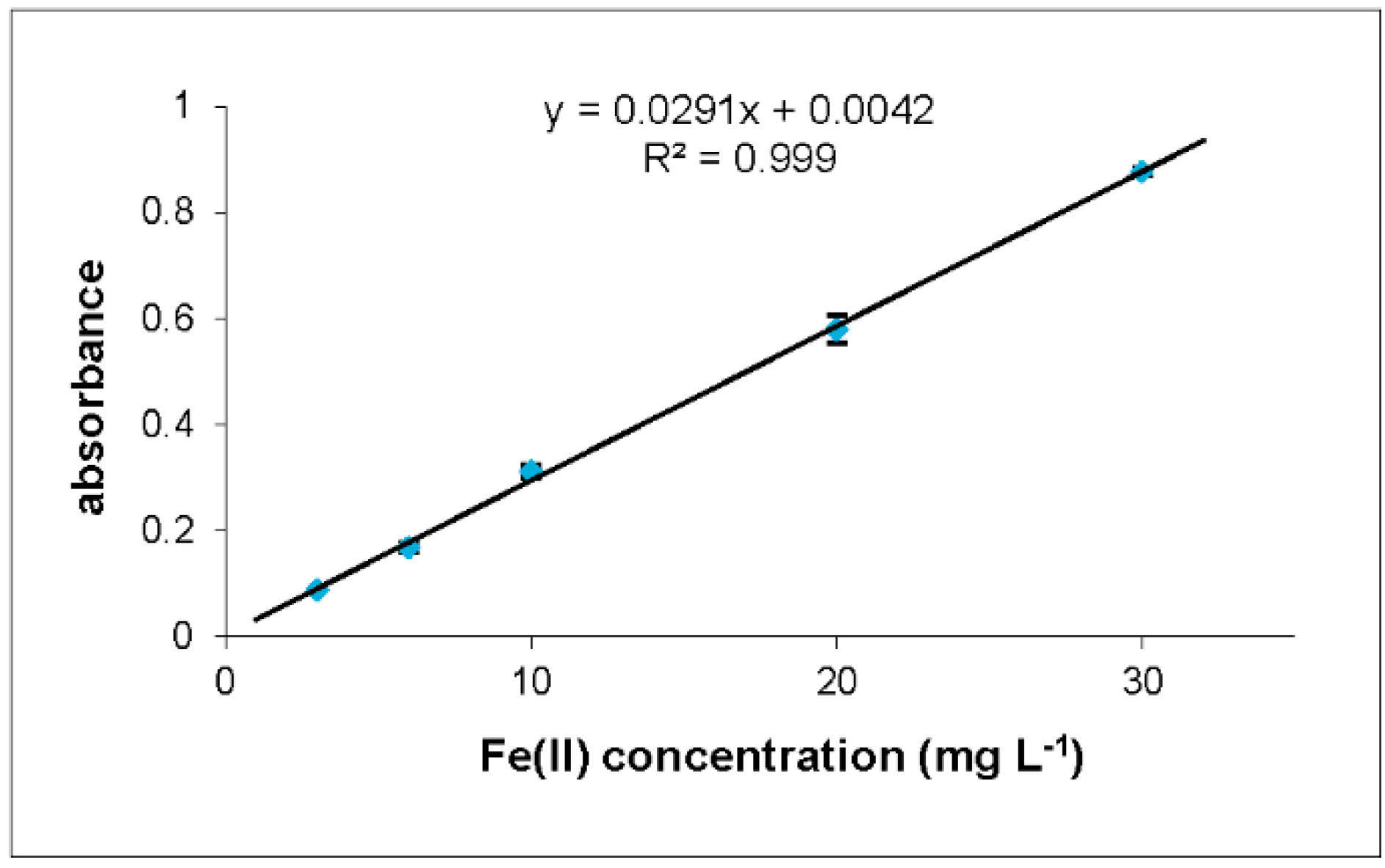
2.2.1. Analytical Parameters
2.2.2. Application to the Real Samples
2.2.3. Recovery Test
2.3. Comparison of Proposed Methodology with Other Modern Metodologies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples
3.3. Apparatus
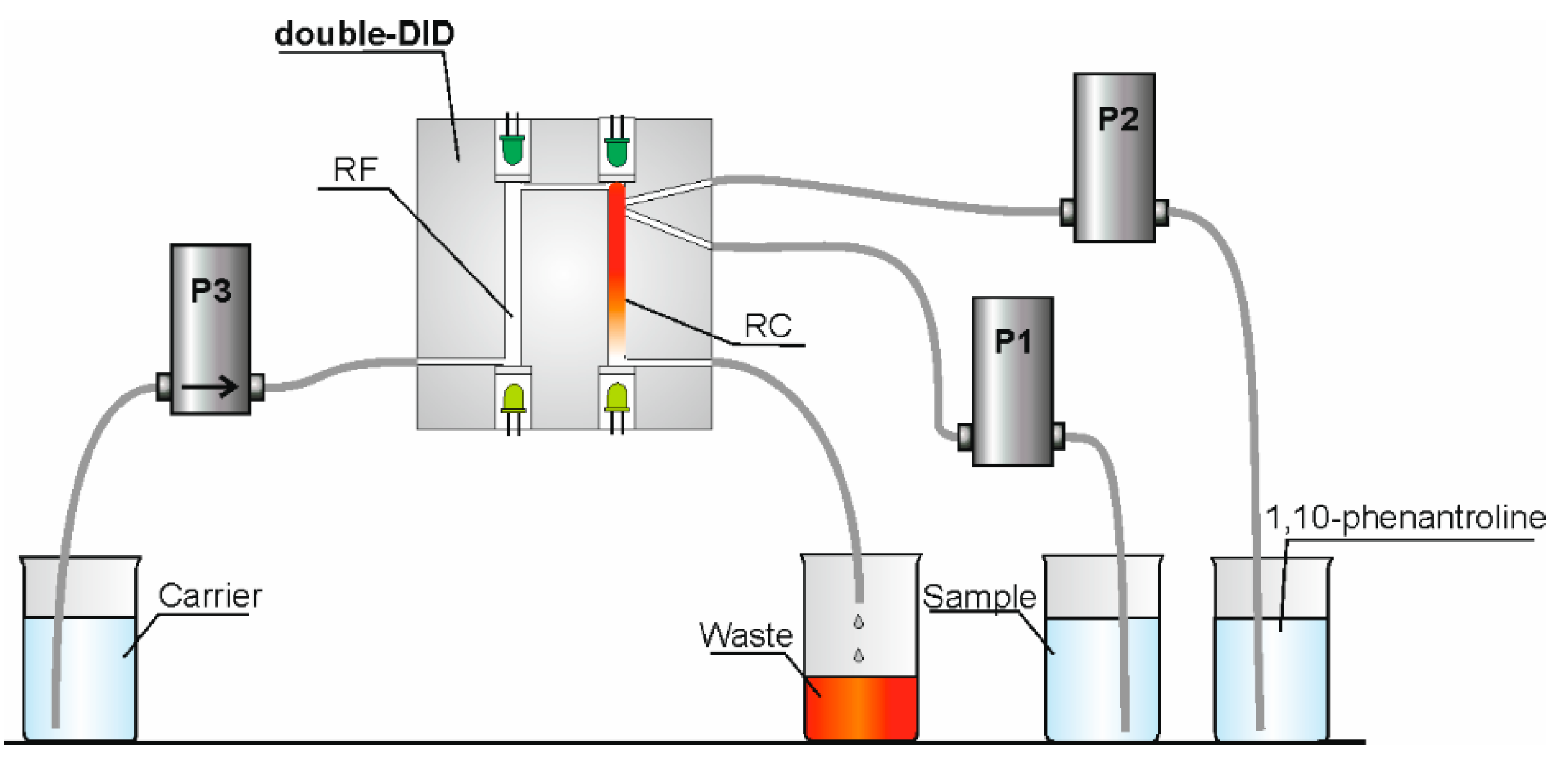
3.3.1. Flow System
3.3.2. Procedure
3.3.3. Reference Method
4. Conclusions
5. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Beard, J.L.; Dawson, H.; Pinero, D.J. Iron metabolism: A comprehensive review. Nutr. Rev. 1996, 54, 296–317. [Google Scholar] [CrossRef]
- Karpinska, J.; Kulikowska, M. Simultaneous determination of zinc (II), manganese (II) and iron (II) in pharmaceutical preparations. J. Pharm. Biomed. 2002, 29, 153–158. [Google Scholar] [CrossRef]
- Vakh, C.; Freze, E.; Pochivalov, A.; Evdokimova, E.; Kamencev, M.; Moskvin, L.; Bulatov, A. Simultaneous determination of iron (II) and ascorbic acid in pharmaceuticals based on flow sandwich technique. J. Pharmacol. Toxicol. Methods 2015, 73, 56–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliveira, P.C.C.; Masini, J.C. Sequential injection of iron (II) in antianemic pharmaceutical formulations with spectrophotometric detection. Anal. Lett. 2001, 34, 389–397. [Google Scholar] [CrossRef]
- Tesfaldet, Z.O.; van Staden, J.F.; Stefan, R.I. Sequential injection spectrophotometric determination of iron as Fe (II) in multi-vitamin preparations using 1,10-phenanthroline as complexing agent. Talanta 2004, 64, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Deng, N.S. Speciation analysis of iron in traditional Chinese medicine by flame atomic absorption spectrometry. J. Pharm. Biomed. Anal. 2003, 32, 51–57. [Google Scholar] [CrossRef]
- Waseem, A.; Yaqoob, M.; Nabi, A. Analytical Applications of Flow Injection Chemiluminescence for the Determination of Pharmaceuticals—A Review. Curr. Pharm. Anal. 2013, 9, 363–395. [Google Scholar] [CrossRef]
- Merli, D.; Profumo, A.; Dossi, C. An analytical method for Fe (II) and Fe (III) determination in pharmaceutical grade iron sucrose complex and sodium ferric gluconate complex. J. Pharm. Anal. 2012, 2, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, W.H. Iron ion-selective electrodes for direct potentiometry and potentiotitrimetry in pharmaceuticals. Anal. Chim. Acta 2001, 436, 199–206. [Google Scholar] [CrossRef]
- Feres, M.A.; Reis, B.F. A downsized flow set up based on multicommutation for the sequential photometric determination of iron(II)/iron(III) and nitrite/nitrate in surface water. Talanta 2005, 68, 422–428. [Google Scholar] [CrossRef]
- Paluch, J.; Kościelniak, P.; Moleda, I.; Machowski, K.; Kalinowski, S.; Koronkiewicz, S.; Kozak, J. Novel approach to determination of Fe(II) using a flow system with direct-injection detector. Mon. Chem. Chem. Mon. 2020, 151, 1305–1310. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Eom, I.Y.; Morris, K.J.; Li, J. Light emitting diode-based detectors: Absorbance, fluorescence and spectroelectrochemical measurements in a planar flow-through cell. Anal. Chim. Acta 2003, 500, 337–364. [Google Scholar] [CrossRef]
- O’ Toole, M.; Lau, K.T.; Diamond, D. Photometric detection in flow analysis systems using integrated PEDDs. Talanta 2005, 66, 1340–1344. [Google Scholar] [CrossRef]
- Li, S.; Pandharipande, A. LED-based color sensing and control. IEEE Sens. J. 2015, 15, 6116–6124. [Google Scholar] [CrossRef]
- Bui, D.A.; Hauser, P.C. Absorbance measuring with light-emitting diodes as sources: Silicon photodiodes or light-emitting diodes as detector? Talanta 2013, 116, 1073–1078. [Google Scholar]
- Pires, C.K.; Reis, B.F.; Morales-Rubio, A.; de la Guardia, M. Speciation of chromium in natural waters by micropumping multicommutated light emitting diode photometry. Talanta 2007, 72, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Tymecki, L.; Pokrzywnicka, M.; Koncki, R. Paired emitter detector diode (PEDD) based photometry—An alternative approach. Analyst 2008, 133, 1501–1504. [Google Scholar] [CrossRef]
- Koronkiewicz, S.; Kalinowski, S. A novel direct-injection detector integrated with solenoid pulse-pump flow system. Talanta 2011, 86, 436–441. [Google Scholar] [CrossRef]
- Kalinowski, S.; Koronkiewicz, S. Double-beam photometric direct-injection detector for multi-pumping flow system. Sens. Actuators A 2017, 258, 146–155. [Google Scholar] [CrossRef]
- Koronkiewicz, S.; Kalinowski, S. Application of direct-injection detector integrated with the multi-pumping flow system to photometric, stop-flow determination of total iron. Talanta 2012, 96, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rybkowska, N.; Koncki, R.; Strzelak, K. Optoelectronic iron detectors for pharmaceutical flow analysis. J. Pharm. Biomed. Anal. 2017, 145, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Lapa, R.A.S.; Lima, J.L.F.C.; Reis, B.F.; Santos, J.L.M.; Zagatto, E.A.G. Multi-pumping in flow analysis: Concepts, instrumentation, potentialities. Anal. Chim. Acta 2002, 466, 125–132. [Google Scholar] [CrossRef]
- Santos, J.L.M.; Ribeiro, M.F.T.; Dias, A.C.B.; Lima, J.L.F.C.; Zagatto, E.E.A. Multi-pumping flow systems: The potential of simplicity. Anal. Chim. Acta 2007, 600, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fortes, P.R.; Feres, M.A.; Sasaki, M.K.; Alves, E.R.; Zagatto, E.A.G.; Prior, J.A.V.; Santos, J.L.M.; Lima, J.L.F.C. Evidences for turbulent mixing in multi-pumping flow systems. Talanta 2009, 79, 978–983. [Google Scholar] [CrossRef]
- Marczenko, Z. Separation and Spectrophotometric Determination of Elements; Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Ferreira, A.M.R.; Lima, J.L.F.C.; Rangel, A.O.S.S. Colorimetric determination of available iron in soil by flow injection analysis. Analusis 1996, 24, 343–346. [Google Scholar]
- Gomes, D.M.C.; Segundo, M.A.; Lima, J.L.F.C.; Rangel, A.O.S.S. Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injection system. Talanta 2005, 66, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Software for Flow Injection Analysis. Available online: http://www.uwm.edu.pl/kchem/el_team/software/fia.htm (accessed on 21 June 2021).
- Kalinowski, S.; Koronkiewicz, S. Instantaneous Photometric Detector. Polish Patent No. Pat.222359, 11 September 2015. [Google Scholar]

| Sample | Proposed Flow System | Manual Procedure | Claimed Value |
|---|---|---|---|
| Sorbifer Durules | 100.63 ± 0.25 | 99.25 ± 0.27 | 100 |
| Hemofer Prolongtum | 113.03 ± 0.28 | 108.41 ± 0.31 | 105 |
| Chela-ferr biocomplex | 13.95 ± 0.06 | 13.63 ± 0.03 | 14 |
| Ascofer | 23.66 ± 0.07 | 24.02 ± 0.08 | 23.2 |
| Actiferol | 28.78 ± 0.09 | 29.05 ± 0.08 | 30 |
| Sample | Determined Concentration of Fe(II) (mg L−1) | Added Fe(II) (mg L−1) | Found Fe(II) (mg L−1) | Recovery (%) |
|---|---|---|---|---|
| Sorbifer Durules | 10.57 ± 0.09 | 5.00 | 4.79 | 96 |
| 10.00 | 9.74 | 97 | ||
| 15.00 | 16.12 | 107 | ||
| Hemofer Prolongatum | 16.72 ± 0.28 | 5.00 | 4.90 | 98 |
| 10.00 | 9.93 | 99 | ||
| 15.00 | 13.98 | 93 | ||
| Chela-ferr biocomplex | 12.28 ± 0.25 | 5.00 | 5.01 | 100 |
| 10.00 | 9.73 | 97 | ||
| 15.00 | 14.61 | 97 |
| Detection | Reagent | Flow System | Sample/Reagent Consumption (Waste) (µL) | LOD or LOQ (mg L−1) | Working Range (mg L−1) | Repeatability R.S.D. (%) | Type of Sample/Throughput (Injection h−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| Conventional spectrophotometer | 4-(2-pyridylazo) resorcinol | not applicable | no inf. | LOD = 0.008 | 0.025–0.2 | 2.9 | pharmaceuticals/10 | [2] |
| Conventional spectrophotometer | 1,10-phenanthroline | FIA | 1000/150 (no inf.) | LOD = 0.2 | 0.5–4.0 | <3.0 | pharmaceuticals/41 | [3] |
| Conventional spectrophotometer FIAlab 3500 instrument | 2,2-bipyridyl | SIA | 50/200 (no inf.) | LOD = 1.0 | 5.0–40 | 5.0 | pharmaceuticals/100 | [4] |
| Conventional spectrophotometer | 1,10-phenanthroline | SIA | 187/140 (no inf.) | LOD = 0.02 | 0.25–5.0 | 3.0 | pharmaceuticals/40 | [5] |
| LED-phototransistor | 1,10-phenanthroline | multicommutated FA | no inf. (2400) | LOD = 0.5 | 0.5–6.0 | 1.0 | surface water/40 | [10] |
| Single-DID | 1,10-phenanthroline | MPFS | 80/20 (500) | LOQ = 0.07 | 0.07–1.00 1.00–7.00 | 14.8 9.6 | wine, wastewater/36 | [11] |
| Double-DID | 1,10-phenanthroline | MPFS | 20/20 (540) | LOD = 0.5 LOQ = 1.7 | 1–30 | 2.0 | pharmaceuticals/90 | this work |
| No. | Name/Supplier | Form | Active Substance |
|---|---|---|---|
| 1 | Sorbifer Durules/Egis Pharmaceuticals PLC (Budapest, Hungary) | tablet | FeSO4 |
| 2 | Hemofer Prolongatum/GlaxoSmithKline Pharmaceuticals (Poznan, Poland) | tablet | FeSO4 |
| 3 | Chela-Ferr/OlimpLabs (Debica, Poland) | capsule with powder | iron(II) diglycinate |
| 4 | Ascofer/Espefa (Cracow, Poland) | tablet | iron(II) gluconate |
| 5 | Actiferol/Sequoia (Warsaw, Poland) | sachet with powder | iron(II) pyrophosphate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koronkiewicz, S. Photometric Determination of Iron in Pharmaceutical Formulations Using Double-Beam Direct Injection Flow Detector. Molecules 2021, 26, 4498. https://doi.org/10.3390/molecules26154498
Koronkiewicz S. Photometric Determination of Iron in Pharmaceutical Formulations Using Double-Beam Direct Injection Flow Detector. Molecules. 2021; 26(15):4498. https://doi.org/10.3390/molecules26154498
Chicago/Turabian StyleKoronkiewicz, Stanislawa. 2021. "Photometric Determination of Iron in Pharmaceutical Formulations Using Double-Beam Direct Injection Flow Detector" Molecules 26, no. 15: 4498. https://doi.org/10.3390/molecules26154498
APA StyleKoronkiewicz, S. (2021). Photometric Determination of Iron in Pharmaceutical Formulations Using Double-Beam Direct Injection Flow Detector. Molecules, 26(15), 4498. https://doi.org/10.3390/molecules26154498





