Hispolon Induces Apoptosis, Suppresses Migration and Invasion of Glioblastoma Cells and Inhibits GBM Xenograft Tumor Growth In Vivo
Abstract
:1. Introduction
2. Results
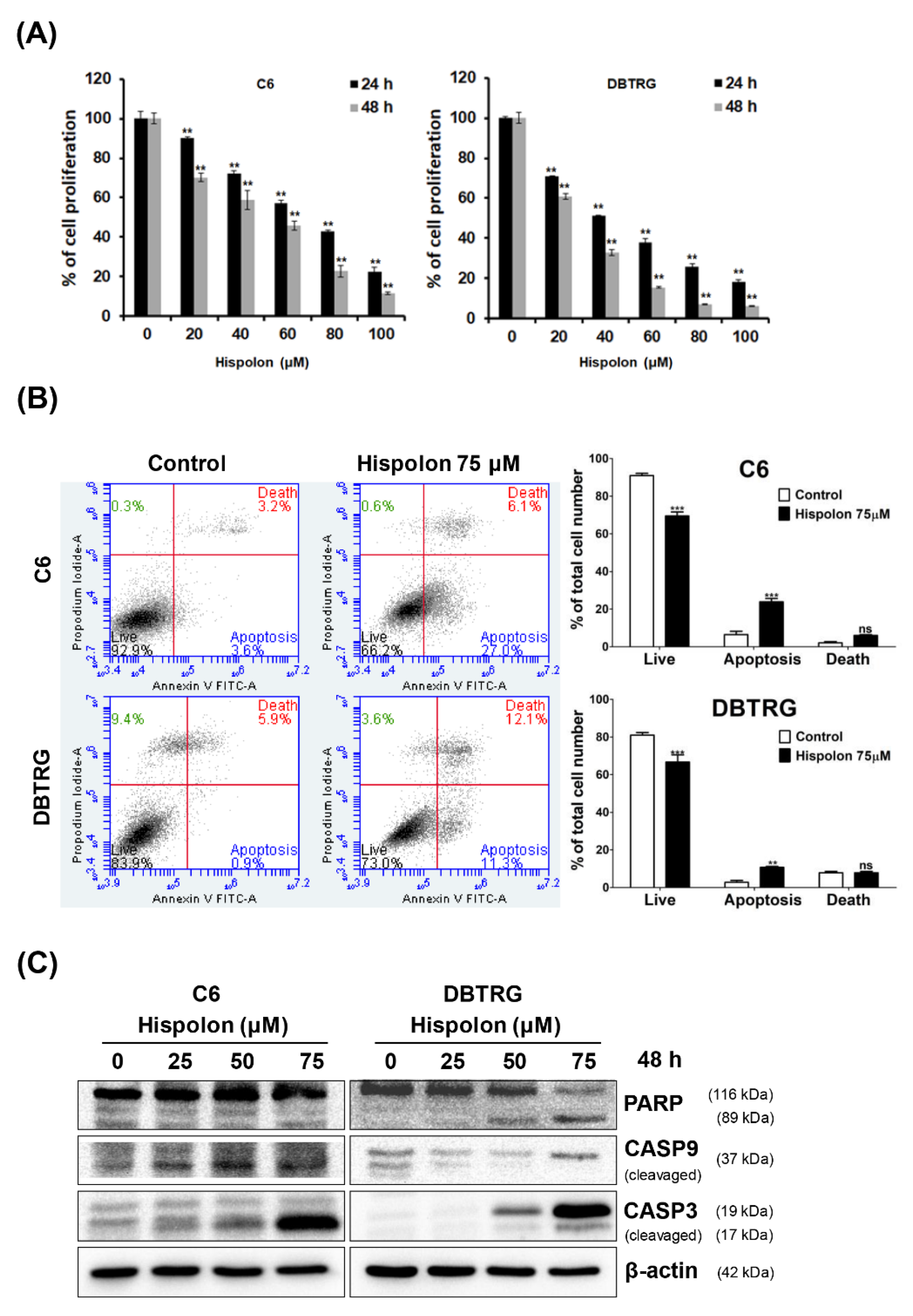
2.1. Effects of Hispolon on the Viability of GBM Cells
2.2. Hispolon Induces Apoptosis and Caspase Activation in GBM Cells
2.3. Hispolon Induces Cell Cycle G2/M Arrest on GBM Cells
2.4. Hispolon Inhibits Cell Migration and Invasion in GBM Cells
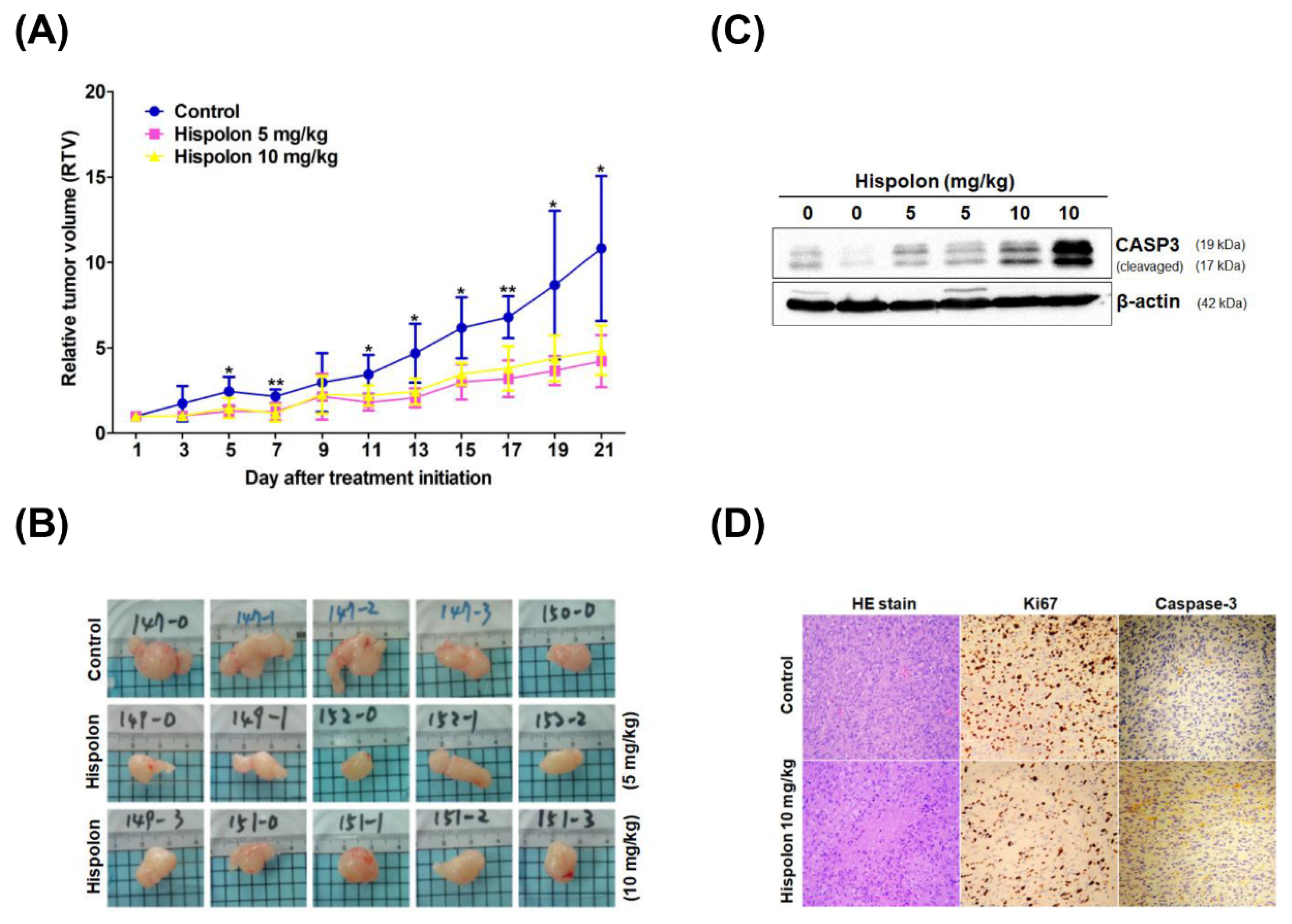
2.5. Hispolon Suppresses Tumor Growth in DBTRG Xenograft Mice
3. Discussion
4. Materials and Methods
4.1. Glioblastoma Cell Lines
4.2. Chemicals and Reagents
4.3. Cell Viability Assay
4.4. RNA Extraction and Real-Time RT-PCR
4.5. Western Blot
4.6. Cell Cycle Analysis
4.7. Cell Migration and Invasion Assay
4.8. Animal Studies
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Merkel, A.; Soeldner, D.; Wendl, C.; Urkan, D.; Kuramatsu, J.B.; Seliger, C.; Proescholdt, M.; Eyupoglu, I.Y.; Hau, P.; Uhl, M. Early postoperative tumor progression predicts clinical outcome in glioblastoma-implication for clinical trials. J. Neurooncol. 2017, 132, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, T.; Collins, L.; Xiao, Z.X.; Kim, S.H.; Chen, C.Y. Modulation of lung cancer growth arrest and apoptosis by Phellinus linteus. Mol. Carcinog. 2007, 46, 144–154. [Google Scholar] [CrossRef]
- Kim, B.C.; Choi, J.W.; Hong, H.Y.; Lee, S.A.; Hong, S.; Park, E.H.; Kim, S.J.; Lim, C.J. Heme oxygenase-1 mediates the anti-inflammatory effect of mushroom Phellinus linteus in LPS-stimulated RAW264.7 macrophages. J. Ethnopharmacol. 2006, 106, 364–371. [Google Scholar] [CrossRef]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S.; et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, H.J.; Lim, E.S.; Ahn, K.S.; Shim, B.S.; Kim, H.M.; Gong, S.J.; Kim, D.K.; Kim, S.H. Cambodian Phellinus linteus inhibits experimental metastasis of melanoma cells in mice via regulation of urokinase type plasminogen activator. Biol. Pharm. Bull. 2005, 28, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sliva, D.; Jedinak, A.; Kawasaki, J.; Harvey, K.; Slivova, V. Phellinus linteus suppresses growth, angiogenesis and invasive behaviour of breast cancer cells through the inhibition of AKT signalling. Br. J. Cancer 2008, 98, 1348–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.Y.; Lee, J.Y.; Lee, J.O.; Ryu, C.H.; Choi, B.T.; Jeong, Y.K.; Lee, K.W.; Jeong, S.C.; Choi, Y.H. Partial characterization and immunostimulatory effect of a novel polysaccharide-protein complex extracted from Phellinus linteus. Biosci. Biotechnol. Biochem. 2006, 70, 1218–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.Y.; Oh, W.K.; Shin, B.C.; Shin, Y.I.; Park, Y.C.; Ahn, S.C.; Lee, J.D.; Bae, Y.S.; Kwak, J.Y.; Park, Y.M. Proteoglycan isolated from Phellinus linteus inhibits tumor growth through mechanisms leading to an activation of CD11c+CD8+ DC and type I helper T cell-dominant immune state. FEBS Lett. 2004, 576, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Kim, S.H.; Chen, C.Y. A medicinal mushroom: Phellinus linteus. Curr. Med. Chem. 2008, 15, 1330–1335. [Google Scholar] [CrossRef]
- Wang, J.; Hu, F.; Luo, Y.; Luo, H.; Huang, N.; Cheng, F.; Deng, Z.; Deng, W.; Zou, K. Estrogenic and anti-estrogenic activities of hispolon from Phellinus lonicerinus (Bond.) Bond. et sing. Fitoterapia 2014, 95, 93–101. [Google Scholar] [CrossRef]
- Lu, T.L.; Huang, G.J.; Lu, T.J.; Wu, J.B.; Wu, C.H.; Yang, T.C.; Iizuka, A.; Chen, Y.F. Hispolon from Phellinus linteus has antiproliferative effects via MDM2-recruited ERK1/2 activity in breast and bladder cancer cells. Food Chem. Toxicol. 2009, 47, 2013–2021. [Google Scholar] [CrossRef]
- Chang, H.Y.; Peng, W.H.; Sheu, M.J.; Huang, G.J.; Tseng, M.C.; Lai, M.T.; Ho, Y.L.; Chang, Y.S. Hepatoprotective and Antioxidant Effects of Ethanol Extract from Phellinus merrillii on carbon tetrachloride-induced liver damage. Am. J. Chin. Med. 2007, 35, 793–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Chiu, C.S.; Liao, J.C.; Hsieh, W.T.; Sheu, M.J.; Wu, C.H. Hispolon Protects against Acute Liver Damage in the Rat by Inhibiting Lipid Peroxidation, Proinflammatory Cytokine, and Oxidative Stress and Downregulating the Expressions of iNOS, COX-2, and MMP-9. Evid. Based Complement. Altern. Med. 2012, 2012, 480714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3, -8 and -9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Ho, H.Y.; Ho, Y.C.; Hsieh, M.J.; Yang, S.F.; Chuang, C.Y.; Lin, C.W.; Hsin, C.H. Hispolon suppresses migration and invasion of human nasopharyngeal carcinoma cells by inhibiting the urokinase-plasminogen activator through modulation of the Akt signaling pathway. Environ. Toxicol. 2017, 32, 645–655. [Google Scholar] [CrossRef]
- Masood, M.; Rasul, A.; Sarfraz, I.; Jabeen, F.; Liu, S.; Liu, X.; Wei, W.; Li, J.; Li, X. Hispolon induces apoptosis against prostate DU145 cancer cells via modulation of mitochondrial and STAT3 pathways. Pak. J. Pharm. Sci. 2019, 32, 2237–2243. [Google Scholar]
- Arcella, A.; Sanchez, M. Natural substances to potentiate canonical glioblastoma chemotherapy. J. Chemother. 2021, 1–12. [Google Scholar] [CrossRef]
- Arcella, A.; Oliva, M.A.; Sanchez, M.; Staffieri, S.; Esposito, V.; Giangaspero, F.; Cantore, G. Effects of hispolon on glioblastoma cell growth. Environ. Toxicol. 2017, 32, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chang, H.Y.; Deng, J.S.; Chen, J.J.; Huang, S.S.; Lin, I.H.; Kuo, W.L.; Chao, W.; Huang, G.J. Hispolon from Phellinus linteus induces G0/G1 cell cycle arrest and apoptosis in NB4 human leukaemia cells. Am. J. Chin. Med. 2013, 41, 1439–1457. [Google Scholar] [CrossRef]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Hu, M.L. Hispolon induces apoptosis and cell cycle arrest of human hepatocellular carcinoma Hep3B cells by modulating ERK phosphorylation. J. Agric. Food Chem. 2011, 59, 7104–7113. [Google Scholar] [CrossRef]
- Li, G.; Kim, D.H.; Kim, T.D.; Park, B.J.; Park, H.D.; Park, J.I.; Na, M.K.; Kim, H.C.; Hong, N.D.; Lim, K.; et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004, 216, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.C.; Hsieh, Y.H.; Chow, J.M.; Yang, S.F.; Hsiao, M.; Hua, K.T.; Lin, C.H.; Chen, H.Y.; Chien, M.H. Hispolon induces apoptosis through JNK1/2-mediated activation of a caspase-8, -9, and -3-dependent pathway in acute myeloid leukemia (AML) cells and inhibits AML xenograft tumor growth in vivo. J. Agric. Food Chem. 2013, 61, 10063–10073. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Yang, C.M.; Chang, Y.S.; Amagaya, S.; Wang, H.C.; Hou, W.C.; Huang, S.S.; Hu, M.L. Hispolon suppresses SK-Hep1 human hepatoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen activator through the PI3K/Akt and ERK signaling pathways. J. Agric. Food Chem. 2010, 58, 9468–9475. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, Z.; Li, L.; Wu, B.; Chen, S.F.; Zhou, H.; Wang, Y.; Li, Y.Q. Hispolon induces apoptosis in human gastric cancer cells through a ROS-mediated mitochondrial pathway. Free Radic. Biol. Med. 2008, 45, 60–72. [Google Scholar] [CrossRef]
- Armento, A.; Ehlers, J.; Schotterl, S.; Naumann, U. Molecular Mechanisms of Glioma Cell Motility. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Chen, D.; Li, D.; Xu, X.B.; Qiu, S.; Luo, S.; Qiu, E.; Rong, Z.; Zhang, J.; Zheng, D. Galangin inhibits epithelial-mesenchymal transition and angiogenesis by downregulating CD44 in glioma. J. Cancer 2019, 10, 4499–4508. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.; Park, M.J.; Jang, E.H.; Jung, B.; Kim, N.J.; Kim, J.H. Hispolon as an inhibitor of TGF-beta-induced epithelial-mesenchymal transition in human epithelial cancer cells by co-regulation of TGF-beta-Snail/Twist axis. Oncol. Lett. 2017, 14, 4866–4872. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.C.; Huang, S.Y.; Chen, S.P.; Su, C.C.; Chiu, T.L.; Pang, C.Y. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013, 16, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chang, S.F.; Liao, K.F.; Chiu, S.C. Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling. Int. J. Mol. Sci. 2017, 18, 1616. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.C.; Huang, S.Y.; Chang, S.F.; Chen, S.P.; Chen, C.C.; Lin, T.H.; Liu, H.H.; Tsai, T.H.; Lee, S.S.; Pang, C.Y.; et al. Potential therapeutic roles of tanshinone IIA in human bladder cancer cells. Int. J. Mol. Sci. 2014, 15, 15622–15637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, S.C.; Chiu, T.L.; Huang, S.Y.; Chang, S.F.; Chen, S.P.; Pang, C.Y.; Hsieh, T.F. Potential therapeutic effects of N-butylidenephthalide from Radix Angelica Sinensis (Danggui) in human bladder cancer cells. BMC Complement. Altern. Med. 2017, 17, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, K.-F.; Chiu, T.-L.; Chang, S.-F.; Wang, M.-J.; Chiu, S.-C. Hispolon Induces Apoptosis, Suppresses Migration and Invasion of Glioblastoma Cells and Inhibits GBM Xenograft Tumor Growth In Vivo. Molecules 2021, 26, 4497. https://doi.org/10.3390/molecules26154497
Liao K-F, Chiu T-L, Chang S-F, Wang M-J, Chiu S-C. Hispolon Induces Apoptosis, Suppresses Migration and Invasion of Glioblastoma Cells and Inhibits GBM Xenograft Tumor Growth In Vivo. Molecules. 2021; 26(15):4497. https://doi.org/10.3390/molecules26154497
Chicago/Turabian StyleLiao, Kuan-Fu, Tsung-Lang Chiu, Shu-Fang Chang, Mei-Jen Wang, and Sheng-Chun Chiu. 2021. "Hispolon Induces Apoptosis, Suppresses Migration and Invasion of Glioblastoma Cells and Inhibits GBM Xenograft Tumor Growth In Vivo" Molecules 26, no. 15: 4497. https://doi.org/10.3390/molecules26154497
APA StyleLiao, K.-F., Chiu, T.-L., Chang, S.-F., Wang, M.-J., & Chiu, S.-C. (2021). Hispolon Induces Apoptosis, Suppresses Migration and Invasion of Glioblastoma Cells and Inhibits GBM Xenograft Tumor Growth In Vivo. Molecules, 26(15), 4497. https://doi.org/10.3390/molecules26154497





