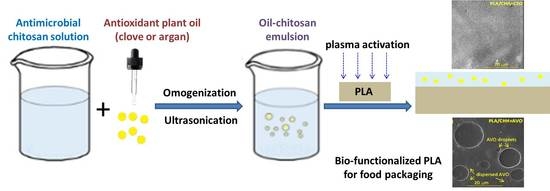
Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Coating-Forming Emulsion
2.3. PLA Activation and Functionalization
2.4. Characterization
2.4.1. FTIR Spectroscopy
2.4.2. Scanning Electron Microscopy
2.4.3. Contact Angle
2.4.4. Gas Permeability
2.4.5. Antioxidant Activity
2.4.6. Evaluation of Microbiological Properties
2.4.7. Statistical Analysis
3. Results and Discussions
3.1. Preliminary Evaluation of the Emulsions
3.2. Coatings Characterization
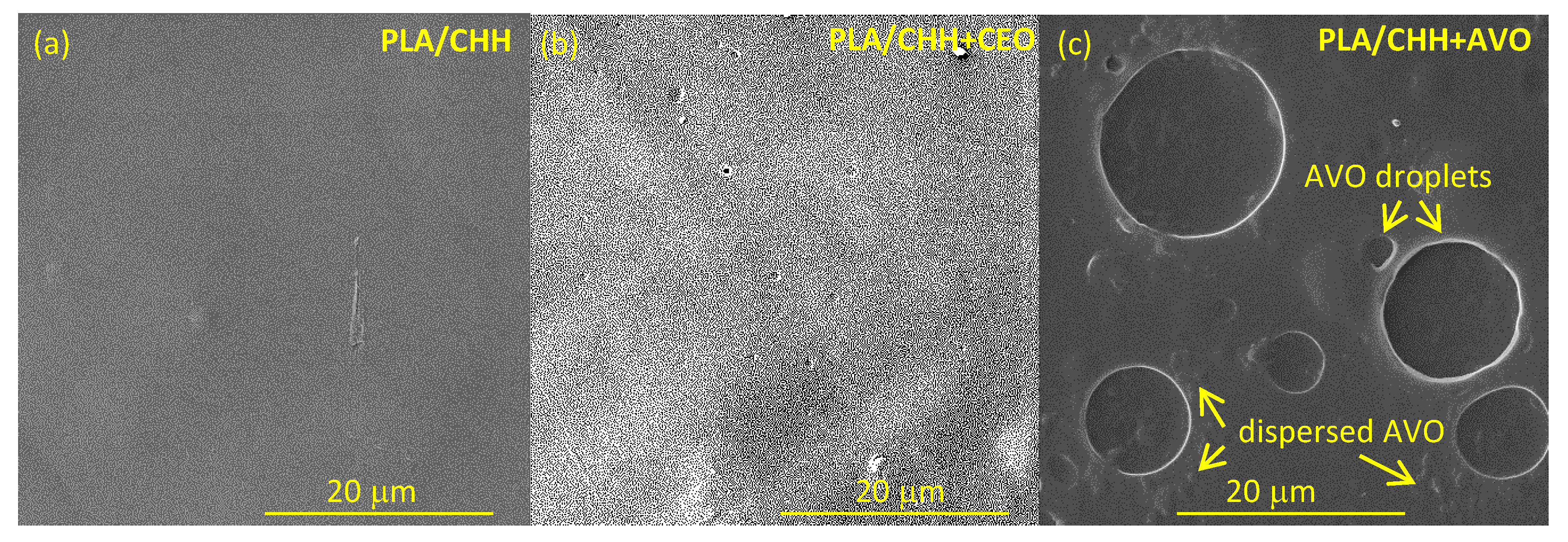
3.2.1. Morphology
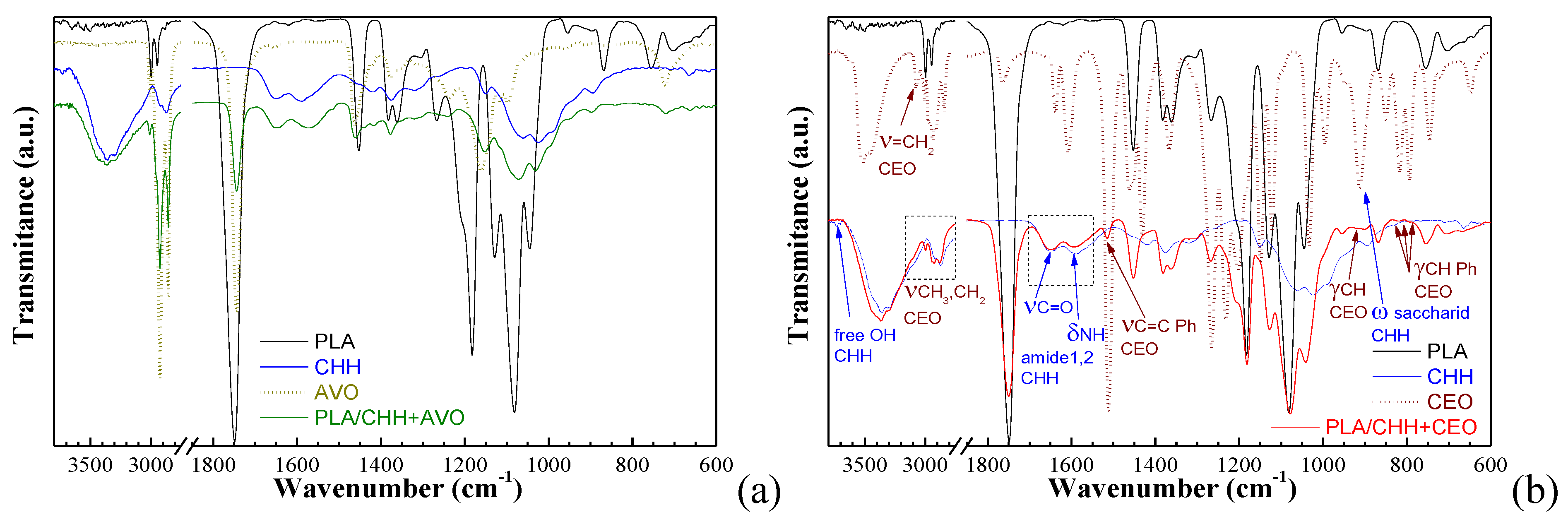
3.2.2. Chemical Structure of Oil-Loaded Chitosan Coatings
3.2.3. Stability to UV Exposure
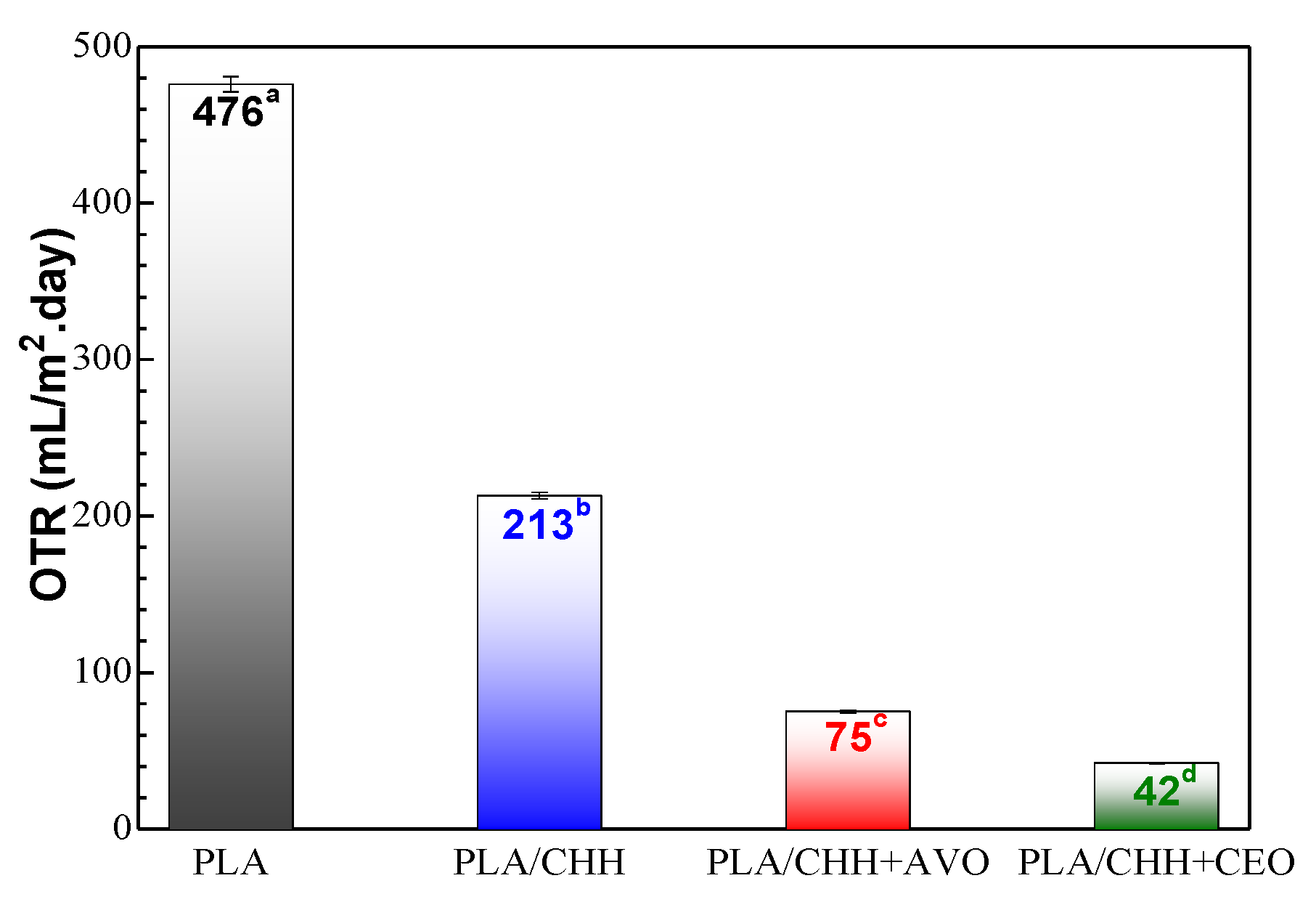
3.2.4. Gas Barrier Properties
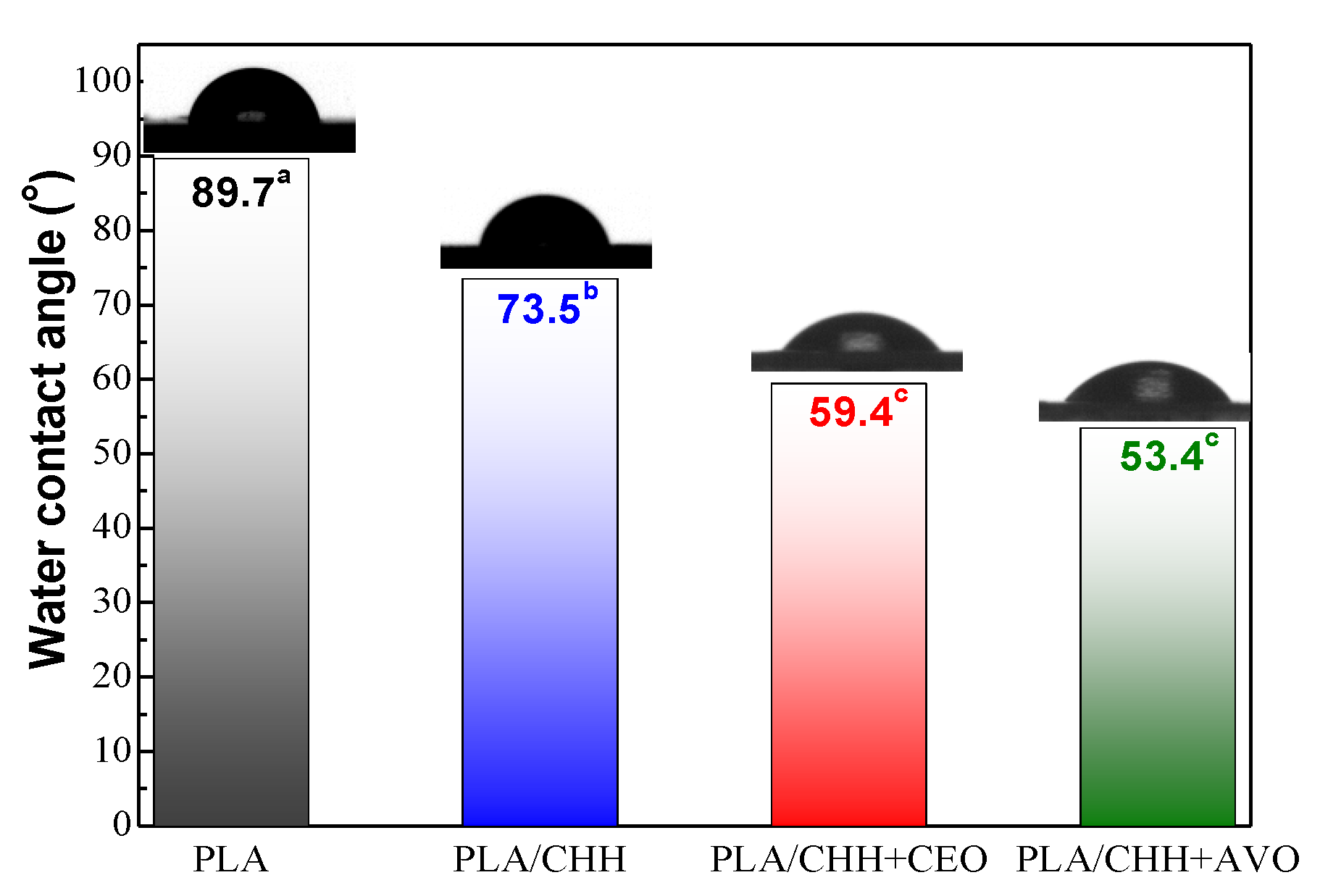
3.2.5. Wettability Evaluation
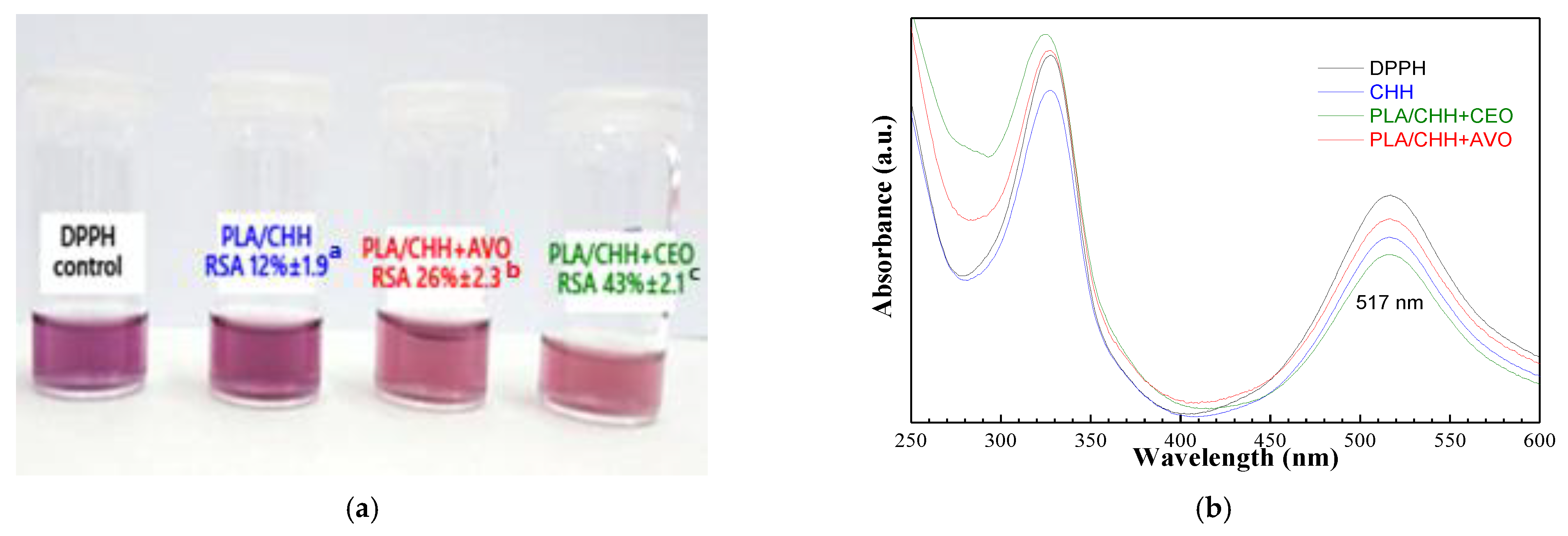
3.2.6. Radical Scavenging Potential
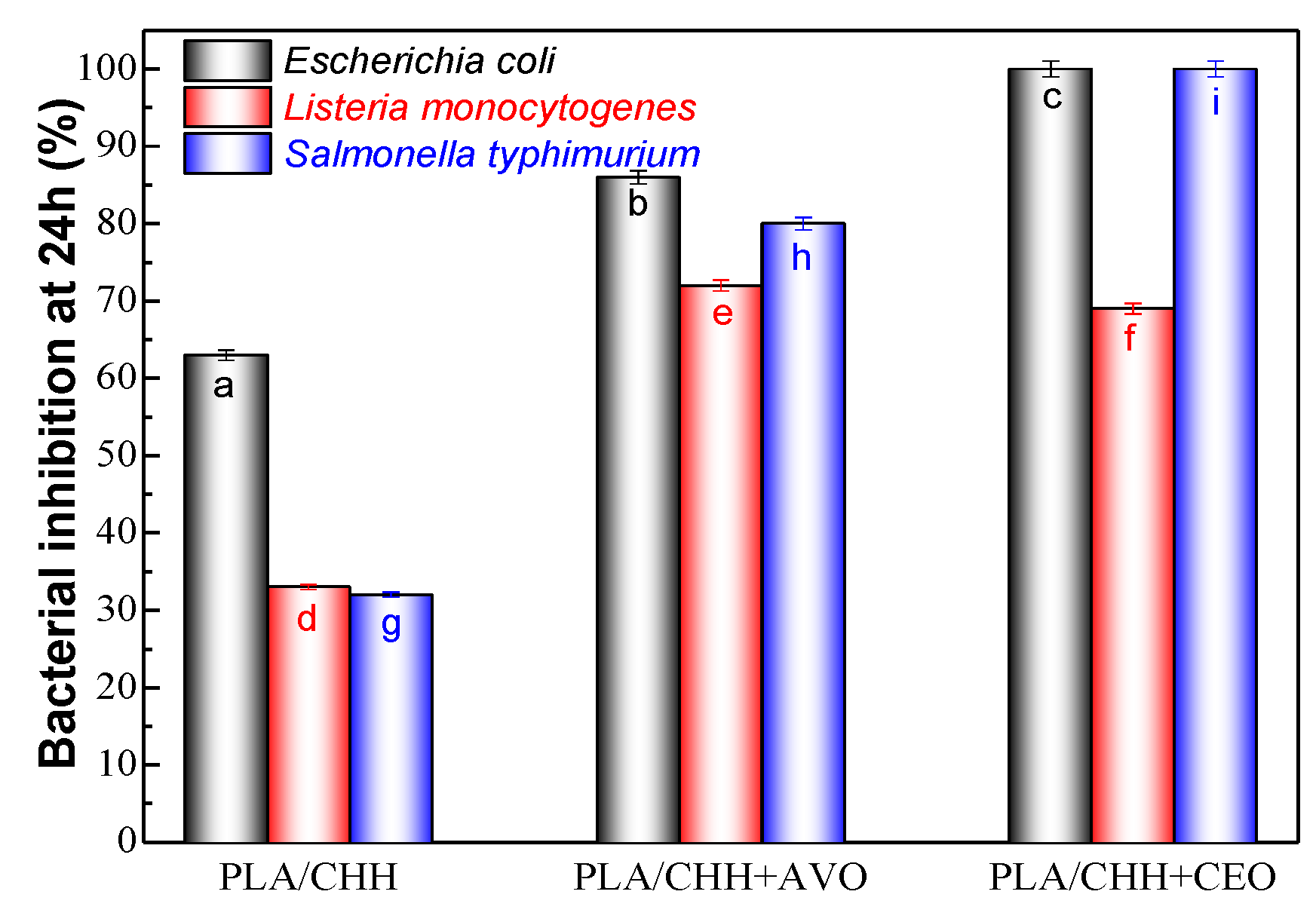
3.2.7. Antimicrobial Results
3.2.8. Microbiological Analysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Mistriotis, A.; Briassoulis, D.; Giannoulis, A.; D’Aquino, S. Design of biodegradable bio-based equilibrium modified atmosphere packaging (EMAP) for fresh fruits and vegetables by using micro-perforated poly-lactic acid (PLA) films. Postharvest Biol. Technol. 2016, 111, 380–389. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.J.; Palade, L.-I.; Cicero, J. Polylactides: Properties and prospects of an environmentally benign plastic from renewable resources. Macromol. Symp. 2001, 175, 55–66. [Google Scholar] [CrossRef]
- Habel, C.; Schöttle, M.; Daab, M.; Eichstaedt, N.J.; Wagner, D.; Bakhshi, H.; Agarwal, S.; Horn, M.A.; Breu, J. High-barrier, biodegradable food packaging. Macromol. Mater. Eng. 2018, 1800333, 1–5. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to come into Contact with Food. Off. J. Eur. Union 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0010&from=EN (accessed on 23 July 2021).
- Claro, P.I.C.; Neto, A.R.S.; Bibbo, A.C.C.; Mattoso, L.H.C.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable blends with potential use in packaging: A comparison of PLA/chitosan and PLA/cellulose acetate films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Dublan-García, O.; Ventura-Aguilar, R.I.; Vázquez-Armenta, F.J.; Aguilar-Montes de Oca, S.; Mardones, C.; Ayala-Zavala, J.F. Physico-chemical and antiadhesive properties of poly(lactic acid)/grapevine cane extract films against food pathogenic microorganisms. Polymers 2020, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.J. Bioprocessing—No Longer a Field of Dreams. Macromol. Symp. 2003, 201, 271–282. [Google Scholar] [CrossRef]
- Bogaert, J.-C.; Coszach, P. Poly(lactic acids): A potential solution to plastic waste dilemma. Macromol. Symp. 2000, 15, 287–303. [Google Scholar] [CrossRef]
- Halász, K.; Hosakun, Y.; Csóka, L. Reducing water vapor permeability of poly(lactic acid) film and bottle through layer-by-layer deposition of green-processed cellulose nanocrystals and chitosan. Int. J. Polym. Sci. 2015, 954290, 6. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tanase, E.E.; Mitelut, A.; Ailiesei, G.-L.; Vasile, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Stoleru, E.; Zaharescu, T.; Hitruc, E.G.; Vesel, A.; Ioanid, E.G.; Coroaba, A.; Safrany, A.; Pricope, G.; Lungu, M.; Schick, C.; et al. Lactoferrin-immobilized surfaces onto functionalized PLA assisted by the gamma-rays and nitrogen plasma to create materials with multifunctional properties. ACS Appl. Mater. Interfaces 2016, 8, 31902–31915. [Google Scholar] [CrossRef]
- Raouche, S.; Mauricio-Iglesias, M.; Peyron, S.; Guillard, V.; Gontard, N. Combined effect of high pressure treatment and anti-microbial bio-sourced materials on microorganisms’ growth in model food during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 426–434. [Google Scholar] [CrossRef]
- Guillard, V.; Issoupov, V.; Redl, A.; Gontard, N. Food preservative content reduction by controlling sorbic acid release from a superficial coating. Innov. Food Sci. Emerg. Technol. 2009, 10, 108–115. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gazdag, Z.; Szente, L.; Meggyes, M.; Horváth, G.; Lemli, B.; Kunsági-Máté, S.; Kuzma, M.; Koszegi, T. Antioxidant and antimicrobial properties of randomly methylated β cyclodextrin—Captured essential oils. Food Chem. 2019, 278, 305–313. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Galvão, J.A.; Mazine, M.R.; Gloria, E.M.; Oetterer, M. Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int. J. Food Microbiol. 2018, 286, 128–138. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Navikaite-Snipaitiene, V.; Ivanauskas, L.; Jakstas, V.; Rüegg, N.; Rutkaite, R.; Wolfram, E.; Yildirim, S. Development of antioxidant food packaging materials containing eugenol for extending display life of fresh beef. Meat Sci. 2018, 145, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and antimicrobial activity of biodegradable active packaging enriched with clove and thyme essential oil for food packaging application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef] [PubMed]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of polyphenols, tocopherols, and antioxidant capacity in virgin argan oil Argania spinosa, Skeels. Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Purwanti, N.; Zehn, A.S.; Pusfitasari, E.D.; Khalid, N.; Febrianto, E.Y.; Mardjan, S.S.; Kobayashi, A.; Kobayashi, I. Emulsion stability of clove oil in chitosan and sodium alginate matrix. Int. J. Food Prop. 2018, 21, 566–581. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; Dublan García, O.; Cabrera-Barjas, G. Corn-starch-based materials incorporated with cinnamon oil emulsion: Physico-chemical characterization and biological activity. Foods 2020, 9, 475. [Google Scholar] [CrossRef]
- Korge, K.; Bajic, M.; Likozar, B.; Novak, U. Active chitosan–chestnut extract films used for packaging and storage of fresh pasta. Int. J. Food Sci. Technol. 2020, 55, 3043–3052. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Rybczynski, P.; Macczak, P.; Smolarkiewicz-Wyczachowski, A.; Ziegler-Borowska, M. Chitosan as a protective matrix for the squaraine dye. Materials 2021, 14, 1171. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C.; Bifani, V. Natural additives in bioactive edible films and coatings: Functionality and applications in foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant migration studies in chitosan films incorporated with plant extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.D.R. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT Food Sci. Technol. 2013, 50, 78–87. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Sacarescu, L.; Vasiliu, A.-L.; Hitruc, G.E.; Pricope, G.M.; Sivertsvik, M.; Rosnes, J.T.; Vasile, C. Antioxidant/antibacterial electrospun nanocoatings applied onto PLA films. Materials 2018, 11, 1973. [Google Scholar] [CrossRef] [PubMed]
- Pâslaru, E.; Fras Zemljic, L.; Bračič, M.; Vesel, A.; Petrinić, I.; Vasile, C. Stability of a chitosan layer deposited onto a polyethylene surface. J. Appl. Polym. Sci. 2013, 130, 2444–2457. [Google Scholar] [CrossRef]
- Stoleru, E.; Munteanu, S.B.; Dumitriu, R.P.; Coroaba, A.; Drobotă, M.; Zemljic, L.F.; Pricope, G.M.; Vasile, C. Polyethylene materials with multifunctional surface properties by electrospraying chitosan/vitamin E formulation destined to biomedical and food packaging applications. Iran. Polym. J. 2016, 25, 295–307. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tarţău, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the rosemary extract effect on the properties of polylactic acid-based materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible materials based on plasticized poly(lactic acid), chitosan and rosemary ethanolic extract i. effect of chitosan on the properties of plasticized poly(lactic acid) materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Sivertsvik, M.; Miteluţ, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tănase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative analysis of the composition and active property evaluation of certain essential oils to assess their potential applications in active food packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef]
- Akretche, H.; Pierre, G.; Moussaoui, R.; Michaud, P.; Delattre, C. Valorization of olive mill wastewater for the development of biobased polymer films with antioxidant properties using eco-friendly processes. Green Chem. 2019, 21, 3065. [Google Scholar] [CrossRef]
- FAO. Technical Guidance Principles of Risk-Based Meat Inspection and Their Application; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/ca5465en/CA5465EN.pdf (accessed on 23 July 2021).
- Vasile, C.; Stoleru, E.; Irimia, A.; Zaharescu, T.; Dumitriu, R.P.; Ioanid, G.E.; Munteanu, B.S. Ionizing radiation and plasma discharge mediating covalent linking of bioactive compounds onto polymeric substrate to obtain stratified composites for food packing. In Proceedings of the Report of the 3rd RCM of the CRP on Application of Radiation Technology in the Development of Advanced Packaging Materials for Food Products, Vienna, Austria, 11–15 July 2016; Available online: http://www-naweb.iaea.org/napc/iachem/working_materials/F22063%20APA416.pdf (accessed on 23 July 2021).
- Rojas, J.; Cabrera, S.; Benavides, J.; Lopera, Y.; Yarce, C.J. Lipidic matrixes containing clove essential oil: Biological activity, microstructural and textural studies. Molecules 2021, 26, 2425. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Charrouf, Z. The origin of virgin argan oils high oxidative stability unraveled. Nat. Prod. Commun. 2012, 7, 621–624. [Google Scholar] [PubMed]
- Guillaume, D.; Charrouf, Z. Argan oil for nutritional and skin care applications. Agro Food Industry Hi Tech 2013, 24, 28–30. [Google Scholar]
- Klinkesorn, U. The role of chitosan in emulsion formation and stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Ethnoeconomical, ethnomedical, and phytochemical study of Argania spinosa (L.) skeels. J. Ethnopharmacol. 1999, 67, 7–14. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein-chitosan-sunflower oil edible film: Mechanical, barrier and structural properties. LWT Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Pearson, F.G.; Marchessault, R.H.; Liang, C.Y. Infrared spectra of crystalline polysaccharides. J. Polym. Sci. 1960, 43, 101–116. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Saraswathy, G.; Pal, S.; Rose, C.; Sastry, T.P. A novel bio-inorganic bone implant containing deglued bone, chitosan and gelatin. Bull. Mater. Sci. 2001, 24, 415–420. [Google Scholar] [CrossRef]
- Alexa, E.; Dragomirescu, A.; Pop, G.; Jianu, C.; Dragoş, D. The use of FT-IR spectroscopy in the identification of vegetable oils adulteration. J. Food Agric. Environ. 2009, 7, 20–24. [Google Scholar]
- Aman, R.M.; Abu Hashim, I.I.; Meshali, M.M. Novel clove essential oil nanoemulgel tailored by Taguchi’s model and scaffold-based nanofibers: Phytopharmaceuticals with promising potential as cyclooxygenase-2 inhibitors in external inflammation. Int. J. Nanomed. 2020, 15, 2171–2195. [Google Scholar] [CrossRef]
- Kadam, S.; Waghmare Jyotsna, S. Identification of major volatile (essential oil) constituents of “carrom seeds” and “clove buds”. Int. J. Sci. Res. Rev. 2014, 3, 85–94. [Google Scholar]
- Darder, M.; Colilla, M.; Ruiz-hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR study of montmorillonite–chitosan nanocomposite materials. Spectrochim. Acta A 2011, 79, 784–788. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R.D.K. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef]
- Silva, S.M.L.; Braga, C.R.C.; Fook, M.V.L.; Raposo, C.M.O.; Carvalho, L.H.; Canedo, E.L. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012. Available online: https://www.intechopen.com/books/infrared-spectroscopy-materials-science-engineering-and-technology/application-of-infrared-spectroscopy-to-analysis-of-chitosan-clay-nanocomposites (accessed on 23 July 2021). [CrossRef]
- Marchessault, R.H.; Ravenelle, F.; Zhu, X.X. Polysaccharides for Drug Delivery and Pharmaceutical Applications, 1st ed.; American Chemical Society: Washington, DC, USA, 2006; Volume 934. [Google Scholar]
- Dhifi, W.; Da Graça Costa Miguel, M.; Mnif, W. Argan Oil: Extraction, Categories, Chemical Composition and Health Benefits, in Seed Oil: Production, uses and Benefits; Nguyen, H.K.D., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 175–188. [Google Scholar]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible Films from Carrageenan/Orange Essential Oil/Trehalose—Structure, Optical Properties, and Antimicrobial Activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Lazic, V.L.; Budinski-Simendic, J.; Gvozdenovic, J.J.; Simendic, B. Barrier properties of coated and laminated polyolefin films for food packaging. Acta Phys. Pol. A 2010, 117, 855–858. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and morphological characterization of chitosan/montmorillonite films incorporated with ginger essential oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/b-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Rullier-Birat, B.; Cazalbou, S.; Nassar, M.A.; Sandrine, C.; Tourrette, A. New backing layer for transdermal drug delivery systems: Coatings based on fatty acid and beeswax on chitosan films. J. Adhes. Sci. Technol. 2015, 29, 245–255. [Google Scholar] [CrossRef][Green Version]
- Ibañez-Peinado, D.; Ubeda-Manzanaro, M.; Martínez, A.; Rodrigo, D. Antimicrobial effect of insect chitosan on Salmonella Typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes survival. PLoS ONE 2020, 15, e0244153. [Google Scholar] [CrossRef] [PubMed]



| Essential/Vegetal Oils | DPPH IC50, mg/mL | ABTS IC50, mg/mL |
|---|---|---|
| Clove (CEO) | 0.017 ± 0.005 a | 0.008 ± 0.002 a |
| Thyme (TEO) | 0.056 ± 0.007 a | 0.056 ± 0.009 b |
| Rosemary (REO) | 0.322 ± 0.081 b | 4.442 ± 0.012 c |
| Ti Tree (TTO) | 0.853 ± 0.121 c | 3.793 ± 0.023 d |
| Argan (AVO) | 10.416 ± 0.963 d | 5.279 ± 0.052 e |
| Apricot (APO) | 14.722 ± 0.825 e | 6.961 ± 0.127 f |
| Grape Seeds (GVO) | 16.784 ± 1.223 f | 7.129 ± 0.861 f |
| Emulsion | ζ-Potential (mV) | Droplet Size (nm) |
| CHH + CEO | 34.32 ± 1.2 a | 12.1 ± 1.6 a |
| CHH + AVO | 18.64 ± 2.3 b | 236.2 ± 16.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules 2021, 26, 4500. https://doi.org/10.3390/molecules26154500
Stoleru E, Vasile C, Irimia A, Brebu M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules. 2021; 26(15):4500. https://doi.org/10.3390/molecules26154500
Chicago/Turabian StyleStoleru, Elena, Cornelia Vasile, Anamaria Irimia, and Mihai Brebu. 2021. "Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils" Molecules 26, no. 15: 4500. https://doi.org/10.3390/molecules26154500
APA StyleStoleru, E., Vasile, C., Irimia, A., & Brebu, M. (2021). Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules, 26(15), 4500. https://doi.org/10.3390/molecules26154500