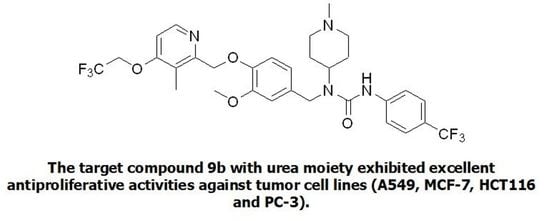
Design, Synthesis and Anticancer Activity of a New Series of N-aryl-N′-[4-(pyridin-2-ylmethoxy)benzyl]urea Derivatives
Abstract
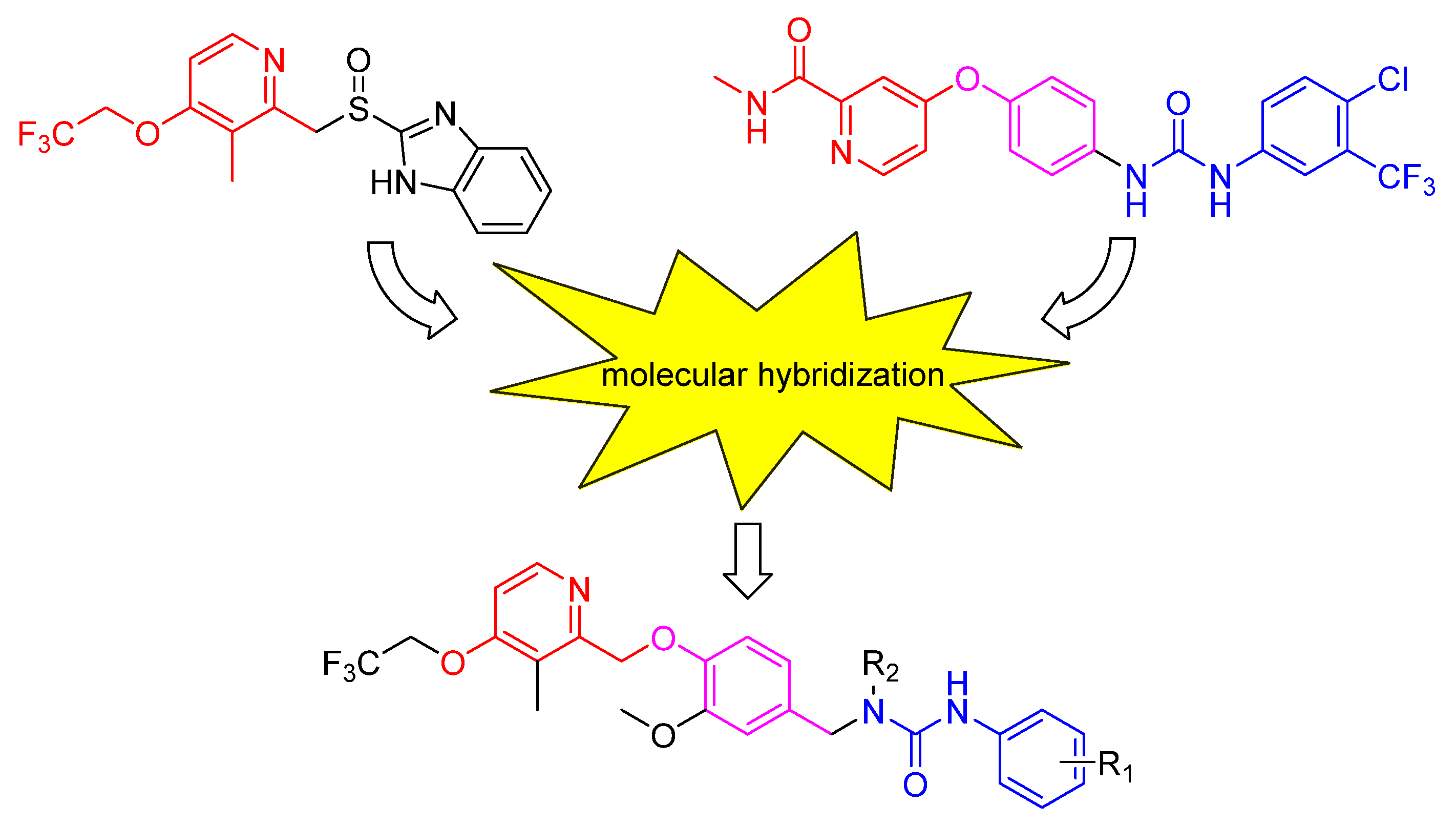
1. Introduction
2. Results and discussion
2.1. Chemistry
2.2. Biological Activity Evaluation
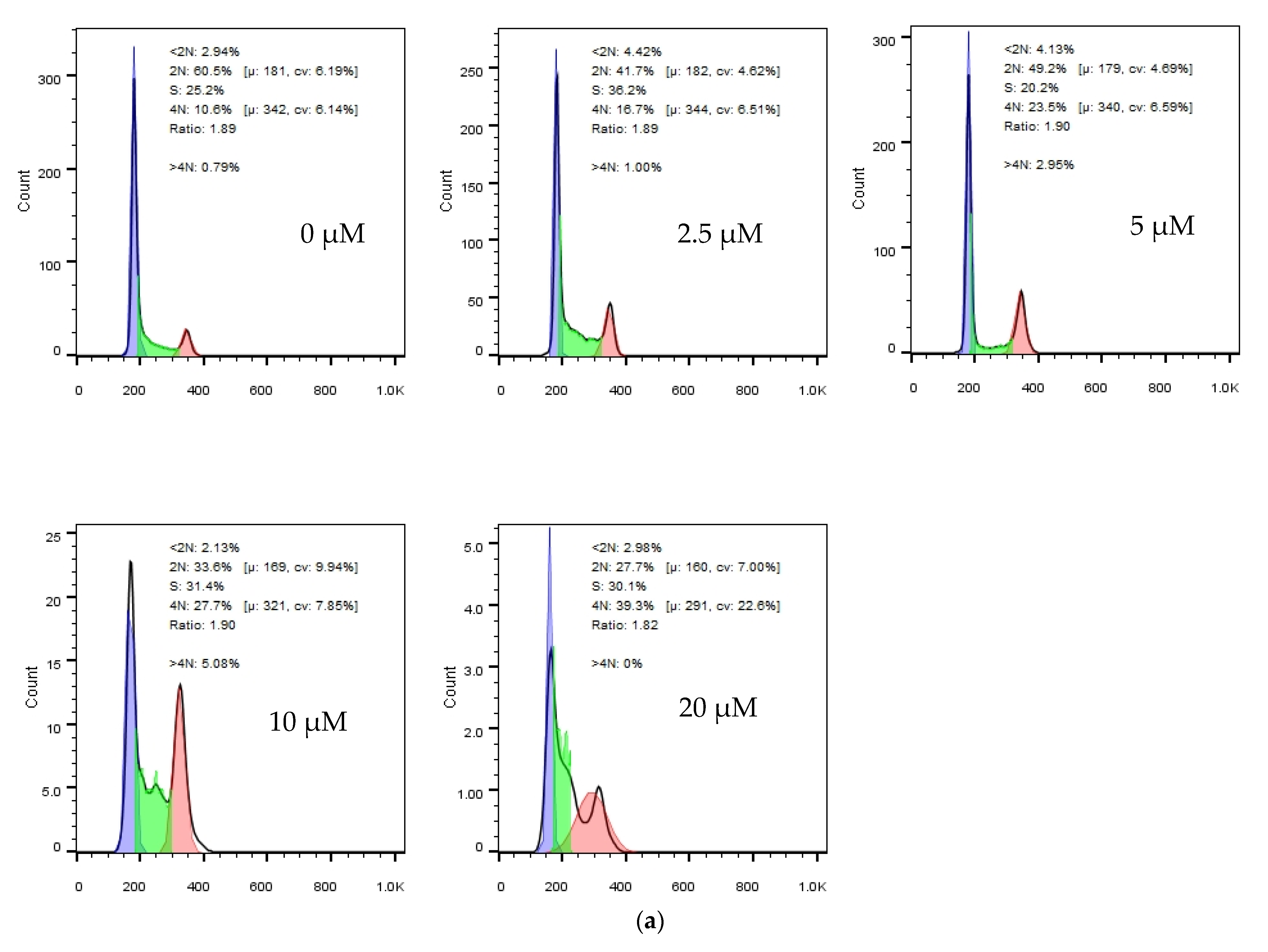
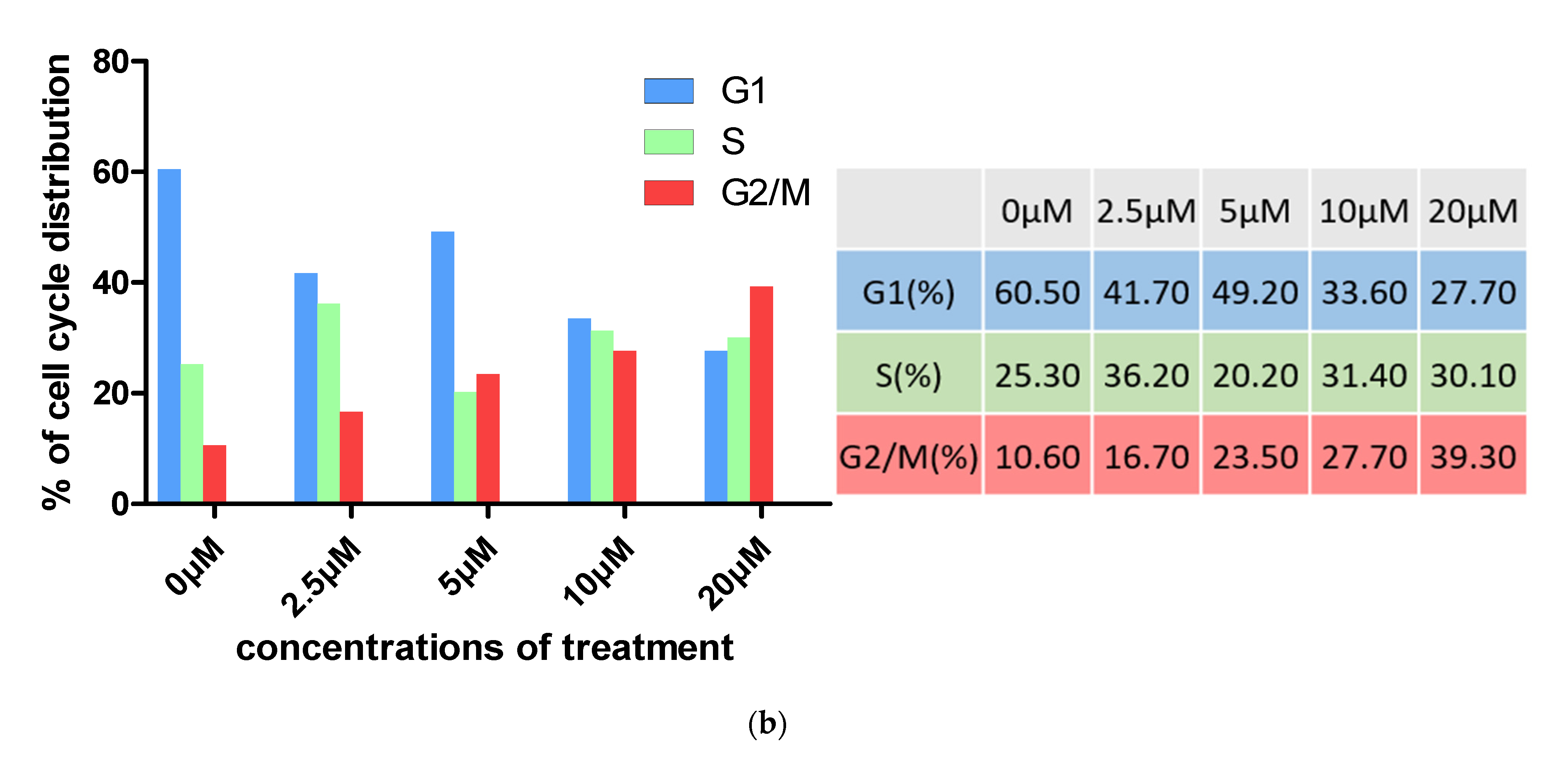
2.3. Cell Cycle Analysis
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Synthesis of 3-methoxy-4-[(3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy] benzaldehyde (3)
3.1.3. Synthesis of (E)-3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl) methoxy)benzaldehyde oxime (4)
3.1.4. Synthesis of (3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)phenyl)methanamine (5)
3.1.5. Synthesis of N-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl) methoxy)benzyl)-1-methylpiperidin-4-amine (6)
3.1.6. General Procedure for the Synthesis of the Target Urea Derivatives
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(4-(trifluoromethoxy)phenyl)urea (8a), white powder 0.99 g, yield 89%; m.p.: 158.8–159.3 °C; MS: 560.4([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.34 (d, J = 5.7 Hz, 1H), 7.54–7.47 (m, 2H), 7.23 (d, J = 8.6 Hz, 2H), 7.14 (d, J = 5.7 Hz, 1H), 7.05 (d, J = 8.2 Hz, 1H), 6.94 (d, J = 2.0 Hz, 1H), 6.81 (dd, J = 8.2, 2.0 Hz, 1H), 6.59 (t, J = 5.9 Hz, 1H), 5.14 (s, 2H), 4.92 (q, J = 8.8 Hz, 2H), 4.23 (d, J = 5.7 Hz, 2H), 3.74 (s, 3H), 2.22 (s, 3H).; 13C NMR (101 MHz, DMSO-d6) δ 161.82, 155.94, 155.51, 149.63, 148.02, 147.18, 142.55, 140.26, 133.67, 124.25 (q, J = 277.9 Hz), 122.08, 121.99, 120.69 (q, J = 254.9 Hz), 119.69, 119.19, 114.27, 112.07, 108.02, 71.56, 65.11 (q, J = 34.7 Hz), 55.95, 43.10, 10.30; IR: 3399, 3053, 3008, 2971, 2829, 1706, 1556, 1507, 1454, 1416, 1256, 1220, 1194, 1154, 1015, 911, 847, 796, 672, 645, 544; HRMS: 558.146914([M − H]−) for C25H22F6N3O5, 560.161467([M + H]+) for C25H24F6N3O5.
- 1-(3-chloro-4-fluorophenyl)-3-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)urea (8b), white powder 0.86 g, yield 82%; m.p.: 167.8–169.1 °C; MS:528.3([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.34 (d, J = 5.7 Hz, 1H), 7.77 (dd, J = 6.8, 2.4 Hz, 1H), 7.32–7.18 (m, 2H), 7.14 (d, J = 5.7 Hz, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.93 (d, J = 2.0 Hz, 1H), 6.80 (dd, J = 8.2, 1.9 Hz, 1H), 6.63 (t, J = 5.8 Hz, 1H), 5.13 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.22 (d, J = 5.7 Hz, 2H), 3.74 (s, 3H), 2.22 (s, 3H).; 13C NMR (101 MHz, DMSO-d6) δ 161.81, 155.94, 155.44, 152.33 (d, J = 240.0 Hz), 149.59, 148.04, 147.15, 138.26 (d, J = 2.8 Hz), 124.27 (q, J = 277.4 Hz), 122.05, 119.69, 119.44 (d, J = 18.9 Hz), 119.31, 118.23 (d, J = 6.5 Hz), 117.13 (d, J = 21.3 Hz), 114.26, 112.08, 108.06, 71.55, 65.08 (q, J = 34.2 Hz), 56.00, 43.09, 10.35; IR: 3313, 2944, 2883, 1641, 1564, 1500, 1477, 1420, 1390, 1308, 1258, 1209, 1164, 1131, 1008, 970, 911, 862, 800, 757, 647, 576, 445; HRMS: 526.116220([M − H]−) for C24H21ClF4N3O4, 528.130773([M + H]+) for C24H23ClF4N3O4.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(4-(trifluoromethyl)phenyl)urea (8c), white powder 0.78 g, yield 72%; m.p: 169.9–171.0 °C; MS:544.5([M + H]+), 566.1([M + Na]+); 1H NMR (400 MHz, DMSO-d6) δ 8.95 (s, 1H), 8.34 (d, J = 5.7 Hz, 1H), 7.59 (q, J = 8.8 Hz, 4H), 7.14 (d, J = 5.7 Hz, 1H), 7.05 (d, J = 8.2 Hz, 1H), 6.95 (d, J = 2.0 Hz, 1H), 6.82 (dd, J = 8.2, 1.9 Hz, 1H), 6.69 (t, J = 5.8 Hz, 1H), 5.14 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.24 (d, J = 5.7 Hz, 2H), 3.74 (s, 3H), 2.22 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 155.93, 155.25, 149.61, 148.03, 147.19, 144.64, 133.47, 126.37, 125.09 (q, J = 270.6 Hz), 124.26 (q, J = 277.5 Hz), 122.06, 119.73, 117.72, 114.26, 112.10, 108.05, 71.55, 65.09 (q, J = 35.0 Hz), 55.98, 43.10, 10.31; IR: 3414, 3376, 2940, 2886, 1703, 1686, 1581, 1534, 1512, 1477, 1408, 1321, 1256, 1220, 1180, 1155, 1135, 1102, 1063, 1008, 979, 862, 842, 812, 595, 554;HRMS: 542.151999 ([M − H]−) for C25H22F6N3O4, 544.166552([M + H]+)+ for C25H24F6N3O4.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(3-(trifluoromethyl)phenyl)urea (8d), white powder 0.83 g, yield 76%; m.p.: 153.2–154.2 °C; MS:544.2([M + H]+), 542.0([M − H]−); 1H NMR (400 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.34 (d, J = 5.7 Hz, 1H), 7.98 (s, 1H), 7.52 (d, J = 8.3 Hz, 1H), 7.45 (t, J = 7.9 Hz, 1H), 7.23 (d, J = 7.6 Hz, 1H), 7.14 (d, J = 5.7 Hz, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.94 (d, J = 1.9 Hz, 1H), 6.81 (dd, J = 8.2, 1.9 Hz, 1H), 6.68 (t, J = 5.9 Hz, 1H), 5.13 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.23 (d, J = 5.8 Hz, 2H), 3.74 (s, 3H), 2.22 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.82, 155.94, 155.46, 149.62, 148.02, 147.18, 141.80, 133.59, 130.11, 124.73 (q, J = 272.3 Hz), 124.25 (q, J = 277.4 Hz), 122.07, 121.62, 119.69, 117.68, 114.28, 114.06, 112.08, 108.02 (d, J = 4.4 Hz), 71.56, 65.10 (q, J = 34.2 Hz), 55.97 (d, J = 3.2 Hz), 43.08, 10.33; IR: 3412, 3374, 2940, 2876, 1702, 1582, 1551, 1514, 1477, 1442, 1380, 1341, 1312, 1255, 1221, 1182, 1158, 1111, 1067, 1028, 1007, 979, 891, 864, 813, 796, 702, 664, 597, 552; HRMS: 542.151999([M − H]−) for C25H22F6N3O4+, 544.161552 ([M + H]+) for C25H24F6N3O4.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(4-methoxyphenyl)urea (8e), white powder 0.59 g, yield 58%; m.p.: 179.5–180.3 °C; MS:566.2([M + H]+), 504.0([M − H]−); 1H NMR (400 MHz, DMSO-d6) δ 8.28 (s, 1H), 7.29 (d, J = 8.9 Hz, 2H), 7.14 (d, J = 5.7 Hz, 1H), 6.93 (d, J = 1.9 Hz, 1H), 6.81 (dd, J = 9.0, 7.1 Hz, 3H), 6.40 (t, J = 5.9 Hz, 1H), 5.13 (s, 2H), 4.92 (q, J = 8.8 Hz, 2H), 4.20 (d, J = 5.8 Hz, 2H), 3.70 (s, 3H), 2.22 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.80, 155.96, 155.86, 154.40, 149.57, 148.05, 147.08, 134.08, 133.97, 124.28 (d, J = 277.9 Hz), 122.05, 119.91, 119.64, 114.33, 114.27, 112.06, 108.07, 71.58, 65.08 (q, J = 34.5 Hz), 55.99, 55.59, 43.09, 10.36; IR: 3312, 2940, 2839, 1631, 1571, 1508, 1467, 1417, 1376, 1363, 1308, 1271, 1241, 1160, 1136, 1030, 973, 862, 827, 669, 578, 524, 423; HRMS: 504.175179 ([M − H]−) for C25H25F3N3O5, 506.189732 ([M + H]+) for C25H27F3N3O5.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(3-nitrophenyl)urea (8f), yellow powder 0.31 g, yield 30%; m.p.: 168.9–170.5 °C; MS: 521.2([M + H]+), 519.0([M − H]−); 1H NMR (400 MHz, DMSO-d6) δ 9.08 (s, 1H), 8.52 (t, J = 2.2 Hz, 1H), 8.33 (d, J = 5.8 Hz, 1H), 7.75 (d, J = 8.3 Hz, 1H), 7.66 (d, J = 8.4 Hz, 1H), 7.50 (dd, J = 9.1, 7.3 Hz, 1H), 7.13 (d, J = 5.9 Hz, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.96–6.92 (m, 1H), 6.85–6.71 (m, 2H), 5.13 (s, 2H), 4.91 (q, J = 8.8 Hz, 2H), 4.24 (d, J = 5.9 Hz, 2H), 3.74 (s, 3H), 2.21 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.83, 155.93, 155.35, 149.63, 148.59, 148.02, 147.20, 142.28, 133.51, 130.31, 124.26 (q, J = 277.8 Hz), 124.16, 122.07, 119.74, 115.95, 114.32, 112.14, 112.06, 108.05, 71.56, 65.11 (q, J = 34.4 Hz), 56.02, 43.11, 10.34; IR: 3410, 3010, 2943, 2882, 2832, 1701, 1584, 1527, 1503, 1480, 1383, 1347, 1318, 1264, 1207, 1161, 1138, 1122, 1033, 1000, 980, 868, 814, 794, 735, 671, 612, 585, 557, 445; HRMS: 519.149693 ([M − H]−) for C24H22F3N4O6, 521.164246([M + H]+) for C24H24F3N4O6.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(4-methoxybenzyl)urea (8g), white powder 0.39 g, yield 38%; m.p.: 153.0–154.2 °C; MS: 520.2([M + H]+), 517.9([M − H]−); 1H NMR (400 MHz, DMSO-d6) δ 9.08 (s, 1H), 8.52 (t, J = 2.2 Hz, 1H), 8.33 (d, J = 5.8 Hz, 1H), 7.75 (d, J = 8.3 Hz, 1H), 7.66 (d, J = 8.4 Hz, 1H), 7.50 (dd, J = 9.1, 7.3 Hz, 1H), 7.13 (d, J= 5.9 Hz, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.96–6.92 (m, 1H), 6.85–6.71 (m, 2H), 5.13 (s, 2H), 4.91 (q, J = 8.8 Hz, 2H), 4.24 (d, J = 5.9 Hz, 2H), 3.74 (s, 3H), 2.21 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.82, 158.54, 158.46, 155.99, 149.57, 148.04, 146.99, 134.47, 133.30, 124.28 (q, J = 277.7 Hz), 122.06, 119.46, 114.26, 114.07, 111.86, 108.08, 71.61, 65.10 (q, J = 34.9 Hz), 55.92, 55.51, 43.25, 42.90, 10.36; IR: 3349, 3301, 2949, 2925, 2884, 2832, 1605, 1579, 1561, 1515, 1468, 1424, 1363, 1363, 1363, 1275, 1251, 1169, 1156, 1133, 1103, 1038, 975, 863, 816, 728, 637, 561; HRMS: 518.190829 ([M − H]−) for C26H27F3N3O5, 520.205382 ([M + H]+) for C26H29F3N3O5.
- 1-(4-ethoxybenzyl)-3-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)urea (8h), white powder 0.57 g, yield 53%; m.p.: 148.9–149.9 °C; MS: 534.3 ([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 5.7 Hz, 1H), 7.20–7.11 (m, 3H), 7.02 (d, J = 8.2 Hz, 1H), 6.90–6.81 (m, 3H), 6.75 (dd, J = 8.3, 1.9 Hz, 1H), 6.30 (td, J = 6.1, 2.3 Hz, 2H), 5.13 (s, 2H), 4.92 (q, J = 8.8 Hz, 2H), 4.15 (d, J = 5.8 Hz, 4H), 3.99 (q, J = 7.0 Hz, 2H), 3.71 (s, 3H), 2.22 (s, 3H), 1.31 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.82, 158.48, 157.77, 155.96, 149.54, 148.02, 146.96, 134.46, 133.16, 128.76, 124.28 (q, J= 277.7, 277.3 Hz), 122.06, 119.44, 114.56, 114.21, 111.81, 108.08, 71.57, 65.08 (q, J = 34.5 Hz), 63.39, 55.89, 43.24, 42.89, 15.11, 10.36; IR: 3331, 2978, 2927, 2883, 1609, 1579, 1556, 1516, 1471, 1423, 1390, 1275, 1249, 1148, 1025, 974, 861, 815, 753, 728, 639, 574, 548; HRMS: 532.206479 ([M − H]−) for C27H29F3N3O5, 534.221032([M + H]+) for C27H31F3N3O5.
- 1-(4-(dimethylamino)benzyl)-3-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)urea (8i), white powder 0.73 g, yield 69%; m.p.: 159.4–161.4 °C; MS:533.9([M + H]+), 555.4([M + Na]+); 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 5.6 Hz, 1H), 7.14 (d, J = 5.7 Hz, 1H), 7.11–7.06 (m, 2H), 7.02 (d, J = 8.2 Hz, 1H), 6.87 (d, J = 2.0 Hz, 1H), 6.74 (dd, J = 8.2, 1.9 Hz, 1H), 6.69–6.65 (m, 2H), 6.22 (dt, J = 21.9, 5.9 Hz, 2H), 5.12 (s, 2H), 4.92 (q, J = 8.7 Hz, 2H), 4.15 (d, J = 5.9 Hz, 2H), 4.10 (d, J = 5.8 Hz, 2H), 3.71 (s, 3H), 2.85 (s, 6H), 2.22 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 158.46, 155.98, 149.97, 149.55, 148.04, 146.96, 134.50, 128.77, 128.49, 124.28 (q, J = 277.7 Hz), 122.05, 119.44, 114.22, 112.85, 111.82, 108.08, 71.59, 65.08 (q, J = 34.3 Hz), 55.92, 43.23, 43.06, 10.36; IR: 3336, 2940, 2918, 2879, 1613, 1570, 1517, 1468, 1421, 1308, 1256, 1233, 1175, 1137, 1039, 1011, 969, 922, 854, 810, 736, 650, 564; HRMS: 531.222464 ([M − H]−) for C27H30F3N4O4, 522.237017 ([M + H]+) for C27H32F3N4O4.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea (9a), white powder 0.79 g, yield 60%; m.p.: 127.3–129.4 °C; MS:657.2([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H), 8.33 (d, J = 5.6 Hz, 1H), 7.58–7.51 (m, 2H), 7.23 (d, J = 8.6 Hz, 2H), 7.14 (d, J = 5.7 Hz, 1H), 7.03 (d, J = 8.3 Hz, 1H), 6.90 (d, J = 2.0 Hz, 1H), 6.75 (dd, J = 8.2, 1.9 Hz, 1H), 5.11 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.52 (s, 2H), 4.10 (tt, J = 12.0, 4.0 Hz, 1H), 3.70 (s, 3H), 2.83 (d, J = 11.6 Hz, 2H), 2.20 (d, J = 5.4 Hz, 6H), 2.06 (d, J = 10.0 Hz, 2H), 1.70 (tt, J = 12.4, 6.7 Hz, 2H), 1.55 (d, J = 14.4 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 155.73, 149.48, 148.05, 146.93, 143.15, 140.30, 133.64, 124.26(q, J =276.1Hz), 121.50, 118.77, 120.66 (q, J = 253.5 Hz), 111.28, 108.07, 71.55, 65.11 (q, J = 34.8, 34.0 Hz), 55.95, 55.08, 53.09, 45.99, 45.70, 45.16, 39.69, 29.90, 10.36; IR: 3397, 2950, 2848, 2799, 1648, 1584, 1513, 1470, 1416, 1377, 1293, 1256, 1227, 1204, 1159, 1132, 1031, 982, 921, 847, 825, 800, 754, 536; HRMS: 655.236063 ([M − H]−) for C31H33F6N4O5, 657.250616 ([M + H]+) for C31H35F6N4O5.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(trifluoromethyl)phenyl)urea (9b), white powder 0.73 g, yield 57%; m.p.: 138.4–140.5 °C; MS: 641.1([M + H]+), 321.5 ([M + 2H]2+), 639.5([M − H]−); 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.33 (d, J = 5.6 Hz, 1H), 7.67 (d, J = 8.6 Hz, 2H), 7.58 (d, J = 8.7 Hz, 2H), 7.14 (d, J = 5.7 Hz, 1H), 7.03 (d, J = 8.3 Hz, 1H), 6.89 (d, J = 2.0 Hz, 1H), 6.75 (dd, J = 8.3, 2.0 Hz, 1H), 5.10 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.54 (s, 2H), 4.16–4.07 (m, 1H), 3.70 (s, 3H), 2.84 (d, J = 10.9 Hz, 2H), 2.19 (s, 6H), 2.06 (t, J = 10.0 Hz, 2H), 1.72 (q, J = 12.6, 11.8 Hz, 2H), 1.57 (d, J = 11.4 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 155.92, 155.50, 149.49, 148.05, 146.97, 144.86, 133.47, 125.91 (q, J = 3.9 Hz), 125.08 (q, J = 270.9 Hz), 124.26 (q, J = 277.6, 277.1 Hz), 122.16 (q, J = 31.8 Hz), 122.08, 118.76, 114.21, 111.26, 108.06, 71.54, 65.11 (q, J = 34.4 Hz), 55.94, 55.06, 53.24, 45.70, 45.25, 29.88, 10.34; IR: 3387, 2943, 2846, 2802, 1650, 1584, 1513, 1468, 1416, 1378, 1313, 1250, 1224, 1162, 1131, 1063, 1030, 1016, 981, 862, 843, 811, 753, 577; HRMS: 639.241148 ([M − H]−) for C31H33F6N4O4, 641.255701([M + H]+) for C31H35F6N4O4.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-3-(4-methoxyphenyl)-1-(1-methylpiperidin-4-yl)urea (9c), white powder 0.71 g, yield 59%; m.p.: 179.1–180.8; MS:603.2([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.33 (d, J = 5.7 Hz, 1H), 8.12 (s, 1H), 7.33–7.26 (m, 2H), 7.14 (d, J = 5.7 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.90 (d, J = 2.0 Hz, 1H), 6.84–6.74 (m, 3H), 5.11 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.49 (s, 2H), 4.08 (tt, J = 11.3, 3.9 Hz, 1H), 3.71 (d, J = 1.9 Hz, 6H), 2.80 (d, J = 11.0 Hz, 2H), 2.21 (s, 3H), 2.17 (s, 3H), 2.06–1.95 (m, 2H), 1.67 (qd, J = 12.1, 3.9 Hz, 2H), 1.54 (d, J = 12.0 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 156.08, 155.96, 155.07, 149.46, 148.06, 146.87, 133.99, 133.88, 124.27 (q, J = 278.0 Hz), 122.70, 122.06, 118.80, 114.19, 113.89, 111.29, 108.08, 71.57, 65.10 (q, J = 33.8 Hz), 55.96, 55.59, 55.28, 52.98, 46.03, 45.03, 30.19, 10.38; IR: 3403, 2943, 2836, 2788, 2759, 1642, 1583, 1511, 1467, 1446, 1416, 1373, 1295, 1250, 1220, 1159, 1128, 1034, 1011, 962, 860, 824, 737, 666, 576, 542, 441; HRMS: 637.241006 ([M + Cl]−) for C31H37ClF3N4O5, 603.278881 ([M + H]+) for C31H38F3N4O5.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-1-(1-methylpiperidin-4-yl)-3-(3-(trifluoromethyl)phenyl)urea (9d), white powder 0.75 g, yield 59%; m.p.: 160.4–162.2 °C; MS: 641.2([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.69 (s, 1H), 8.33 (d, J = 5.7 Hz, 1H), 7.91 (d, J = 2.0 Hz, 1H), 7.74 (dd, J = 8.2, 2.0 Hz, 1H), 7.46 (t, J = 8.0 Hz, 1H), 7.27 (d, J = 7.8 Hz, 1H), 7.14 (d, J = 5.7 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.90 (d, J = 2.0 Hz, 1H), 6.76 (dd, J = 8.3, 2.0 Hz, 1H), 5.11 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.54 (s, 2H), 4.11 (tt, J = 12.1, 3.9 Hz, 1H), 3.70 (s, 3H), 2.84 (d, J = 11.2 Hz, 2H), 2.20 (s, 6H), 2.05 (d, J = 13.2 Hz, 2H), 1.72 (tt, J = 12.4, 6.8 Hz, 2H), 1.57 (d, J = 11.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 155.93, 155.63, 149.48, 148.06, 146.94, 141.89, 133.50, 129.78, 129.51 (q, J = 32.3, 31.7 Hz), 124.76 (d, J = 272.5 Hz), 124.26 (q, J = 278.9, 278.5 Hz), 123.84, 118.75, 118.39 (d, J = 3.9 Hz), 116.39 (d, J = 4.4 Hz), 114.22, 111.25, 108.08, 71.54, 65.10 (q, J = 34.6 Hz), 55.94, 55.05, 53.16, 46.04, 45.68, 45.21, 29.82, 10.35; IR: 3385, 2940, 2839, 2791, 2771, 2735, 1645, 1583, 1513, 1493, 1468, 1444, 1376, 1326, 1247, 1222, 1152, 1122, 1030, 1000, 972, 909, 835, 788, 749, 701, 667, 540, 458; HRMS: 639.241148 ([M − H]−) for C31H33F6N4O4, 641.255701 ([M + H]+) for C31H35F6N4O4.
- 3-(3-chloro-4-fluorophenyl)-1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-1-(1-methylpiperidin-4-yl)urea (9e), white powder 0.69 g, yield 55%; m.p.: 176.2–177.7 °C; MS:625.2([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.33 (d, J = 5.6 Hz, 1H), 7.74 (dd, J = 6.9, 2.6 Hz, 1H), 7.41 (ddd, J = 9.2, 4.4, 2.6 Hz, 1H), 7.27 (t, J = 9.1 Hz, 1H), 7.14 (d, J = 5.7 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.89 (d, J = 2.0 Hz, 1H), 6.75 (dd, J = 8.2, 1.9 Hz, 1H), 5.11 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.51 (s, 2H), 4.08 (tt, J = 12.1, 3.9 Hz, 1H), 3.71 (s, 3H), 2.83 (d, J = 11.2 Hz, 2H), 2.20 (d, J = 2.9 Hz, 6H), 2.05 (d, J = 11.7 Hz, 2H), 1.71 (qd, J = 12.3, 3.7 Hz, 2H), 1.59–1.51 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 161.81, 155.93, 155.62, 152.83 (d, J = 240.9 Hz), 149.46, 148.06, 146.93, 138.30, 133.52, 124.27 (q, J = 277.7 Hz), 122.06, 121.79, 120.59 (d, J = 6.5 Hz), 119.01 (d, J = 18.2 Hz), 118.75, 116.71 (d, J = 21.4 Hz), 114.20, 111.26, 108.08, 71.54, 65.10 (q, J = 34.6 Hz), 55.97, 55.09, 53.16, 45.77, 45.19, 29.89, 10.37; IR: 3403, 3365, 2940, 2881, 2822, 1702, 1598, 1515, 1448, 1407, 1370, 1319, 1260, 1217, 1151, 1107, 1063, 965, 906, 842, 785, 713, 593, 509, 455; HRMS: 623.205307 ([M − H]−) for C30H33ClF4N4O4, 625.219923 ([M + H]+) for C30H35ClF4N4O4.
- 1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-1-(1-methylpiperidin-4-yl)-3-(3-nitrophenyl)urea (9f), yellow powder 0.79 g, yield 64%; m.p.: 157.5–159.1 °C; MS:618.2([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.47 (t, J = 2.3 Hz, 1H), 8.33 (d, J = 5.7 Hz, 1H), 7.92 (dd, J = 8.2, 2.0 Hz, 1H), 7.79 (dd, J = 8.1, 2.2 Hz, 1H), 7.51 (t, J = 8.2 Hz, 1H), 7.13 (d, J = 5.7 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.91 (d, J = 2.0 Hz, 1H), 6.76 (dd, J = 8.3, 2.0 Hz, 1H), 5.10 (s, 2H), 4.91 (q, J = 8.7 Hz, 2H), 4.55 (s, 2H), 4.10 (tt, J = 11.9, 4.0 Hz, 1H), 3.71 (s, 3H), 2.80 (d, J = 11.0 Hz, 2H), 2.20 (s, 3H), 2.16 (s, 3H), 2.00 (t, J = 11.3 Hz, 2H), 1.71 (qd, J = 12.1, 3.8 Hz, 2H), 1.61–1.52 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 161.80, 155.93, 155.52, 149.48, 148.28, 148.06, 146.95, 142.46, 133.45, 129.97, 126.24, 124.27 (q, J = 277.6 Hz), 122.06, 118.74, 116.58, 114.26, 114.21, 111.25, 108.07, 71.54, 65.10 (q, J = 34.4 Hz), 55.97, 55.21, 53.48, 46.01, 45.24, 30.08, 10.37; IR: 3366, 2940, 2842, 2781, 1657, 1585, 1512, 1467, 1426, 1376, 1343, 1248, 1222, 1161, 1131, 1032, 1011, 967, 861, 824, 737, 667, 584, 454; HRMS: 616.238842 ([M − H]−) for C30H33F3N5O6, 618.253395 ([M + H]+) for C30H35F3N5O6.
- 3-(4-ethoxybenzyl)-1-(3-methoxy-4-((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methoxy)benzyl)-1-(1-methylpiperidin-4-yl)urea (9g), white powder 0.41 g, yield 33%; m.p.: 119.0–121.2 °C; MS 631.3([M + H]+); 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 5.7 Hz, 1H), 7.13 (dd, J = 15.2, 7.1 Hz, 3H), 7.01 (d, J = 8.3 Hz, 1H), 6.84–6.75 (m, 4H), 6.72 (dd, J = 8.3, 2.0 Hz, 1H), 5.11 (s, 2H), 4.92 (q, J = 8.7 Hz, 2H), 4.36 (s, 2H), 4.18 (d, J = 5.6 Hz, 2H), 3.98 (q, J = 6.9 Hz, 3H), 3.63 (s, 3H), 2.79 (d, J = 11.0 Hz, 2H), 2.21 (s, 3H), 2.17 (s, 3H), 2.00 (t, J = 11.8 Hz, 2H), 1.61 (qd, J = 12.2, 3.8 Hz, 2H), 1.48 (d, J = 9.9 Hz, 2H), 1.32 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.82, 157.98, 157.62, 155.99, 149.46, 148.06, 146.82, 134.24, 133.60, 128.63, 124.28 (q, J = 277.9 Hz), 122.08, 118.80, 114.39, 114.08, 111.14, 108.09, 71.61, 65.12 (q, J = 34.4 Hz), 63.37, 55.79, 55.18, 52.47, 45.79, 44.88, 43.52, 29.99, 15.12, 10.38; IR: 3348, 2937, 2881, 2837, 2793, 1612, 1584, 1509, 1477, 1447, 1395, 1374, 1292, 1253, 1162, 1131, 1033, 1003, 971, 916, 804, 772, 577; HRMS: 665.272306 ([M + Cl]−) for C33H41ClF3N4O5, 631.310182 ([M + H]+) for C33H42F3N4O5.
3.2. Biological Evaluation
3.2.1. Antiproliferative Activity Assays
3.2.2. Cell Cycle Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Levêque, D.; Veal, G.; Astier, A. Review of therapeutic drug monitoring of anticancer drugs. Part two—targeted therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Piro, G.; Tamburrino, A.; Carbone, C.; Tortora, G. Rationale and clinical use of multitargeting anticancer agents. Curr. Opin. Pharmacol. 2013, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Ma, S. Recent advances in the discovery of multitargeted tyrosine kinase inhibitors as anticancer agents. ChemMedChem 2020, 16, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S.V.U.M. Dual or multi-targeting inhibitors: The next generation anticancer agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kinarivala, N.; Sharma, S. Multi-targeting anticancer agents: Rational approaches, synthetic routes and structure activity relationship. Anti-Cancer Agent. Me. 2019, 19, 842–874. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Marqués-López, E.; Romanos, E.; Fernández-Moreira, V.; Gimeno, M.C.; Marzo, I.; Herrera, R.P. Novel ureido-dihydropyridine scaffolds as theranostic agents. Bioorganic Chem. 2020, 105, 104364. [Google Scholar] [CrossRef] [PubMed]
- Türe, A.; Kahraman, D.C.; Cetin-Atalay, R.; Helvacıoğlu, S.; Charehsaz, M.; Küçükgüzel, İ. Synthesis, anticancer activity, toxicity evaluation and molecular docking studies of novel phenylaminopyrimidine—(thio)urea hybrids as potential kinase inhibitors. Comput. Biol. Chem. 2019, 78, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Kurt, Z.; Ozmen, N.; Bakar-Ates, F. Synthesis and anticancer activity of some pyrimidine derivatives with aryl urea moieties as apoptosis-inducing agents. Bioorganic Chem. 2020, 101, 104028. [Google Scholar] [CrossRef] [PubMed]
- Zi, M.; Liu, F.; Wu, D.; Li, K.; Zhang, D.; Zhu, C.; Zhang, Z.; Li, L.; Zhang, C.; Xie, M.; et al. Discovery of 6-arylurea-2-arylbenzoxazole and 6-arylurea-2-arylbenzimidazole derivatives as angiogenesis inhibitors: Design, synthesis and in vitro biological evaluation. ChemMedChem 2019, 14, 1291–1302. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Zheng, B.; Wang, H.-Y.; Chen, L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacolo. Sin. 2017, 38, 614–622. [Google Scholar] [CrossRef]
- Arai, H.; Battaglin, F.; Wang, J.; Lo, J.H.; Soni, S.; Zhang, W.; Lenz, H.J. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat. Rev. 2019, 81, 101912. [Google Scholar] [CrossRef]
- Azimian, F.; Hamzeh-Mivehroud, M.; Shahbazi Mojarrad, J.; Hemmati, S.; Dastmalchi, S. Synthesis and biological evaluation of diaryl urea derivatives designed as potential anticarcinoma agents using de novo structure-based lead optimization approach. Eur. J. Med. Chem. 2020, 201, 112461. [Google Scholar] [CrossRef]
- Aghcheli, A.; Toolabi, M.; Ayati, A.; Moghimi, S.; Firoozpour, L.; Bakhshaiesh, T.O.; Nazeri, E.; Norouzbahari, M.; Esmaeili, R.; Foroumadi, A. Design, synthesis, and biological evaluation of 1-(5-(benzylthio)-1,3,4-thiadiazol-2-yl)-3-phenylurea derivatives as anticancer agents. Med. Chem. Res 2020, 29, 2000–2010. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Domínguez, E.; Font, M.; Calvo, A.; Prior, C.; Encío, I.; Palop, J.A. Synthesis and pharmacological screening of several aroyl and heteroaroyl selenylacetic acid derivatives as cytotoxic and antiproliferative agents. Molecules 2009, 14, 3313–3338. [Google Scholar] [CrossRef]
- Fako, V.E.; Wu, X.; Pflug, B.; Liu, J.-Y.; Zhang, J.-T. Repositioning proton pump inhibitors as anticancer drugs by targeting the thioesterase domain of human fatty acid synthase. J. Med. Chem. 2015, 58, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ling, T.; Zhaxi, P.; Cao, Y.; Qian, L.; Zhao, D.; Kang, W.; Zhang, W.; Wang, L.; Xu, G.; et al. Proton pump inhibitor pantoprazole inhibits gastric cancer metastasis via suppression of telomerase reverse transcriptase gene expression. Cancer Lett. 2019, 452, 23–30. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; Bolzani, V.D.; Barreir, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Foster, S.A.; Whalen, D.M.; Ozen, A.; Wongchenko, M.J.; Yin, J.; Yen, I.; Schaefer, G.; Mayfield, J.D.; Chmielecki, J.; Stephens, P.J.; et al. Activation mechanism of oncogenic deletion mutations in BRAF, EGFR, and HER2. Cancer Cell 2016, 29, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.R.; Getlik, M.; Grutter, C.; Pawar, V.; Wulfert, S.; Rabiller, M.; Rauh, D. Development of a fluorescent-tagged kinase assay system for the detection and characterization of allosteric kinase inhibitors. J. Am. Chem. Soc. 2009, 131, 13286–13296. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.S.; Zhang, C.; Meng, G.P.; Wu, J.F.; Li, X.S.; Zhao, Q.C.; Hu, C. Design, synthesis and cytotoxic evaluation of a novel series of benzo[d]thiazole-2-carboxamide derivatives as potential EGFR inhibitors. Med. Chem. Res. 2017, 26, 2180–2189. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, X.; Feng, J.; Ding, N.; Li, Y.; Jin, Z.; Meng, Q.; Liu, X.; Hu, C. Design, synthesis and biological evaluation of a new series of 1-aryl-3-{4-[(pyridin-2-ylmethyl)thio]phenyl}urea derivatives as antiproliferative agents. Molecules 2019, 24, 2108. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Youns, M.M.; Soltan, M.K.; Said, A.M. Design, synthesis, molecular modeling and antitumor evaluation of novel indolyl-pyrimidine derivatives with EGFR inhibitory activity. Molecules 2021, 26, 1838. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Jing, D.W.; Chen, R.; Rashid, U.H.; Jiang, J.; Liu, X.; Wang, L.S.; Xie, P. Design, synthesis and evaluation of novel sophoridinic imine derivatives containing conjugated planar structure as potent anticancer agents. Bioorg. Med. Chem. 2018, 26, 4136–4144. [Google Scholar] [CrossRef] [PubMed]



| No. | Structure | IC50(μM) | ||||
|---|---|---|---|---|---|---|
| A549 | MCF7 | HCT116 | PC3 | HL7702 | ||
| 8a |  | 5.30 ± 1.45 | >50 | 7.25 ± 0.87 | >50 | >50 |
| 8b |  | 17.65 ± 5.65 | 10.98 ± 1.68 | 9.33 ± 1.38 | 29.13 ± 5.81 | >50 |
| 8c | 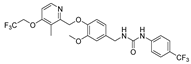 | 4.88 ± 1.94 | >50 | 11.38 ± 3.28 | >50 | >50 |
| 8d |  | 26.47 ± 5.66 | >50 | 9.44 ± 1.22 | >50 | >50 |
| 8e | 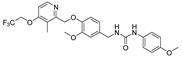 | 29.80 ± 5.09 | >50 | 28.81 ± 3.11 | >50 | >50 |
| 8f | 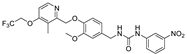 | 12.40 ± 0.60 | 13.26 ± 2.27 | 13.35 ± 2.78 | 15.87 ± 0.73 | >50 |
| 8g | 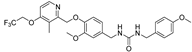 | >50 | >50 | 7.84 ± 1.40 | >50 | >50 |
| 8h |  | 10.93 ± 2.02 | >50 | 23.31 ± 3.12 | >50 | >50 |
| 8i | 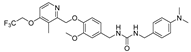 | 9.85 ± 4.40 | >50 | 10.88 ± 2.85 | >50 | >50 |
| 9a | 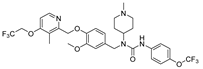 | 17.53 ± 2.95 | 2.59 ± 0.29 | 4.41 ± 0.14 | 4.10 ± 0.19 | >50 |
| 9b | 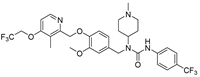 | 4.93 ± 0.46 | 2.56 ± 0.07 | 2.90 ± 0.16 | 3.36 ± 0.17 | >50 |
| 9c |  | 15.76 ± 1.51 | 4.65 ± 0.73 | 12.90 ± 1.59 | 12.35 ± 1.75 | >50 |
| 9d |  | 3.17 ± 0.22 | 2.63 ± 0.08 | 2.56 ± 0.26 | 3.62 ± 0.27 | >50 |
| 9e | 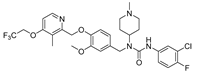 | 5.48 ± 4.36 | 2.56 ± 0.16 | 5.82 ± 0.21 | 4.53 ± 0.33 | >50 |
| 9f |  | 6.09 ± 0.29 | 3.18 ± 0.30 | 5.18 ± 0.33 | 7.39 ± 0.63 | >50 |
| 9g |  | 6.04 ± 0.41 | 4.23 ± 0.47 | 2.93 ± 0.26 | 6.25 ± 0.43 | >50 |
| sorafenib | 6.16 ± 0.46 | 3.54 ± 0.19 | 3.88 ± 0.36 | 5.26 ± 0.46 | >50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Liang, S.; Zhang, C.; Han, Y.; Liang, J.; Hu, H.; Zhang, X.; Hu, C.; Liu, X.; Zhang, H. Design, Synthesis and Anticancer Activity of a New Series of N-aryl-N′-[4-(pyridin-2-ylmethoxy)benzyl]urea Derivatives. Molecules 2021, 26, 3496. https://doi.org/10.3390/molecules26123496
Hou S, Liang S, Zhang C, Han Y, Liang J, Hu H, Zhang X, Hu C, Liu X, Zhang H. Design, Synthesis and Anticancer Activity of a New Series of N-aryl-N′-[4-(pyridin-2-ylmethoxy)benzyl]urea Derivatives. Molecules. 2021; 26(12):3496. https://doi.org/10.3390/molecules26123496
Chicago/Turabian StyleHou, Shicheng, Shishao Liang, Chao Zhang, Yingmei Han, Jianhui Liang, Hongyu Hu, Xingeng Zhang, Chun Hu, Xiaoping Liu, and Hong Zhang. 2021. "Design, Synthesis and Anticancer Activity of a New Series of N-aryl-N′-[4-(pyridin-2-ylmethoxy)benzyl]urea Derivatives" Molecules 26, no. 12: 3496. https://doi.org/10.3390/molecules26123496
APA StyleHou, S., Liang, S., Zhang, C., Han, Y., Liang, J., Hu, H., Zhang, X., Hu, C., Liu, X., & Zhang, H. (2021). Design, Synthesis and Anticancer Activity of a New Series of N-aryl-N′-[4-(pyridin-2-ylmethoxy)benzyl]urea Derivatives. Molecules, 26(12), 3496. https://doi.org/10.3390/molecules26123496