Design of a Near Infrared Fluorescent Ureter Imaging Agent for Prevention of Ureter Damage during Abdominal Surgeries
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Synthesis of PEGylated S0456 NIR Dyes
2.3. Characterization of Spectral Properties
2.4. Animal Husbandry
2.5. In Vivo Biodistribution
2.6. Effect of pH on UreterGlow-11 Emission Spectra
3. Results and Discussion
3.1. Design and Synthesis of the Ureter Probes
3.2. Characterization of Physical Properties
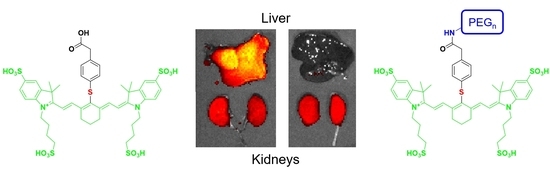
3.3. In Vivo Imaging and Biodistribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Silva, G.; Boutros, M.; Wexner, S. Role of prophylactic ureteric stents in colorectal surgery. Asian J. Endosc. Surg. 2012, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Packiam, V.T.; Andrew, C.; Joseph, P.; Charles, N.; Sarah, F.; Gregory, B. The Impact of Minimally Invasive Surgery on Major Iatrogenic Ureteral Injury and Subsequent Ureteral Repair during Hysterectomy: A National Analysis of Risk Factors and Outcomes. Urology 2016, 98, 183–188. [Google Scholar] [CrossRef]
- Barber, M.D.; Visco, A.G.; Weidner, A.C.; Amundsen, C.L.; Bump, R.C. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am. J. Obstet. Gynecol. 2000, 183, 1402–1411. [Google Scholar] [CrossRef]
- Sandberg, E.M.; Cohen, S.L.; Hurwitz, S.; Einarsson, J.I. Utility of cystoscopy during hysterectomy. Obstet. Gynecol. 2012, 120, 1363–1370. [Google Scholar] [CrossRef]
- Sharp, H.T.; Adelman, M.R. Prevention, Recognition, and Management of Urologic Injuries during Gynecologic Surgery. Obstet. Gynecol. 2016, 127, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.T.; Wang, C.J.; Lien, R.C. Prophylactic ureteral catheterization in gynecologic surgery: A 12-year randomized trial in a community hospital. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009, 20, 689–693. [Google Scholar] [CrossRef]
- Speicher, P.J.; Goldsmith, Z.G.; Nussbaum, D.P.; Turley, R.S.; Peterson, A.C.; Mantyh, C.R. Ureteral stenting in laparoscopic colorectal surgery. J. Surg. Res. 2014, 190, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, F.; Yari, H.; Ali Asgari, M.; Fallahnezhad, M.H.; Tavoosian, A.; Ghadian, A. Benefits and Complications of Removing Ureteral Stent Based on the Elapsed Time after Renal Transplantation Surgery. Nephrourol. Mon. 2016, 8, e31108. [Google Scholar] [CrossRef]
- Fanning, J.; Fenton, B.; Jean, G.M.; Chae, C. Cost analysis of prophylactic intraoperative cystoscopic ureteral stents in gynecologic surgery. J. Am. Osteopath. Assoc. 2011, 111, 667–669. [Google Scholar]
- Cherrick, G.R.; Stein, S.W.; Leevy, C.M.; Davidson, C.S. Indocyanine green: Observations on its physical properties, plasma decay, and hepatic extraction. J. Clin. Investig. 1960, 39, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.M.; Dip, F.; Castillo, M.; Roy, M.; Wexner, S.D.; Rosenthal, R.J.; Low, P.S. Intraoperative Ureter Visualization Using a Novel Near-Infrared Fluorescent Dye. Mol. Pharm. 2018, 15, 3442–3447. [Google Scholar] [CrossRef] [PubMed]
- Siddighi, S.; Yune, J.J.; Hardesty, J. Indocyanine green for intraoperative localization of ureter. Am. J. Obstet. Gynecol. 2014, 211, 436.e1–436.e2. [Google Scholar] [CrossRef] [PubMed]
- Rajanbabu, A.; Patel, V.J. Ureteric mapping with Indocyanine green: A new tool to prevent ureteral injury in complex gynecological surgery. J. Endometr. Pelvic Pain Disord. 2020, 12, 190–192. [Google Scholar] [CrossRef]
- Lee, Z.; Moore, B.; Giusto, L.; Eun, D.D. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur. Urol. 2015, 67, 291–298. [Google Scholar] [CrossRef]
- Cabanes, M.; Boria, F.; Gutiérrez, A.H.; Zapardiel, I. Intra-operative identification of ureters using indocyanine green for gynecological oncology procedures. Int. J. Gynecol. Cancer 2019, 30, 278. [Google Scholar] [CrossRef] [PubMed]
- Slooter, M.; Janssen, A.; Bemelman, W.; Tanis, P.; Hompes, R. Currently available and experimental dyes for intraoperative near-infrared fluorescence imaging of the ureters: A systematic review. Tech. Coloproctol. 2019, 23, 305–313. [Google Scholar] [CrossRef]
- Korb, M.L.; Hartman, Y.E.; Kovar, J.; Zinn, K.R.; Bland, K.I.; Rosenthal, E.L. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J. Surg. Res. 2014, 188, 119–128. [Google Scholar] [CrossRef]
- Shrivastava, A.; Ding, H.; Kothandaraman, S.; Wang, S.-H.; Gong, L.; Williams, M.; Milum, K.; Zhang, S.; Tweedle, M.F. A High-Affinity Near-Infrared Fluorescent Probe to Target Bombesin Receptors. Mol. Imaging Biol. 2014, 16, 661–669. [Google Scholar] [CrossRef][Green Version]
- Kanduluru, A.K.; Srinivasarao, M.; Low, P.S. Design, Synthesis, and Evaluation of a Neurokinin-1 Receptor-Targeted Near-IR Dye for Fluorescence-Guided Surgery of Neuroendocrine Cancers. Bioconjug. Chem. 2016, 27, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Wayua, C.; Low, P.S. Evaluation of a cholecystokinin 2 receptor-targeted near-infrared dye for fluorescence-guided surgery of cancer. Mol. Pharm. 2014, 11, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Rafiei, P.; Azadi, A. Designing PEGylated therapeutic molecules: Advantages in ADMET properties. Expert Opin. Drug Discov. 2008, 3, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Mozar, F.S.; Chowdhury, E.H. Impact of PEGylated nanoparticles on tumor targeted drug delivery. Curr. Pharm. Des. 2018, 24, 3283–3296. [Google Scholar] [CrossRef] [PubMed]
- De Boer, E.; Harlaar, N.J.; Taruttis, A.; Nagengast, W.B.; Rosenthal, E.L.; Ntziachristos, V.; van Dam, G.M. Optical innovations in surgery. Br. J. Surg. 2015, 102, e56–e72. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, J.; Al-Taher, M.A.; Bouvy, N.D.; Stassen, L.P.S. Near-infrared fluorescence laparoscopy of the ureter with three preclinical dyes in a pig model. Surg Endosc. 2019, 33, 986–991. [Google Scholar] [CrossRef]
- Bono, M.J.; Reygaert, W.C. Urinary Tract Infection. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]







| NIR Dyes | Excitation Maximum (nm) | Emission Maximum (nm) | Stokes Shift (nm) |
|---|---|---|---|
| IR800CW | 780 | 795 | 15 |
| LS288 | 770 | 785 | 15 |
| ZW800-1 | 770 | 788 | 18 |
| ICG | 780 | 802 | 22 |
| OTL38 | 776 | 796 | 20 |
| UreterGlow | 800 | 830 | 30 |
| UreterGlow-0 | 800 | 830 | 30 |
| UreterGlow-3 | 800 | 830 | 30 |
| UreterGlow-11 | 800 | 830 | 30 |
| UreterGlow-45 | 800 | 830 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalingam, S.M.; Putt, K.S.; Srinivasarao, M.; Low, P.S. Design of a Near Infrared Fluorescent Ureter Imaging Agent for Prevention of Ureter Damage during Abdominal Surgeries. Molecules 2021, 26, 3739. https://doi.org/10.3390/molecules26123739
Mahalingam SM, Putt KS, Srinivasarao M, Low PS. Design of a Near Infrared Fluorescent Ureter Imaging Agent for Prevention of Ureter Damage during Abdominal Surgeries. Molecules. 2021; 26(12):3739. https://doi.org/10.3390/molecules26123739
Chicago/Turabian StyleMahalingam, Sakkarapalayam M., Karson S. Putt, Madduri Srinivasarao, and Philip S. Low. 2021. "Design of a Near Infrared Fluorescent Ureter Imaging Agent for Prevention of Ureter Damage during Abdominal Surgeries" Molecules 26, no. 12: 3739. https://doi.org/10.3390/molecules26123739
APA StyleMahalingam, S. M., Putt, K. S., Srinivasarao, M., & Low, P. S. (2021). Design of a Near Infrared Fluorescent Ureter Imaging Agent for Prevention of Ureter Damage during Abdominal Surgeries. Molecules, 26(12), 3739. https://doi.org/10.3390/molecules26123739