Nanomedicine Reformulation of Chloroquine and Hydroxychloroquine
Abstract
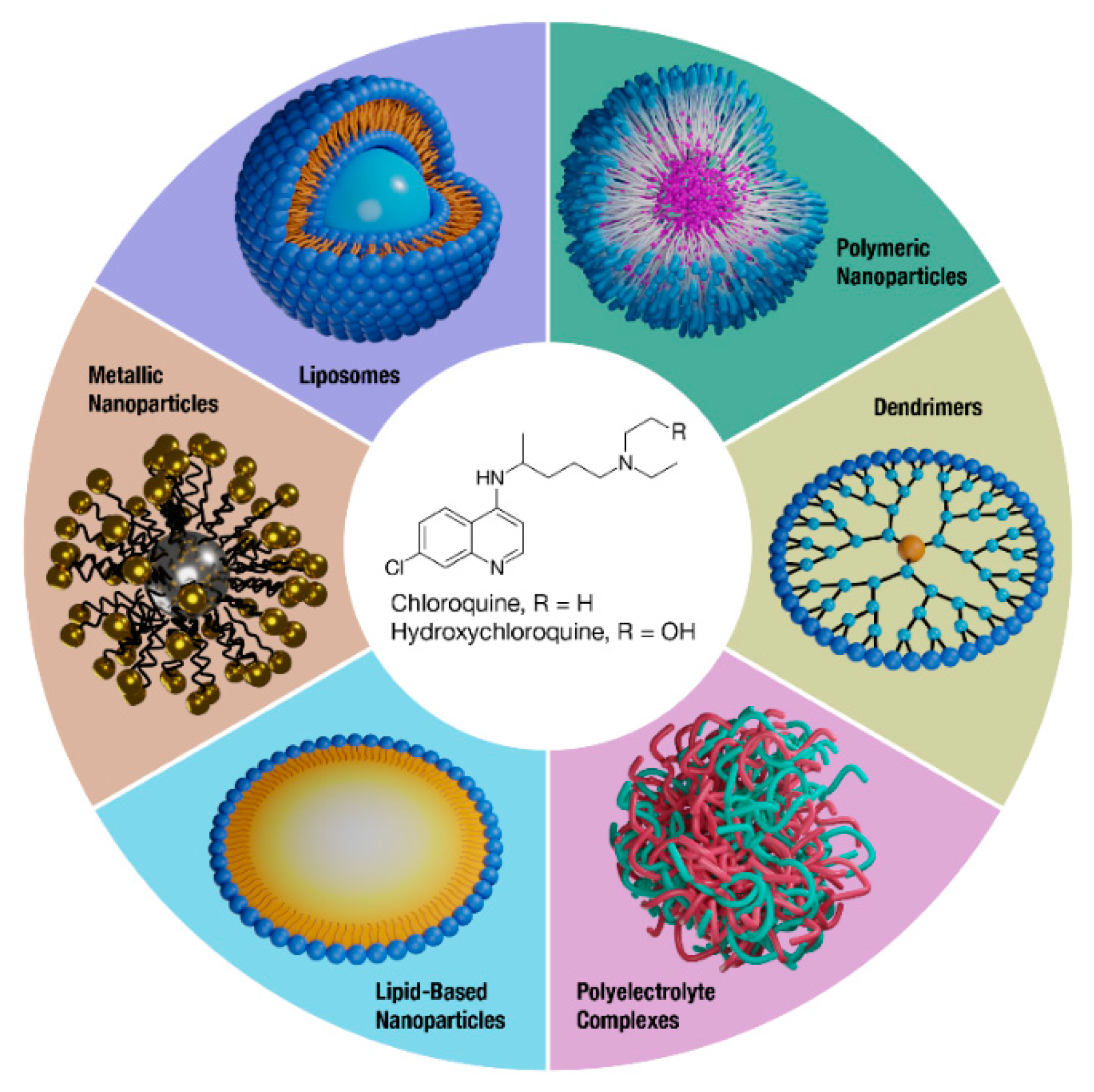
1. Introduction
2. Liposomes
3. Polymeric Nanoparticles
4. Dendrimers
5. Polyelectrolyte Complexes
6. Non-Liposomal Lipid-Based Nanoparticles
7. Metal Nanoparticles
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chew, C.Y.; Mar, A.; Nikpour, M.; Saracino, A.M. Hydroxychloroquine in dermatology: New perspectives on an old drug. Australas J. Dermatol. 2020, 61, e150–e157. [Google Scholar] [CrossRef]
- Mian, A.; Ibrahim, F.; Scott, D.L. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol. 2019, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Taherian, E.; Rao, A.; Malemud, C.J.; Askari, A.D. The biological and clinical activity of anti-malarial drugs in autoimmune disorders. Curr. Rheumatol. Rev. 2013, 9, 45–62. [Google Scholar] [CrossRef]
- Dolgin, E. Anticancer autophagy inhibitors attract ‘resurgent’ interest. Nat. Rev. Drug Discov. 2019, 18, 408–410. [Google Scholar] [CrossRef]
- Shi, T.T.; Yu, X.X.; Yan, L.J.; Xiao, H.T. Research progress of hydroxychloroquine and autophagy inhibitors on cancer. Cancer Chemother. Pharmacol. 2017, 79, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Maes, H.; Kuchnio, A.; Peric, A.; Moens, S.; Nys, K.; De Bock, K.; Quaegebeur, A.; Schoors, S.; Georgiadou, M.; Wouters, J.; et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 2014, 26, 190–206. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective autophagy elicited by RAF-->MEK-->ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef]
- Dyall, J.; Coleman, C.M.; Hart, B.J.; Venkataraman, T.; Holbrook, M.R.; Kindrachuk, J.; Johnson, R.F.; Olinger, G.G., Jr.; Jahrling, P.B.; Laidlaw, M.; et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014, 58, 4885–4893. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Van Ranst, M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and (accessed on 18 November 2020).
- Mehra, M.R.; Ruschitzka, F.; Patel, A.N. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet 2020, 395, 1820. [Google Scholar] [CrossRef]
- Hoffmann, M.; Mosbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Kruger, N.; Gassen, N.C.; Muller, M.A.; Drosten, C.; Pohlmann, S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 2020, 585, 588–590. [Google Scholar] [CrossRef]
- Browning, D.J. Pharmacology of Chloroquine and Hydroxychloroquine. In Hydroxychloroquine and Chloroquine Retinopathy; Springer: New York, NY, USA, 2014. [Google Scholar]
- Leung, L.S.; Neal, J.W.; Wakelee, H.A.; Sequist, L.V.; Marmor, M.F. Rapid Onset of Retinal Toxicity From High-Dose Hydroxychloroquine Given for Cancer Therapy. Am. J. Ophthalmol. 2015, 160, 799–805 e1. [Google Scholar] [CrossRef]
- Michaelides, M.; Stover, N.B.; Francis, P.J.; Weleber, R.G. Retinal toxicity associated with hydroxychloroquine and chloroquine: Risk factors, screening, and progression despite cessation of therapy. Arch. Ophthalmol. 2011, 129, 30–39. [Google Scholar] [CrossRef]
- Offerhaus, J.A.; Wilde, A.A.M.; Remme, C.A. Prophylactic (hydroxy)chloroquine in COVID-19: Potential relevance for cardiac arrhythmia risk. Heart Rhythm. 2020, 17, 1480–1486. [Google Scholar] [CrossRef]
- Furst, D.E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 1996, 5 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Abra, R.M.; Bankert, R.B.; Chen, F.; Egilmez, N.K.; Huang, K.; Saville, R.; Slater, J.L.; Sugano, M.; Yokota, S.J. The next generation of liposome delivery systems: Recent experience with tumor-targeted, sterically-stabilized immunoliposomes and active-loading gradients. J. Liposome Res. 2002, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.C. Surface-modified liposomes: Assessment and characterization for increased stability and prolonged blood circulation. Chem. Phys. Lipids 1993, 64, 249–262. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Singhal, A.; Gupta, C.M. Functional drug targeting to erythrocytes in vivo using antibody bearing liposomes as drug vehicles. Biochem. Biophys. Res. Commun. 1987, 148, 357–361. [Google Scholar] [CrossRef]
- Owais, M.; Varshney, G.C.; Choudhury, A.; Chandra, S.; Gupta, C.M. Chloroquine encapsulated in malaria-infected erythrocyte-specific antibody-bearing liposomes effectively controls chloroquine-resistant Plasmodium berghei infections in mice. Antimicrob. Agents Chemother. 1995, 39, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.A.; Brunink, B.G.; Eling, W.M.; Crommelin, D.J. Therapeutic effect of chloroquine(CQ)-containing immunoliposomes in rats infected with Plasmodium berghei parasitized mouse red blood cells: Comparison with combinations of antibodies and CQ or liposomal CQ. Biochim. Biophys. Acta 1989, 981, 269–276. [Google Scholar] [CrossRef]
- Peeters, P.A.; de Leest, K.; Eling, W.M.; Crommelin, D.J. Chloroquine blood levels after administration of the liposome-encapsulated drug in relation to therapy of murine malaria. Pharm. Res. 1989, 6, 787–793. [Google Scholar] [CrossRef]
- Peeters, P.A.; Huiskamp, C.W.; Eling, W.M.; Crommelin, D.J. Chloroquine containing liposomes in the chemotherapy of murine malaria. Parasitology 1989, 98 Pt 3, 381–386. [Google Scholar] [CrossRef]
- Titulaer, H.A.; Eling, W.M.; Crommelin, D.J.; Peeters, P.A.; Zuidema, J. The parenteral controlled release of liposome encapsulated chloroquine in mice. J. Pharm. Pharmacol. 1990, 42, 529–532. [Google Scholar] [CrossRef]
- Fotoran, W.L.; Muntefering, T.; Kleiber, N.; Miranda, B.N.M.; Liebau, E.; Irvine, D.J.; Wunderlich, G. A multilamellar nanoliposome stabilized by interlayer hydrogen bonds increases antimalarial drug efficacy. Nanomedicine 2019, 22, 102099. [Google Scholar] [CrossRef]
- Gabizon, A.; Chisin, R.; Amselem, S.; Druckmann, S.; Cohen, R.; Goren, D.; Fromer, I.; Peretz, T.; Sulkes, A.; Barenholz, Y. Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br. J. Cancer 1991, 64, 1125–1132. [Google Scholar] [CrossRef]
- Khan, M.A.; Jabeen, R.; Mohammad, O. Prophylactic role of liposomized chloroquine against murine cryptococcosis less susceptible to fluconazole. Pharm. Res. 2004, 21, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Jabeen, R.; Nasti, T.H.; Mohammad, O. Enhanced anticryptococcal activity of chloroquine in phosphatidylserine-containing liposomes in a murine model. J. Antimicrob. Chemother. 2005, 55, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Moles, E.; Urban, P.; Jimenez-Diaz, M.B.; Viera-Morilla, S.; Angulo-Barturen, I.; Busquets, M.A.; Fernandez-Busquets, X. Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J. Control. Release 2015, 210, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar]
- Price, L.S.L.; Stern, S.T.; Deal, A.M.; Kabanov, A.V.; Zamboni, W.C. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci. Adv. 2020, 6, eaay9249. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xu, Y.; Qiu, L. Enhanced combination therapy effect on paclitaxel-resistant carcinoma by chloroquine co-delivery via liposomes. Int. J. Nanomed. 2015, 10, 6615–6632. [Google Scholar]
- Gao, M.; Xu, Y.; Qiu, L. Sensitization of multidrug-resistant malignant cells by liposomes co-encapsulating doxorubicin and chloroquine through autophagic inhibition. J. Liposome Res. 2017, 27, 151–160. [Google Scholar] [CrossRef]
- Wang, Y.; Tai, X.; Zhang, L.; Liu, Y.; Gao, H.; Chen, J.; Shi, K.; Zhang, Q.; Zhang, Z.; He, Q. A novel antitumour strategy using bidirectional autophagic vesicles accumulation via initiative induction and the terminal restraint of autophagic flux. J. Control. Release 2015, 199, 17–28. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Chen, W.; Qin, M.; Zhang, Z.; Gong, T.; Sun, X. Potentiating bacterial cancer therapy using hydroxychloroquine liposomes. J. Control. Release 2018, 280, 39–50. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, K.; Zhang, L.; Hu, G.; Wan, J.; Tang, J.; Yin, S.; Duan, J.; Qin, M.; Wang, N.; et al. Significantly enhanced tumor cellular and lysosomal hydroxychloroquine delivery by smart liposomes for optimal autophagy inhibition and improved antitumor efficiency with liposomal doxorubicin. Autophagy 2016, 12, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, Q.; Liu, Y.; Sheng, Q.; Shi, K.; Wang, Y.; Li, M.; Zhang, Z.; He, Q. Synergistic cytotoxicity and co-autophagy inhibition in pancreatic tumor cells and cancer-associated fibroblasts by dual functional peptide-modified liposomes. Acta Biomater. 2019, 99, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xia, C.; Wang, Y.; Wan, D.; Rao, J.; Tang, X.; Wei, J.; Wang, X.; Li, M.; Zhang, Z.; et al. Dual receptor recognizing liposomes containing paclitaxel and hydroxychloroquine for primary and metastatic melanoma treatment via autophagy-dependent and independent pathways. J. Control. Release 2018, 288, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, Y.; Yu, Q.; Li, H.; Chen, X.; Li, M.; Long, Y.; Liu, Y.; Lu, L.; Tang, J.; et al. Enhanced glioma therapy by synergistic inhibition of autophagy and tyrosine kinase activity. Int. J. Pharm. 2018, 536, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, P.; Zhang, K.; Shi, Y.; Li, Y.; Li, C.; Lu, J.; Liu, Q.; Wang, X. Manipulation of Mitophagy by “All-in-One” nanosensitizer augments sonodynamic glioma therapy. Autophagy 2020, 16, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, J.; He, Z.; Men, K.; Mao, Y.; Ye, T.; Chen, H.; Li, L.; Xu, B.; Wei, Y.; et al. Cholesterol-modified Hydroxychloroquine-loaded Nanocarriers in Bleomycin-induced Pulmonary Fibrosis. Sci. Rep. 2017, 7, 10737. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhu, W.; Lv, X.; Yang, Q.; Liu, X.; Xie, Y.; Tang, P.; Sun, L. Encapsulation of chloroquine and doxorubicin by MPEG-PLA to enhance anticancer effects by lysosomes inhibition in ovarian cancer. Int. J. Nanomed. 2018, 13, 8231–8245. [Google Scholar] [CrossRef]
- Yang, C.; Hu, T.; Cao, H.; Zhang, L.; Zhou, P.; He, G.; Song, X.; Tong, A.; Guo, G.; Yang, F.; et al. Facile Construction of Chloroquine Containing PLGA-Based pDNA Delivery System for Efficient Tumor and Pancreatitis Targeting in Vitro and in Vivo. Mol. Pharm. 2015, 12, 2167–2179. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Han, Y.; Zhang, J.; Liu, D.; Ma, G.; Li, C.; Liu, L.; Kong, D. Nanovaccine Incorporated with Hydroxychloroquine Enhances Antigen Cross-Presentation and Promotes Antitumor Immune Responses. ACS Appl. Mater. Interfaces 2018, 10, 30983–30993. [Google Scholar] [CrossRef]
- Mezzaroba, N.; Zorzet, S.; Secco, E.; Biffi, S.; Tripodo, C.; Calvaruso, M.; Mendoza-Maldonado, R.; Capolla, S.; Granzotto, M.; Spretz, R.; et al. New potential therapeutic approach for the treatment of B-Cell malignancies using chlorambucil/hydroxychloroquine-loaded anti-CD20 nanoparticles. PLoS ONE 2013, 8, e74216. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, X.; Gu, J.; Chang, D.; Zhang, J.; Chen, Z.; Ye, Y.; Wang, C.; Tao, W.; Zeng, X.; et al. Investigation and intervention of autophagy to guide cancer treatment with nanogels. Nanoscale 2017, 9, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Marti Coma-Cros, E.; Lancelot, A.; San Anselmo, M.; Neves Borgheti-Cardoso, L.; Valle-Delgado, J.J.; Serrano, J.L.; Fernandez-Busquets, X.; Sierra, T. Micelle carriers based on dendritic macromolecules containing bis-MPA and glycine for antimalarial drug delivery. Biomater. Sci. 2019, 7, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Gupta, U.; Jain, N.K. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials 2007, 28, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, D.; Bhadra, S.; Jain, N.K. PEGylated peptide dendrimeric carriers for the delivery of antimalarial drug chloroquine phosphate. Pharm. Res. 2006, 23, 623–633. [Google Scholar] [CrossRef]
- Panagiotaki, K.N.; Sideratou, Z.; Vlahopoulos, S.A.; Paravatou-Petsotas, M.; Zachariadis, M.; Khoury, N.; Zoumpourlis, V.; Tsiourvas, D. A Triphenylphosphonium-Functionalized Mitochondriotropic Nanocarrier for Efficient Co-Delivery of Doxorubicin and Chloroquine and Enhanced Antineoplastic Activity. Pharmaceuticals 2017, 10, 91. [Google Scholar] [CrossRef]
- Marti Coma-Cros, E.; Biosca, A.; Marques, J.; Carol, L.; Urban, P.; Berenguer, D.; Riera, M.C.; Delves, M.; Sinden, R.E.; Valle-Delgado, J.J.; et al. Polyamidoamine Nanoparticles for the Oral Administration of Antimalarial Drugs. Pharmaceutics 2018, 10, 225. [Google Scholar] [CrossRef]
- Urban, P.; Valle-Delgado, J.J.; Mauro, N.; Marques, J.; Manfredi, A.; Rottmann, M.; Ranucci, E.; Ferruti, P.; Fernandez-Busquets, X. Use of poly(amidoamine) drug conjugates for the delivery of antimalarials to Plasmodium. J. Control. Release 2014, 177, 84–95. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, S.; Chakraborty, S.P.; Sahu, S.K.; Pramanik, P.; Roy, S. Synthesis, characterization of chitosan-tripolyphosphate conjugated chloroquine nanoparticle and its in vivo anti-malarial efficacy against rodent parasite: A dose and duration dependent approach. Int. J. Pharm. 2012, 434, 292–305. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, S.; Dash, S.K.; Chattopadhyay, S.; Roy, S. The Impact of Nanochloroquine on Restoration of Hepatic and Splenic Mitochondrial Damage against Rodent Malaria. J. Nanoparticls 2013, 2013, 106152. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, S.; Dash, S.K.; Mahapatra, S.K.; Chattopadhyay, S.; Majumdar, S.; Roy, S. A prospective strategy to restore the tissue damage in malaria infection: Approach with chitosan-trypolyphosphate conjugated nanochloroquine in Swiss mice. Eur. J. Pharmacol. 2014, 737, 11–21. [Google Scholar] [CrossRef]
- Tripathy, S.; Mahapatra, S.K.; Chattopadhyay, S.; Das, S.; Dash, S.K.; Majumder, S.; Pramanik, P.; Roy, S. A novel chitosan based antimalarial drug delivery against Plasmodium berghei infection. Acta Trop. 2013, 128, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Bhalekar, M.R.; Upadhaya, P.G.; Madgulkar, A.R. Fabrication and efficacy evaluation of chloroquine nanoparticles in CFA-induced arthritic rats using TNF-alpha ELISA. Eur. J. Pharm. Sci. 2016, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baruah, U.K.; Gowthamarajan, K.; Ravisankar, V.; Karri, V.; Simhadri, P.K.; Singh, V. Optimisation of chloroquine phosphate loaded nanostructured lipid carriers using Box-Behnken design and its antimalarial efficacy. J. Drug Target. 2018, 26, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Bendas, E.R.; Abdullah, H.; El-Komy, M.H.; Kassem, M.A. Hydroxychloroquine niosomes: A new trend in topical management of oral lichen planus. Int. J. Pharm. 2013, 458, 287–295. [Google Scholar] [CrossRef]
- Ruan, S.; Xie, R.; Qin, L.; Yu, M.; Xiao, W.; Hu, C.; Yu, W.; Qian, Z.; Ouyang, L.; He, Q.; et al. Aggregable Nanoparticles-Enabled Chemotherapy and Autophagy Inhibition Combined with Anti-PD-L1 Antibody for Improved Glioma Treatment. Nano Lett. 2019, 19, 8318–8332. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, X.; Hao, Y.; Wang, N.; Feng, X.; Hou, L.; Zhang, Z. Cancer Cell Membrane-Biomimetic Nanoplatform for Enhanced Sonodynamic Therapy on Breast Cancer via Autophagy Regulation Strategy. ACS Appl. Mater. Interfaces 2019, 11, 32729–32738. [Google Scholar] [CrossRef]
- Kanvinde, S.; Chhonker, Y.S.; Ahmad, R.; Yu, F.; Sleightholm, R.; Tang, W.; Jaramillo, L.; Chen, Y.; Sheinin, Y.; Li, J.; et al. Pharmacokinetics and efficacy of orally administered polymeric chloroquine as macromolecular drug in the treatment of inflammatory bowel disease. Acta Biomater. 2018, 82, 158–170. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, M.; Sheng, Z.; Chen, Y.; Yeh, C.K.; Chen, W.; Liu, J.; Liu, X.; Yan, F.; Zheng, H. Theranostic nanosensitizers for highly efficient MR/fluorescence imaging-guided sonodynamic therapy of gliomas. J. Cell Mol. Med. 2018, 22, 5394–5405. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Wang, P.; Zhang, K.; Geng, X.; Liu, Q.; Wang, X. Tumor targeting DVDMS-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater. Sci. 2019, 7, 985–994. [Google Scholar] [CrossRef]
- Signorell, R.D.; Luciani, P.; Brambilla, D.; Leroux, J.C. Pharmacokinetics of lipid-drug conjugates loaded into liposomes. Eur. J. Pharm. Biopharm. 2018, 128, 188–199. [Google Scholar] [CrossRef]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Poly(ethylene oxide)-block-poly(L-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 169–190. [Google Scholar] [CrossRef]
- Norvaisas, P.; Ziemys, A. The role of payload hydrophobicity in nanotherapeutic pharmacokinetics. J. Pharm. Sci. 2014, 103, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, D.C.; Steele, J.C.; Adagu, I.S.; Craig, J.C.; Cullander, C. Hydroxychloroquine is much less active than chloroquine against chloroquine-resistant Plasmodium falciparum, in agreement with its physicochemical properties. J. Antimicrob. Chemother. 2003, 52, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mo, L.; Lu, C.H.; Fu, T.; Yang, H.H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef]
- Seymour, L.W.; Ferry, D.R.; Anderson, D.; Hesslewood, S.; Julyan, P.J.; Poyner, R.; Doran, J.; Young, A.M.; Burtles, S.; Kerr, D.J.; et al. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Cui, F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J. Pharm. Pharmacol. 2004, 56, 1527–1535. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Yang, W.; Yu, M.; Sun, S.; Xie, B. Improved Oral Bioavailability and Liver Targeting of Sorafenib Solid Lipid Nanoparticles in Rats. AAPS PharmSciTech 2018, 19, 761–768. [Google Scholar] [CrossRef]
- Krishna, S.; White, N.J. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin. Pharm. 1996, 30, 263–299. [Google Scholar] [CrossRef]
- Tulpule, A.; Krishnaswamy, K. Effect of food on bioavailability of chloroquine. Eur. J. Clin. Pharmacol. 1982, 23, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Hasan, G.M.; Garg, N.; Dogra, E.; Surolia, R.; Ghosh, P.C. Inhibition of the Growth of Plasmodium falciparum in Culture by Stearylamine-Phosphatidylcholine Liposomes. J. Parasitol. Res. 2011, 2011, 120462. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Ambardekar, V.V.; Stern, S.T. NBCD Pharmacokinetics and Drug Release Methods. In Non-Biological Complex Drugs; The Science and the Regulatory Landscape, 1st ed.; Crommelin, D.J.A., de Vlieger, J.S.B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 261–287. [Google Scholar]
- Stern, S.T.; Hall, J.B.; Yu, L.L.; Wood, L.J.; Paciotti, G.F.; Tamarkin, L.; Long, S.E.; McNeil, S.E. Translational considerations for cancer nanomedicine. J. Control. Release 2010, 146, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Skoczen, S.L.; Snapp, K.S.; Crist, R.M.; Kozak, D.; Jiang, X.; Liu, H.; Stern, S.T. Distinguishing Pharmacokinetics of Marketed Nanomedicine Formulations Using a Stable Isotope Tracer Assay. ACS Pharmacol. Transl. Sci. 2020, 3, 547–558. [Google Scholar] [CrossRef]
- Starpharma. DEP® Docetaxel Positive Phase 1 Results; Phase 2 Commences. Available online: https://www.starpharma.com/news/339 (accessed on 18 November 2020).
- Starpharma. Starpharma to Commence DEP® Cabazitaxel Phase 1/2 Trial. Available online: http://www.starpharma.com/news/356 (accessed on 18 November 2020).
- El Maghraby, G.M.; Arafa, M.F. Liposomes for Enhanced Cellular Uptake of Anticancer Agents. Curr. Drug Deliv. 2020, 17, 861–873. [Google Scholar] [CrossRef]
- Charrois, G.J.; Allen, T.M. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim. Biophys. Acta 2004, 1663, 167–177. [Google Scholar] [CrossRef]
- Shmeeda, H.; Amitay, Y.; Gorin, J.; Tzemach, D.; Mak, L.; Stern, S.T.; Barenholz, Y.; Gabizon, A. Coencapsulation of alendronate and doxorubicin in pegylated liposomes: A novel formulation for chemoimmunotherapy of cancer. J. Drug Target. 2016, 24, 878–889. [Google Scholar] [CrossRef]
- Silverman, L.; Barenholz, Y. In vitro experiments showing enhanced release of doxorubicin from Doxil(R) in the presence of ammonia may explain drug release at tumor site. Nanomedicine 2015, 11, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Drug Loading Method | Drug | Co-drug | Indication | Reference |
|---|---|---|---|---|---|
| Liposome (Anti-mouse erythrocyte F(ab’)2 targeted- egg PC/Chol/gangliosides) | Passive loading by thin film sonication | CQ | - | Malaria | [25] |
| Liposome (Mouse monoclonal antibody F10 targeted- egg PC/chol/gangliosides) | Passive loading by thin film sonication | CQ | - | Malaria | [26] |
| Liposome (Soybean PC/egg PG/Chol) | Passive loading by reverse-phase evaporation, sonication, and extrusion | CQ | - | Malaria | [28,29,30] |
| Liposome (Anti-mouse erythrocyte Fab’ targeted-MPC-PE, Chol/PC/PS) | Passive loading by reverse-phase evaporation, sonication, and extrusion | CQ | - | Malaria | [27] |
| Liposome (Multilamellar- DOPC/DPGG/amine-N-[4-(p- maleimidophenyl) butyramide)]) | Passive loading by thin film sonication | CQ | - | Malaria | [31] |
| Liposome (Glycophorin A targeted- DOPC/DSPC/DSPE-PEG2000-Mal) | Passive loading by thin film hydration, sonication, and extrusion | CQ | - | Malaria | [35] |
| Liposome (Egg PC/Chol) | Passive loading by thin film hydration and sonication | CQ | Fluconazole | C. neoformans | [33,34] |
| Liposome (Soybean PC/Chol) | Thin film hydration, sonication, and active loading using a citrate pH-gradient | CQ | PTX | Lung cancer | [39] |
| Liposome (Soybean PC/Chol) | Thin film hydration, sonication, and extrusion, followed by active loading using a citrate pH-gradient | CQ | DXR | Breast cancer | [40] |
| Liposome (Soybean PC/Chol/DSPE-mPEG2000) | Thin film hydration, sonication, and active loading using a citrate pH-gradient | HCQ | Tat-Beclin1 peptide | Breast cancer | [41] |
| Liposome (Soybean PC/Chol/DSPE-mPEG2000) | Thin film hydration, sonication, and active loading using a citrate pH-gradient | HCQ | VNP20009 | Melanoma | [42] |
| Liposome (ITGAV-ITGB3/integrin αvβ3 receptor-targeted- soybean PC/Chol/DSPE-PEG2000-Mal) | Thin film hydration, sonication, and active loading using a citrate pH-gradient | HCQ | DXR | Melanoma | [43] |
| Liposome (ITGAV-ITGB3/integrin αvβ3 receptor-targeted- soybean PC/Chol/DSPE-PEG2000-Mal) | Thin film hydration, sonication, and active loading using a sulfate pH-gradient | HCQ | PTX | Pancreatic cancer | [44] |
| Liposome (Neuropilin-1/integrin αvβ3 receptor-targeted- soybean PC/Chol/DSPE-PEG2000-Mal) | Thin film hydration, sonication, and active loading using a sulfate pH-gradient | HCQ | PTX | Melanoma | [45] |
| Liposome (Neuropilin-1/integrin αvβ3 receptor-targeted- soybean PC/Chol/DSPE-PEG2000-Mal) | Thin film hydration, sonication, and active loading using a sulfate pH-gradient | HCQ | ZD6473 | Glioma | [46] |
| Liposome (LRP1-targeted-DSPC/DOPC/DSPE-PEG2000-Mal) | Thin film hydration, sonication, and extrusion, followed by active loading using a citrate pH-gradient | HCQ | Chlorin e6 | Glioma | [47] |
| Liposome (Chol-HCQ/PC) | Passive loading by thin film hydration and sonication | HCQ (cholesterol modified) | - | Pulmonary fibrosis | [48] |
| Polymeric micelle (mPEG-PLA) | Thin film hydration | CQ | DXR, PTX, cis-platin | Ovarian cancer | [49] |
| Polymeric NP (PLGA) | Water-in-oil-in-water (w/o/w) double emulsion solvent evaporation method | CQ | pDNA | Colon cancer | [50] |
| Polymeric NP (PLGA) | Water-in-oil-in-water (w/o/w) double emulsion solvent evaporation method | HCQ | OVA | Vaccine | [51] |
| Polymeric NP (CD-20 antibody-targeted- PCL/PLA) | Not described | HCQ | Chlorambucil | [52] | |
| Acrylamide nanogel | Electrostatic complexation | CQ | DXR | Breast cancer | [53] |
| Dendrimer (Bis-MPA/glycine) | Emulsion evaporation method | CQ | - | Malaria | [54] |
| Dendrimer (PEG-PLL/galactose) | Equilibrium dialysis | CQ | - | Malaria | [55] |
| Dendrimer (PEG-PLL/chondroitin A sulfate) | Equilibrium dialysis | CQ | - | Malaria | [56] |
| Dendrimer (PEI/triphenylphosphate) | Precipitation | CQ | DXR | Prostate cancer | [57] |
| Polyelectrolyte complex (Poly(amidoamine)) | Electrostatic interaction | CQ | - | Malaria | [58,59] |
| Polyelectrolyte complex (Chitosan/tripolyphosphate) | Electrostatic interaction | CQ | - | Malaria | [60,61,62,63] |
| SLN (Compritol® proprietary lipid) | Melt homogenization method | CQ | - | Arthritis | [64] |
| Lipid nanoemulsion | Microemulsion method | CQ | - | Malaria | [65] |
| Niosome gel | Emulsion evaporation method | HCQ | - | Oral lichen planus | [66] |
| Gold NP | Conjugated to gold np via HCQ thiol prodrug | HCQ | DXR | Glioma | [67] |
| Titanium dioxide NP | Inclusion complex | HCQ | - | Breast cancer | [68] |
| Polymer prodrug(poly(N-(2-hydroxypropyl) methacrylamide-co-methacryloylated HCQ)) | Polymer ester prodrug | HCQ | - | Inflammatory bowel disease | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, D.M.; Crist, R.M.; Stern, S.T. Nanomedicine Reformulation of Chloroquine and Hydroxychloroquine. Molecules 2021, 26, 175. https://doi.org/10.3390/molecules26010175
Stevens DM, Crist RM, Stern ST. Nanomedicine Reformulation of Chloroquine and Hydroxychloroquine. Molecules. 2021; 26(1):175. https://doi.org/10.3390/molecules26010175
Chicago/Turabian StyleStevens, David M., Rachael M. Crist, and Stephan T. Stern. 2021. "Nanomedicine Reformulation of Chloroquine and Hydroxychloroquine" Molecules 26, no. 1: 175. https://doi.org/10.3390/molecules26010175
APA StyleStevens, D. M., Crist, R. M., & Stern, S. T. (2021). Nanomedicine Reformulation of Chloroquine and Hydroxychloroquine. Molecules, 26(1), 175. https://doi.org/10.3390/molecules26010175






