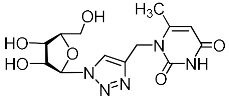
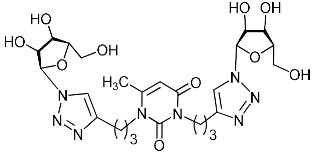
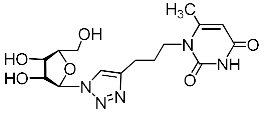
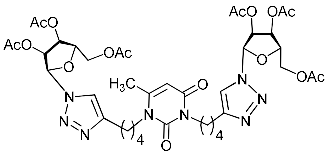
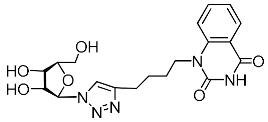
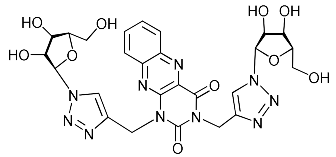
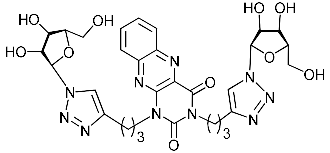
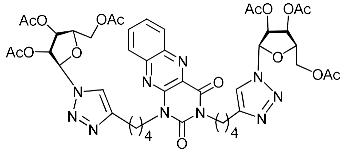
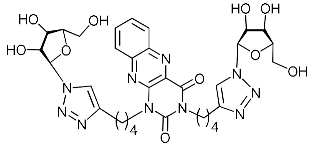
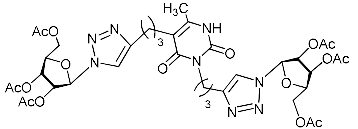
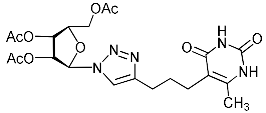
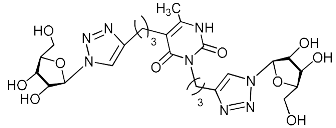
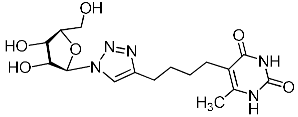
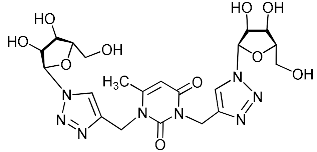
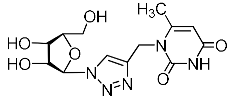
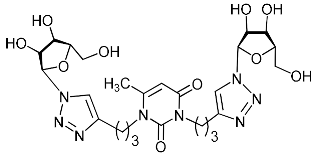
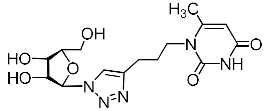
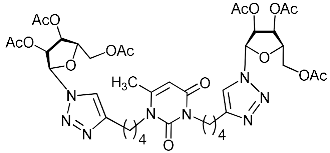
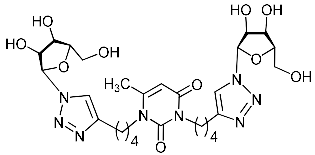
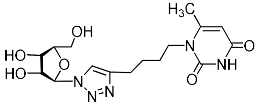
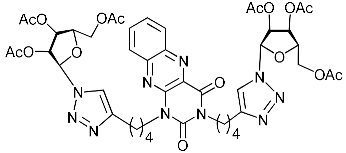
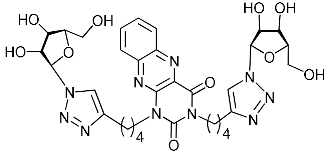
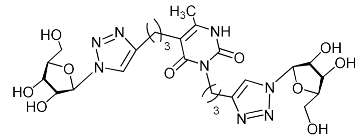
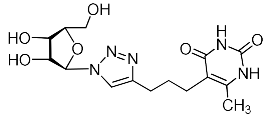
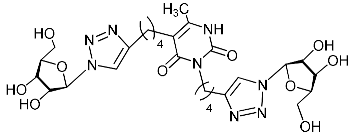
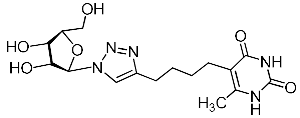
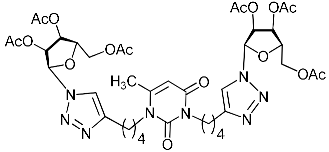
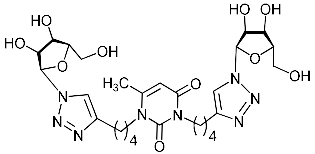
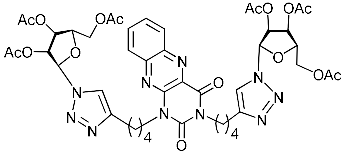
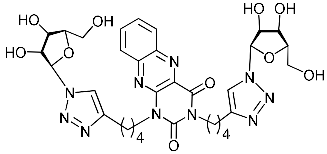
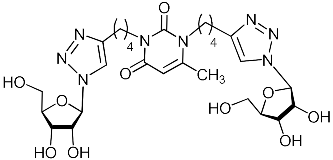
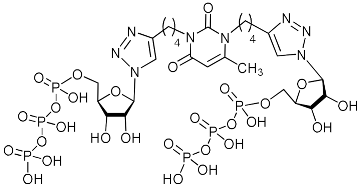
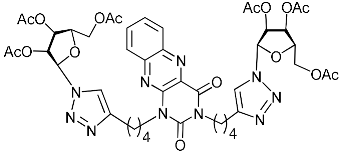
Abstract
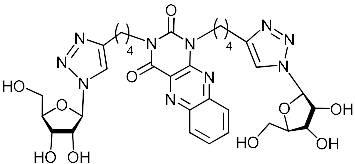
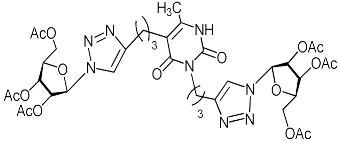
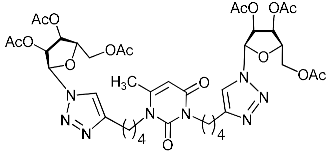
A series of 1,2,3-triazolyl nucleoside analogues in which 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments are attached via polymethylene linkers to both nitrogen atoms of the heterocycle moiety (uracil, 6-methyluracil, thymine, quinazoline-2,4-dione, alloxazine) or to the C-5 and N-3 atoms of the 6-methyluracil moiety was synthesized. All compounds synthesized were evaluated for antiviral activity against influenza virus A/PR/8/34/(H1N1) and coxsackievirus B3. Antiviral assays revealed three compounds, 2i, 5i, 11c, which showed moderate activity against influenza virus A H1N1 with IC50 values of 57.5 µM, 24.3 µM, and 29.2 µM, respectively. In the first two nucleoside analogues, 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments are attached via butylene linkers to N-1 and N-3 atoms of the heterocycle moiety (6-methyluracil and alloxazine, respectively). In nucleoside analogue 11c, two 1,2,3-triazol-4-yl-2′,3′,5′-tri-O-acetyl-β-d-ribofuranose fragments are attached via propylene linkers to the C-5 and N-3 atoms of the 6-methyluracil moiety. Almost all synthesized 1,2,3-triazolyl nucleoside analogues showed no antiviral activity against the coxsackie B3 virus. Two exceptions are 1,2,3-triazolyl nucleoside analogs 2f and 5f, in which 1,2,3-triazol-4-yl-2′,3′,5′-tri-O-acetyl-β-d-ribofuranose fragments are attached to the C-5 and N-3 atoms of the heterocycle moiety (6-methyluracil and alloxazine respectively). These compounds exhibited high antiviral potency against the coxsackie B3 virus with IC50 values of 12.4 and 11.3 µM, respectively, although both were inactive against influenza virus A H1N1. According to theoretical calculations, the antiviral activity of the 1,2,3-triazolyl nucleoside analogues 2i, 5i, and 11c against the H1N1 (A/PR/8/34) influenza virus can be explained by their influence on the functioning of the polymerase acidic protein (PA) of RNA-dependent RNA polymerase (RdRp). As to the antiviral activity of nucleoside analogs 2f and 5f against coxsackievirus B3, it can be explained by their interaction with the coat proteins VP1 and VP2.
1. Introduction
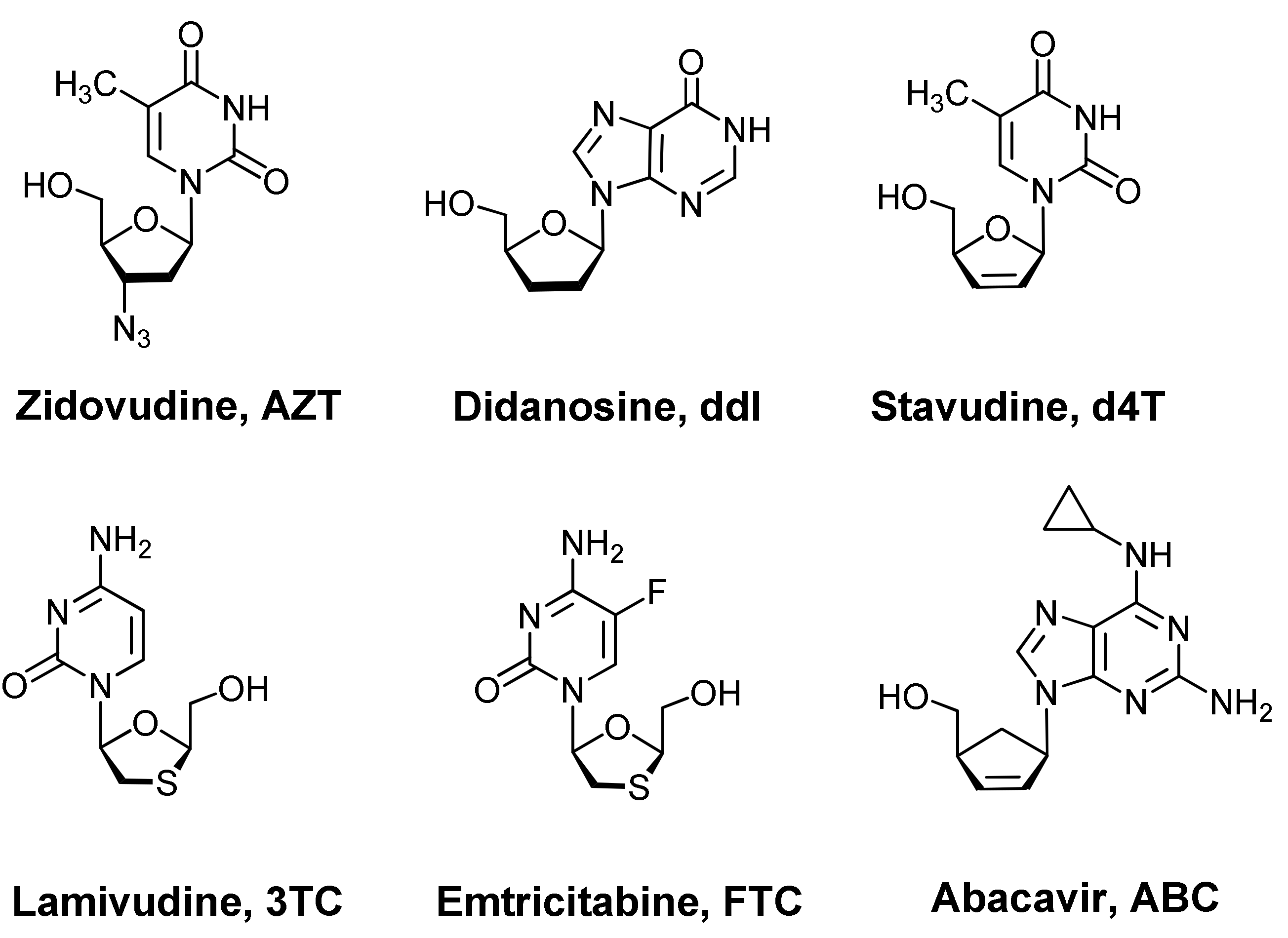
Nucleoside analogues represent an efficient scaffold for the development of antiviral drugs. For example, the first six antivirals approved by the FDA (Silver Spring, MD, USA) for the treatment of HIV infection, namely zidovudine, didanosine, stavudine, lamivudine, emtricitabine, abacavir, are synthetic analogues of thymidine, cytidine, adenosine, and guanosine [1]. In general, the following methods are known for obtaining analogues of natural nucleosides: (a) functionalizing a nucleic base with various substituents [2]; (b) the introduction or removal of nitrogen atoms in the nucleic base [3]; (c) annulation to the nucleic base with another heterocycle [2]; (d) replacing the nucleic base with another nitrogen-containing heterocycle [4]; (e) functionalization of the d-ribofuranosyl residue with various substituents [5,6]; (f) removing one or both hydroxyl groups from the C-2′ and C-3′ atoms of the d-ribofuranosyl residue [1,2,6]; (g) removing both hydroxyl groups and hydrogen atoms from the C-2′ and C-3′ atoms of the d-ribofuranosyl residue [1,2]; (h) introducing additional sulfur or oxygen atoms into a d-ribofuranosyl residue [7,8]; (i) replacement of the d-ribofuranosyl residue with a cyclopentane or cyclopentene ring [1,2]; (j) phosphorylation of the hydroxyl group at the C-5′ atom of the d-ribofuranose residue, that is, the transition to nucleotides [9]; (k) replacement of a C-N glycosidic bond with a C-C bond [1,2]; synthesis of l-nucleoside analogues [1,2,7]. However, as a rule, in the synthesis of new nucleoside analogues several of the above methods are used simultaneously. So, for example, didanosine (Figure 1), unlike guanosine, has neither an amino group at the C-2 atom nor hydroxyl groups at the C-2′ and C-3′ atoms [1,2]. Abacavir (Figure 1), in comparison with adenosine, underwent both a modification of the adenine moiety (an amino group appeared at the C-2 position and a cyclopropane ring was attached to the amino group at the C-6 atom) and the sugar residue (the d-ribofuranosyl moiety was replaced by a cyclopentene ring) [1,2].
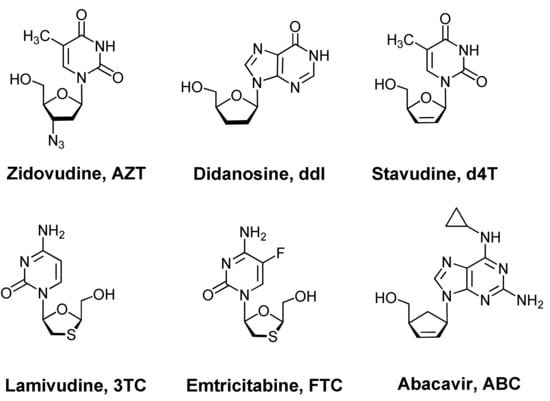
Figure 1.
Example of nucleoside analogues—antiretroviral drugs.
The emergence of click-chemistry reactions attracted the attention of medical chemists to 1,2,3-triazole as a bridge group, which easily could connect two pharmacophores to form a new biologically active molecule [10]. In addition, 1,2,3-triazole itself is a pharmacophore and is present in many drugs [11]. In recent years, nucleoside analogues with substituted 1,2,3-triazole rings in the nucleic base [3,12,13,14] in the d-ribofuranosyl residue [5,15,16] as well as nucleoside analogues in which the nucleic base was coupled to the d-ribofuranosyl residue through a 1,2,3-triazole bridge have been synthesized [17,18,19].
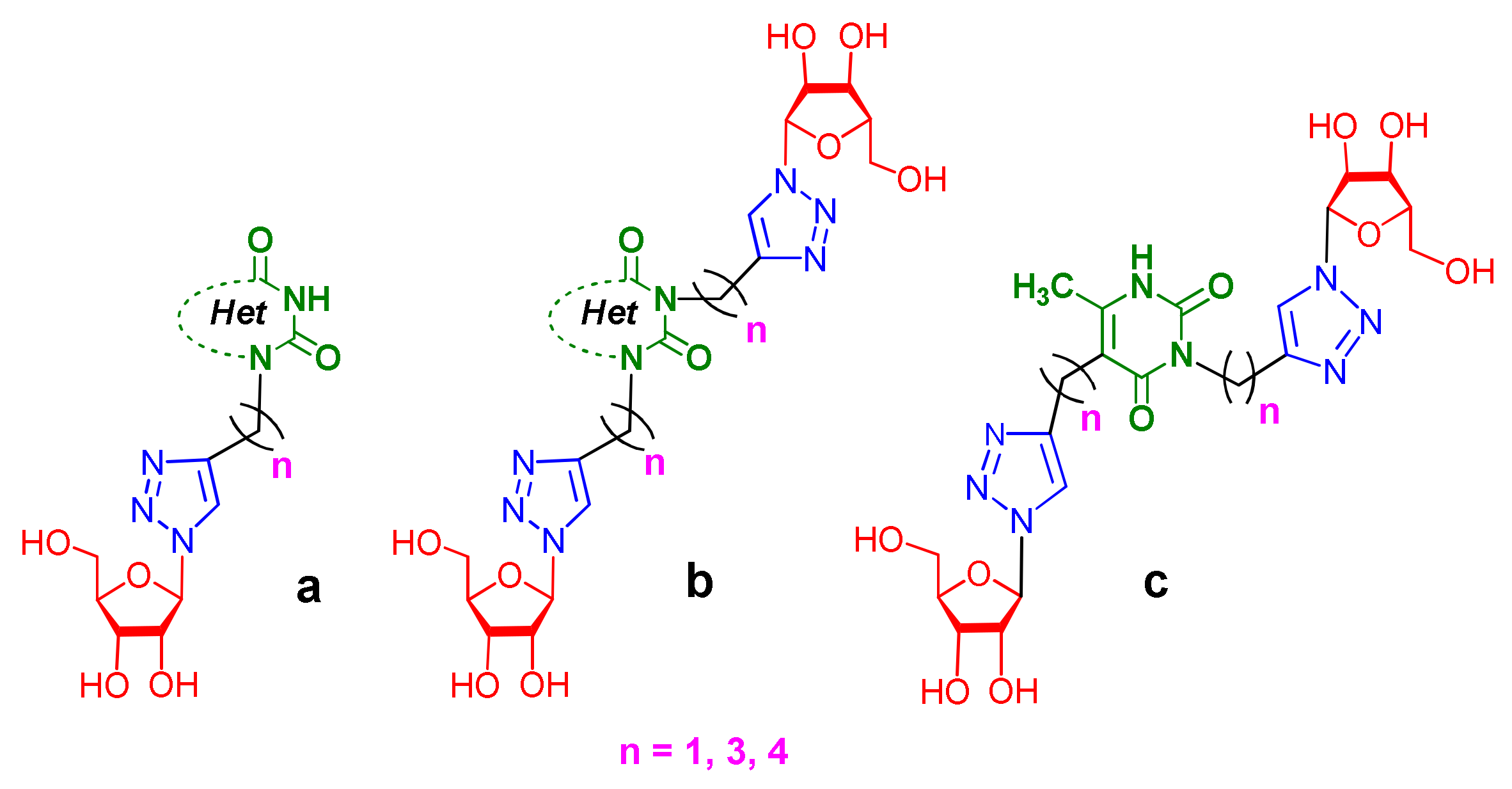
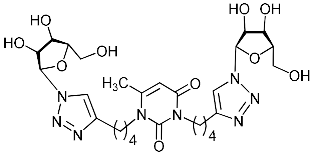
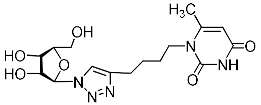
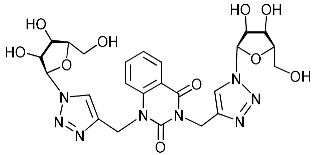
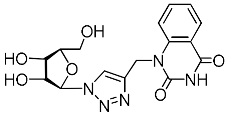
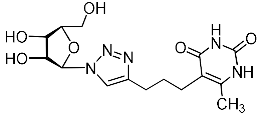
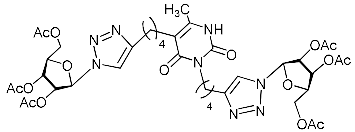
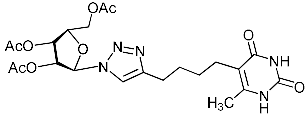
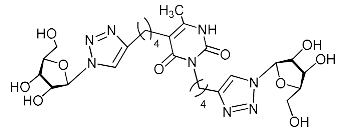
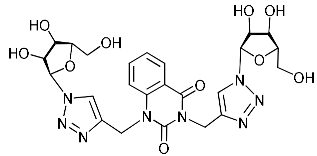
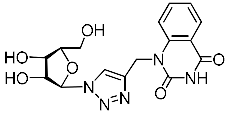
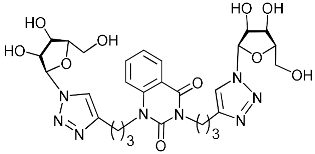
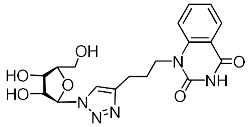
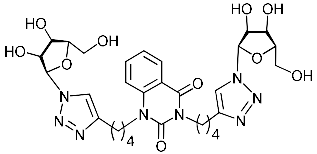
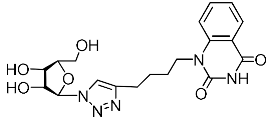
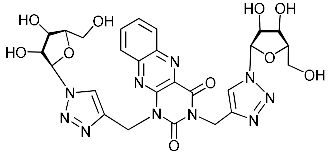
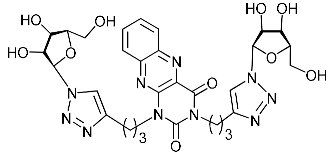
Recently, we synthesized nucleoside analogues in which a 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment was attached to the pyrimidine moiety (uracil, 6-methyluracil, thymine, quinazoline-2,4-dione) through a polymethylene linker of variable length [20,21] (Figure 2). Biological testing has revealed several compounds showing moderate (IC50 = 15, 30, 42–48 µM) antiviral activity against the influenza A (H1N1) virus. Herein, we report on the synthesis and antiviral evaluation of novel nucleoside analogues in which a 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment is attached via a polymethylene linker of variable length to both nitrogen atoms of the pyrimidine moiety (uracil, 6-methyluracil, thymine, quinazoline-2,4-dione, alloxazine) or to the C-5 and N-3 atoms of the 6-methyluracil moiety (Figure 2).
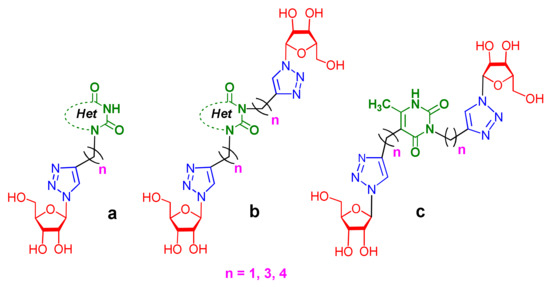
Figure 2.
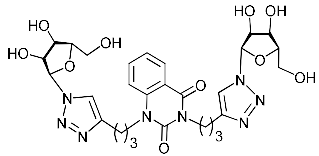
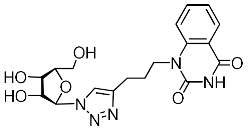
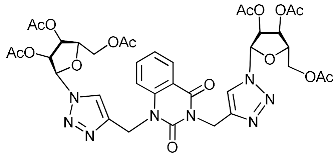
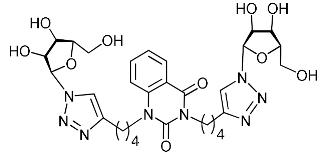
Nucleoside analogues being investigated by our group: (a) already obtained and evaluated compounds [20,21]; (b,c) the target compounds of this study. Het = uracil, 6-methyluracil, thymine, quinazoline-2,4-dione, alloxazine.
2. Results and Discussion
2.1. Chemistry
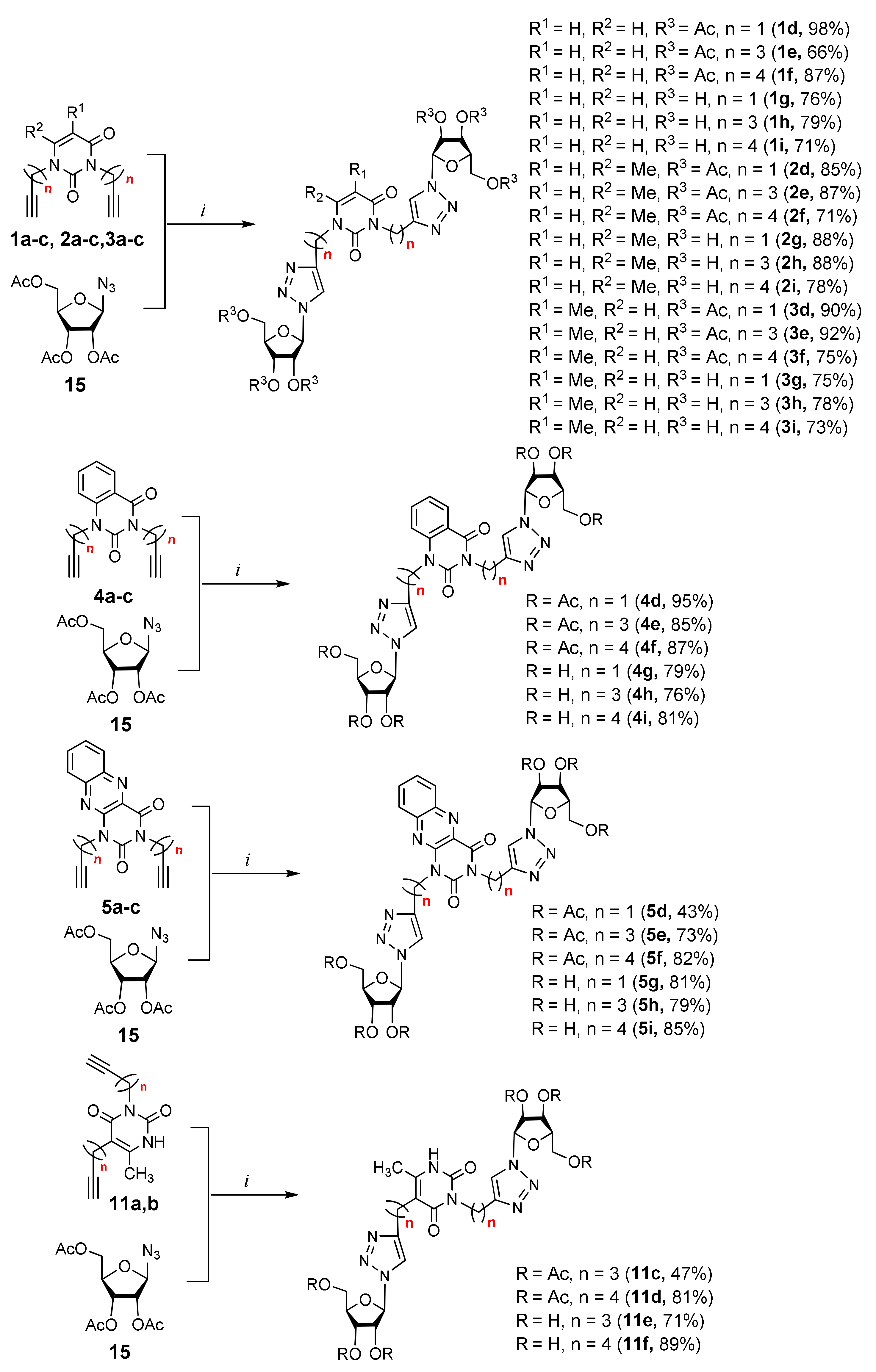
The strategy for the synthesis of the target nucleoside analogues (Figure 2) was based upon the copper-catalyzed alkyne–azide cycloaddition (CuAAC) reaction and was carried out according to a convergent scheme consisting of pyrimidine and carbohydrate routes completed with the formation of alkyne precursors related to alkynyl substituted pyrimidines and an azide precursor related to β-d-ribofuranose, respectively. At the final stage, the precursors were coupled by a CuAAC reaction.
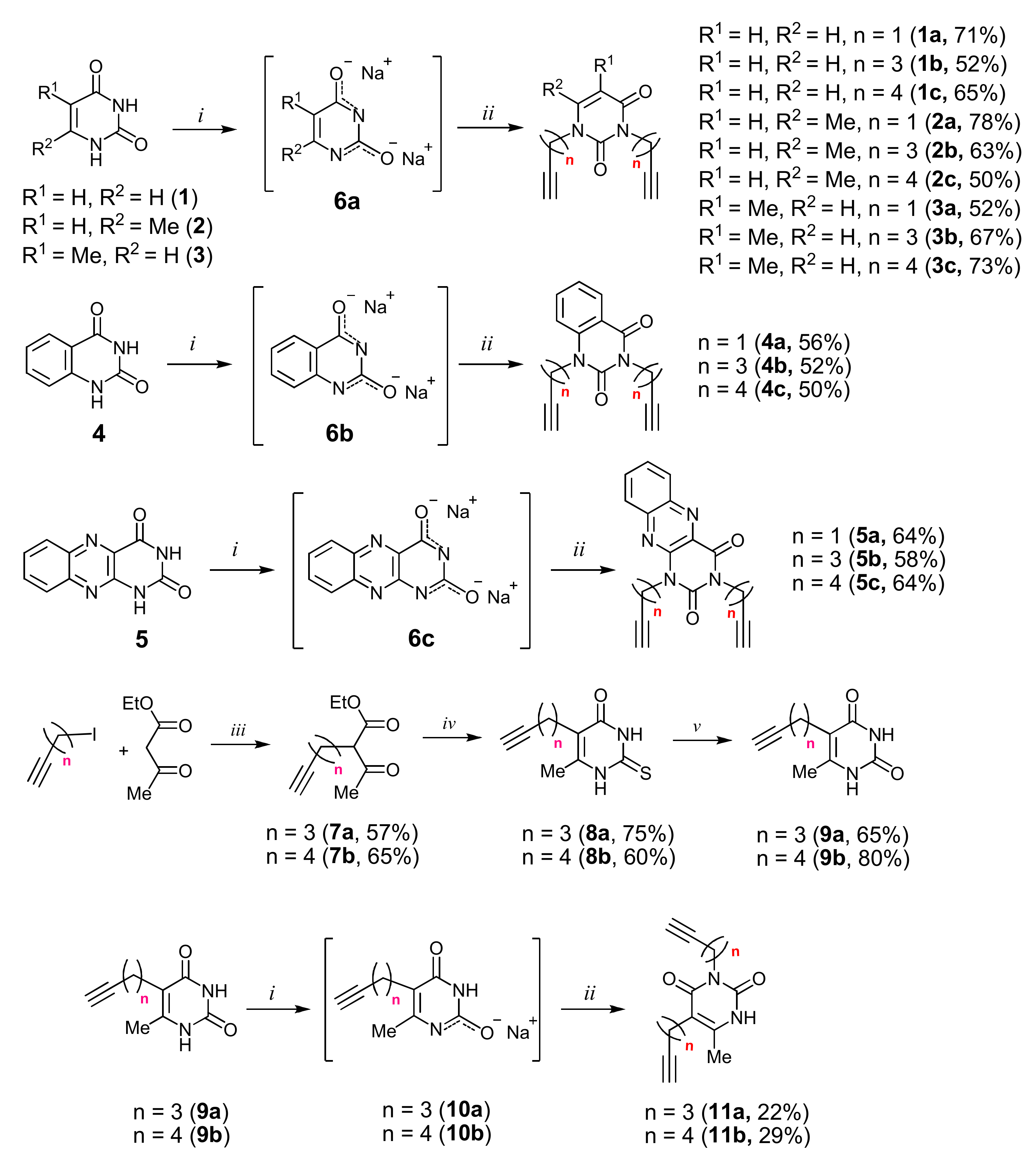
Alkyne precursors related to alkynyl-substituted pyrimidines were prepared according to the synthetic routes summarized in Scheme 1. Uracil 1, 6-methyluracil 2, thymine 3, quinazoline-2,4-dione 4, and alloxazine 5 were converted with the excess of sodium hydride into corresponding disodium salts 6, which were alkylated with propargyl bromide, 5-iodo-pent-1-yne, and 6-iodo-hex-1-yne without any purification to afford 1,3-bis(α,ω-alkynyl)derivatives of uracil 1a–c, 6-methyluracil 2a,b,c, thymine 3a,b,c, quinazoline-2,4-dione 4a,b,c, and alloxazine 5a,b,c. Subsequently, 3,5-bis(α,ω-alkynyl)-6-methyluracils 11a,b were prepared in two steps. At the first stage, acetoacetic ester was reacted with 5-iodo-1-pentyne or 6-iodo-1-hexyne to afford 3-(α,ω-alkyne)acetoacetic esters 7, condensation of which with thiourea provided 2-thio-5-(α,ω-alkynyl)-6-methyluracils 8. The hydrolysis of thiopyrimidines 8 with an aqueous solution of chloroacetic acid led to 5-alkynyl-6-methyluracils 9a,b in 65–80% yields. At the second stage, pyrimidines 9 were converted to monosodium salts 10, which were alkylated in situ with 5-iodo-1-pentyne and 6-iodo-1-hexyne to give 3,5-bis(α,ω-alkynyl)-6-methyluracils 11a,b with 22–29% yields (Scheme 1).
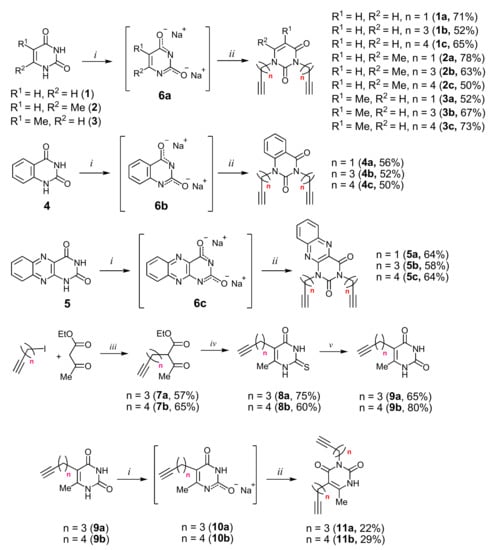
Scheme 1.
Synthesis of 1,3- and 3,5-bis(α,ω-alkinyl)pyrimidines. Reagents and conditions: (i) NaH, DMF, 67–70 °C, 8 h; (ii) HC≡C-(CH2)n-Hlg, DMF, 115–120 °C, 16 h; (iii) NaOEt, EtOH, 0–78 °C, 10 h; (iv) thiourea, K2CO3, MeOH, reflux, 15 h; (v) ClCH2COOH, H2O, reflux, 20 h.
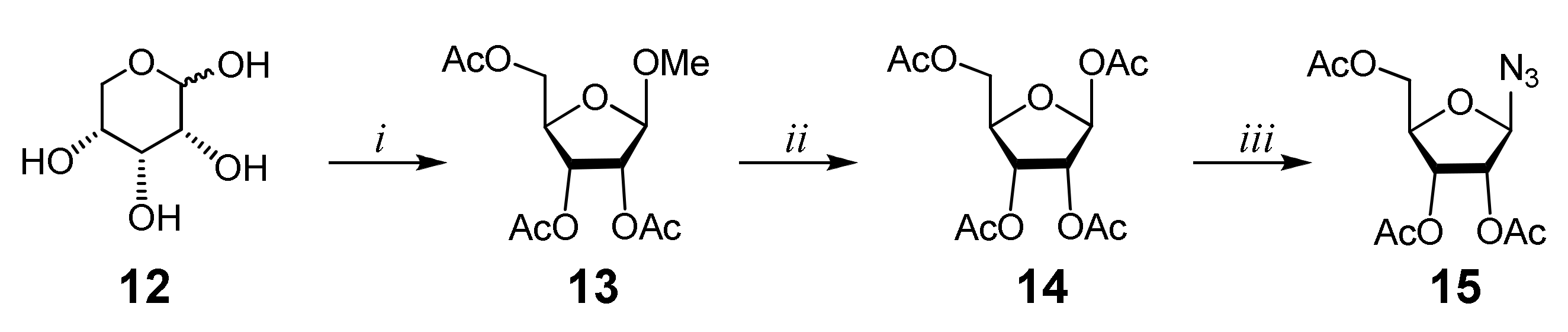
Afterward, 2,3,5-tri-O-acetyl-β-d-ribofuranosylazide 15 (Scheme 2), whose preparation has recently been reported by our group [20,21], was used as the azide precursor of a CuAAC reaction. Commercially available d-ribose 12 was reacted with MeOH to afford methyl d-α/β-ribofuranoside, which was immediately acetylated to obtain a mixture of α- and β-anomers of methyl-2,3,5-tri-O-acetyl-d-ribofuranoside 13, which were separated through silica gel flash column chromatography. The methoxy group of the β-anomer of monosaccharide 13 was then replaced by an acetoxy group by the reaction with a mixture of glacial acetic acid and Ac2O in the presence of H2SO4. The obtained 1,2,3,5-tetra-O-acetyl-β-d-ribofuranoside 14 was treated with trimethylsilyl azide (TMSN3) in the presence of tin tetrachloride to provide the azide precursor 15 in 95% yield (Scheme 2).
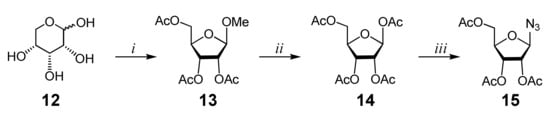
Scheme 2.
Synthesis of azido 2,3,5-tri-O-acetyl-β-d-ribofuranoside. Reagents and conditions: (i) a. MeOH, H2SO4, r.t., 6 h, Na2CO3; b. Py, Ac2O, r.t., 24 h (42%); (ii) Ac2O, AcOH, 5% H2SO4, 0 °C, 3 h, NaHCO3 (43%); (iii) TMSN3, SnCl4, r.t., 24 h (95%).
Finally, a CuAAC reaction was utilized to complete the convergent scheme and couple alkyne precursors 1a–c, 2a–c, 3a–c, 4a–c, 5a–c, 11a,b with azide precursor 15 to afford nucleoside analogues 1d–f, 2d–f, 3d–f, 4d–f, 5d–f, 11c,d bearing one pyrimidine moiety and two 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments with protected hydroxyl groups (Scheme 3). Removal of the acetyl protecting groups in a methanolic sodium methoxide solution provided the target nucleoside analogues 1g–i, 2g–i, 3g–i, 4g–i, 5g–i, 11e,f having sugar free hydroxyls (Scheme 3).
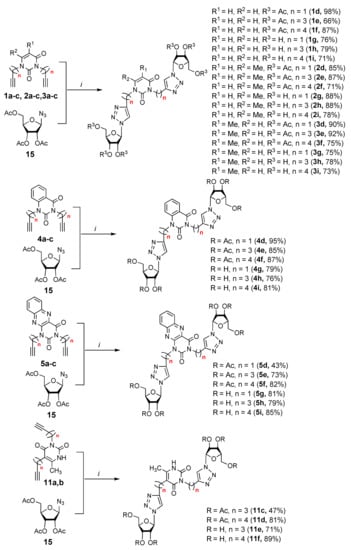
Scheme 3.
Synthesis of the target nucleoside analogues. Reagents and conditions: (i) a. tert-BuOH/H2O, CuSO4·5H2O, Na l-ascorbate, 40 °C, 48 h; b. MeOH, MeONa, Amberlist®15H+, r.t.
Procedures for the synthesis of the compounds, characterization of the compounds synthesized are detalized in Supplementary Materials. NMR spectra of the compounds are also shown there, Figures S1–S96.
2.2. Antiviral Evaluation
Several synthesized 1,2,3-triazolyl nucleoside analogues were evaluated for their in vitro antiviral activity against the A/Puerto Rico/8/34 (H1N1) influenza virus (for MDCK cells) and coxsackievirus B3 (for Vero cells). The resulting data expressed as virus-inhibiting activity (IC50), cytotoxicity (CC50), as well as selectivity index (SI), which is the ratio CC50/IC50, are presented in Table 1 and Table 2. The data of Table 1 demonstrate that the antiviral activity against influenza virus A H1N1 depends more on the length of the linker connecting the heterocyclic moiety to the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments than on the nature of the heterocycle. Both compounds 2i and 5i, in which the heterocyclic moiety is coupled to the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments through butylene linkers, exhibited moderate activity (IC50 values 57.5 µM and 24.3 µM, respectively) in contrast to compounds 2g, 4g, 5g and 2h, 4h, 5h possessing methylene or propylene linkers, respectively, which were found to be inactive (IC50 > 100 µM). Thus, both in the series of compounds 2g, 2h, 2f, 2i possessing 6-methyluracil as the heterocyclic moiety and in the series of compounds 5g, 5h, 5f, 5i possessing alloxazine as the heterocyclic moiety with an increase in the length of polymethylene linkers, the antiviral activity increased by 9 and 7 times, respectively. The dependence of the antiviral activity of compounds 2g, 2h, 2f, 2i and 5g, 5h, 5f, 5i on the nature of the heterocyclic moiety was less pronounced, but present. The antiviral activity of the lead compound 5i possessing the alloxazine moiety coupled to the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments via butylene linkers (IC50 = 24.3 μM, SI = 17) exceeded the antiviral activity of compound 2i, which possessed the 6-methyluracil moiety coupled to the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments also via butylene linkers (IC50 = 57.5 µM, SI = 8) twice. That is, the increase in the size and the lipophilicity of the heterocyclic moiety of 1,2,3-triazole nucleoside analogues upon passing from compound 2i to compound 5i was accompanied by an increase in antiviral activity. However, this pattern was violated by compound 4i, which has the quinazoline-2,4-dione moiety coupled to the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments also via butylene linkers.

Table 1.
Antiviral activity against A/Puerto Rico/8/34 (H1N1) influenza virus and cytotoxicity of 1,2,3-triazolyl nucleoside analogues synthesized in this study and earlier ones published.

Table 2.
Antiviral activity against the Coxsackie B3 virus and cytotoxicity of synthesized 1,2,3-triazolyl nucleoside analogues.
Since the size and the lipophilicity of quinazoline-2,4-dione is average between the sizes and the lipophilicities of 6-methyluracil and alloxazin, it could be assumed that compound 4i has to show the antiviral activity in the range between the activities of compound 2i possessing the 6-methyluracil moiety and compound 5i possessing the alloxazine moiety. However, compound 4i was found to be inactive against influenza virus A H1N1 (IC50 = 297 µM). At the same time, it should be noted that the analogue of compound 4i with only one 1,2,3-triazolylribofuranosyl fragment attached to the N-1 atom of the quinazoline-2,4-dione moiety, namely, compound 4m, showed moderate antiviral activity against the H1N1 influenza virus (IC50 = 30 µM, Table 1, [22]). This fact indicates the significant dependence of the antiviral activity of 1,2,3-triazole nucleoside analogues against the influenza A H1N1 virus on the attachment of the second 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment to the heterocyclic moiety via a polymethylene linker. Indeed, the addition of the second 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment to the 6-methyluracil moiety of nucleoside analogue 2k with one 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment, showed moderate antiviral activity (IC50 = 43 µM [22]), that is, the transition from compound 2k to compound 2h led to a complete loss of activity (IC50 > 492.9 µM, Table 1). A similar loss of activity occurred in the result of the addition of the second 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment to the nucleoside analogues 4j, 4m, 11h, which showed moderate antiviral activity (the values of IC50 are 42, 30, and 15 μM, respectively, Table 1). The IC50 values of their derivatives 4g, 4i, 11e possessing two 1,2,3-triazolylribofuranosyl fragments are 180.1, 297.3, and 164.3 μM, respectively (Table 1). However, the addition of the second 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragment to the heterocyclic moiety of nucleoside analogues 2j, 2m, 4k, 11i, 11j, that is, the transition to disubstituted nucleoside analogues 2g, 2i, 4h, 11d, 11f, did not cause changes in the antiviral activity (Table 1). Disubstituted nucleoside analogues 2g, 4h, 11d, 11f did not demonstrate antiviral activity against the influenza A H1N1 virus just like monosubstituted nucleoside analogues 2j, 4k, 11i, 11j, and disubstituted nucleoside analogue 2i showed approximately the same moderate activity (IC50 = 57.5 μM) as its monosubstituted precursor 2m (IC50 = 48 μM, Table 1).
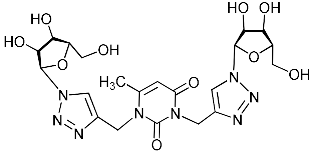
Unexpectedly, antiviral activity against the influenza A H1N1 virus (IC50 = 29.2 µM, CC50 > 349 µM, SI = 12) was demonstrated by nucleoside analogue 11c with protected (acylated) hydroxyl groups of the sugar residue (Table 1). Moreover, its derivative 11e possessing free hydroxyl groups turned out to be completely inactive (Table 1). It should be noted that other nucleoside analogues with protected hydroxyl groups 2f, 4f, 5f, 11d evaluated in this study were also inactive against the influenza A H1N1 virus. So, for reasons so far unclear, compound 11c was the only one among the investigated nucleoside analogues with two 1,2,3-triazolyl-2′,3′,5′-tri-O-acetyl-ribofuranosyl fragments that showed the same antiviral activity against the influenza A H1N1virus as compound 5i with two 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments with free hydroxyl groups. A similar case was described earlier [22] when we found that the analogue of compound 11c with one 1,2,3-triazolyl-2′,3′,5′-tri-O-acetyl-β-d-ribofuranosyl fragment attached via a propylene linker to the C-5 atom of the 6-methyluracil moiety (compound 11g in Table 1) was able to inhibit in vitro replication of the influenza A H1N1 virus and the coxsackie B3 virus with IC50 values of 67 and 9 µM, respectively.
The results of an in vitro study of cytotoxic and antiviral properties of several synthesized compounds against the coxsackie B3 virus (for Vero cells) are presented in Table 2. It can be seen that almost all the compounds studied were inactive against this virus. Two exceptions are 1,2,3-triazolyl nucleoside analogues 2f and 5f, in which the heterocyclic moiety (6-methyluracil in the case of 2f and alloxazine in the case of 5f) is coupled to 1,2,3-triazol-4-yl-2′,3′,5′-tri-O-acetyl-β-d-ribofuranosyl fragments via butylene linkers. These compounds exhibited high antiviral potency against the coxsackie B3 virus, with IC50 values of 12.4 and 11.3 µM, respectively (Table 2), although both were inactive against influenza virus A H1N1 (Table 1). Moreover, these 1,2,3-triazolyl nucleoside analogues showed very high cytotoxicity (CC50 = 18 µM) against Vero cells that were used as a cell host for the growth of the coxsackie B3 virus, that is, infected cells were dying almost immediately as soon as the inhibition of virus replication began (Table 2). Interestingly, these compounds possessing protected hydroxyl groups of β-d-ribofuranosyl residues as well as their derivatives 2i and 5i with free hydroxyl groups appeared to be not cytotoxic against human cancer cell lines M-HeLa, HuTu-80, PC-3, normal cell line WI-38, and the MDCK cell line, which was used as a cell host to grow the influenza A H1N1 virus (Table 1 and Table 3). By the way, analogues of nucleosides 2i and 5i with free hydroxyl groups of ribofuranosyl residues, in contrast to their precursors 2f and 5f, were not cytotoxic also against the Vero cell line (Table 2).

Table 3.
Cytotoxic activity of several 1,2,3-triazolyl nucleoside analogues against human cancer and normal human cell lines (IC50 values in µM with standard errors) 1.
2.3. Molecular Docking
2.3.1. Influenza Virus A H1N1
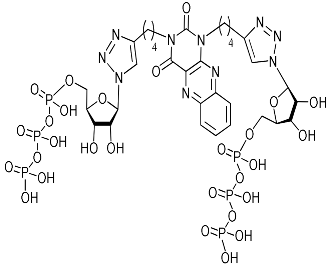
Of all drug targets of influenza virus A H1N1, like all other RNA viruses, including SARS-CoV-2, RNA-dependent RNA polymerase (RdRp) is the most promising drug target since it plays a critical role in RNA viruses replication [24,25]. The RdRp consists of three separate polypeptides called polymerase basic 2 (PB2), polymerase basic 1 (PB1), and polymerase acidic (PA) [26]. It has been shown that the antiviral activity of nucleoside analogues is due to interactions with the fragment of PA containing the N-terminal endonuclease domain (PA-Nter) of RdRp [24,27]. Therefore, we decided to test the ability of the lead compounds 2i, 5i, 11c to bind to RdRp of influenza virus A H1N1 in molecular docking simulations described in Supplementary Materials [28,29,30,31,32,33]. As a potential target we chose the ligand-binding domain (LBD) of acidic polymerase (PA) of RdRp. The PA-Nter domain has a cation-dependent endonuclease active-site core, the catalytic residues His41, Glu80, Asp108, and Glu119 being conserved among all influenza A subtypes and strains [29,34]. The literature has revealed that cellular kinases and virally encoded kinases metabolize nucleoside analogues to their 5-phosphorylated derivatives [35,36]. At first, nucleoside kinases encoded by the host cell or the virus infecting the host cell catalyze phosphorylation of a nucleoside analogue to its 5-monophosphate form. Subsequently, the transformation of 5-monophosphates to the corresponding 5-diphosphates and triphosphates is carried out by nucleoside, nucleotidyl, and nucleoside diphosphate kinases, respectively [35,36,37]. Accordingly, 5-triposphate (TP) derivatives of the lead compounds 2i-TP and 5i-TP were also taken into consideration.
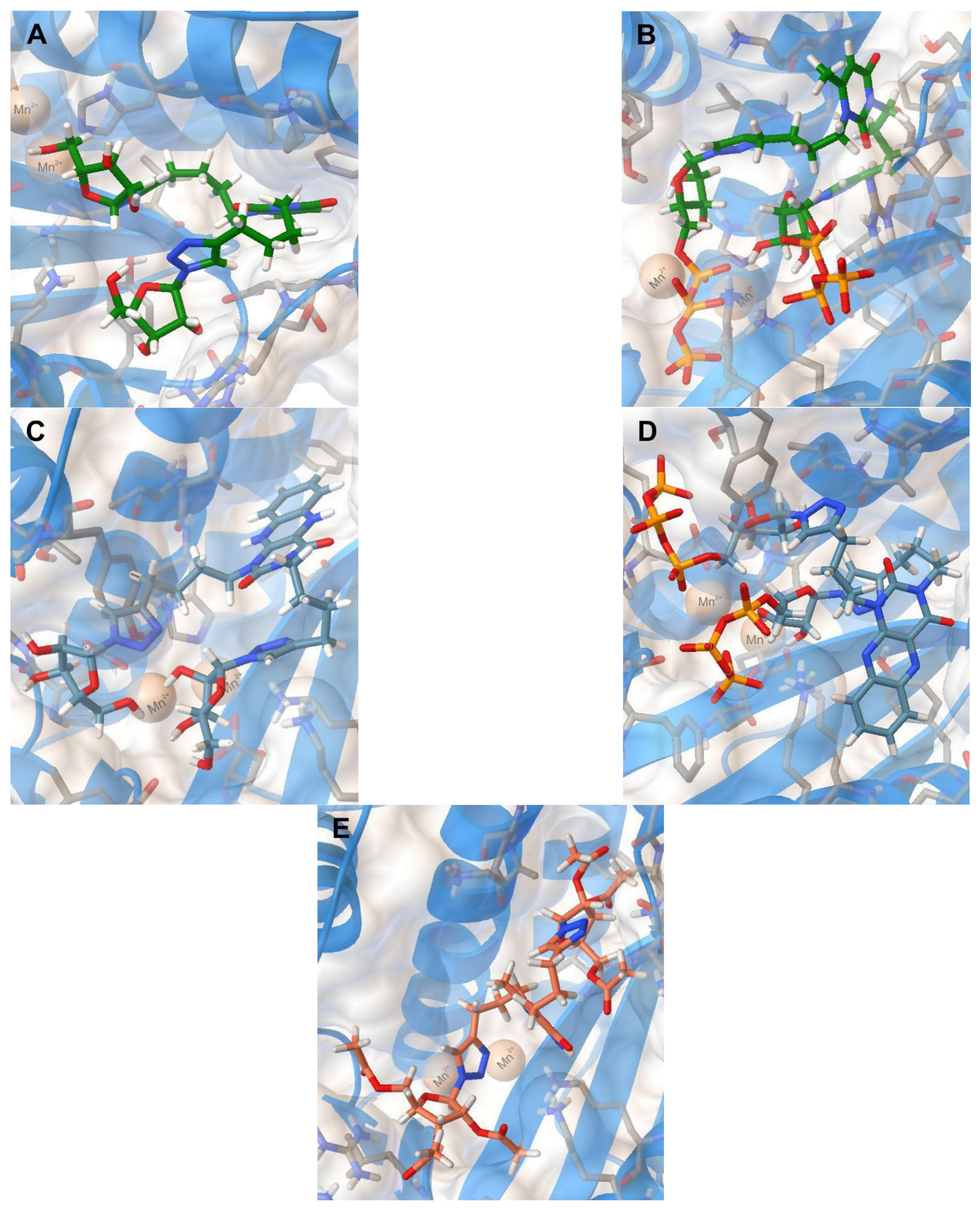
Thus, we have evaluated the binding of 2i, 2i-TP, 5i, 5i-TP, and 11c to the N-terminal domain of the RdRp acidic subunit of influenza virus A/Puerto Rico/8/1934/H1N1 [29] (PDB code 5I13). The positions of the optimized docking models of compounds 2i, 2i-TP, 5i, 5i-TP, and 11c exhibiting the best binding energies are shown in Figure 3. According to the docking simulations, all studied molecules are located deep in the cavity of the active site of PA N-ter, being approximately in one area of space, but the conformations of compounds 2i, 2i-TP, 5i, 5i-TP differ significantly from the conformation of compound 11c. The conformations of the first four compounds are folded with the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments oriented in one direction while the conformation of 11c is unfolded with the 1,2,3-triazol-4-yl-β-d-ribofuranosyl fragments oriented orthogonally to the plane of the 6-methyluracil ring in different directions (Figure 3). Nevertheless, 11c has interactions with the same protein residues Lys134, Lys137, Arg196, Leu106 as compounds 2i, 2i-TP, 5i, 5i-TP (Table 4).
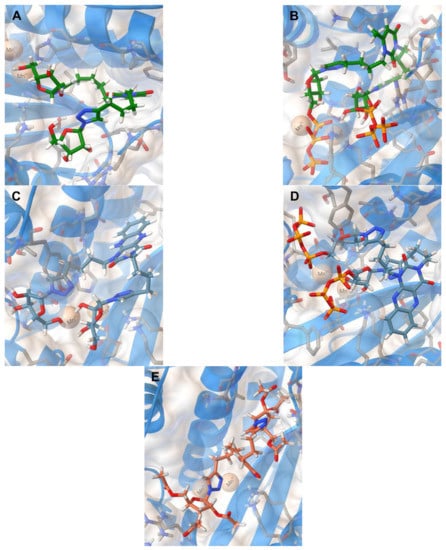
Figure 3.
Molecular docking simulations of the optimized docking model of compounds 2i (A), 2i-TP (B), 5i (C), 5i-TP (D), 11c (E) in the PA-Nter (PDB code 5I13) active site obtained in the lowest-energy conformations.

Table 4.
Inhibitory activity against influenza virus A H1N1 of the lead compounds as well as their binding energies and ligand–protein interactions obtained by molecular docking simulations.
The main structural fragments of compounds 2i, 5i, and 11c that ensure their interaction with the PA-N-ter active center are ribofuranosyl residues and 1,2,3-triazole rings. The ribofuranosyl residues of 2i are linked to the amino groups of the side chains of Lys134 and Lys137 protein residues via hydrogen bonds and the 1,2,3-triazole ring of this compound has H-bonding with the Val122 residue. In the case of compound 5i, one of the ribofuranosyl moieties has interaction with the Leu106 residue, the other one with the Gly81 residue. The 1,2,3-triazole ring of 5i has H-bonding with the Tyr24 residue and, in addition, is engaged in the π-cationic interaction with one of the two Mn2+ cations located in the active site of the PA protein. The other 1,2,3-triazole ring of 5i and the protonated amino group of the side chain of Lys134 residue interact via π-cationic interaction. Compound 11c is retained in the PA-Nter cavity via the π-cationic interaction of the 1,2,3-triazole ring with Mn2+ cation and H-bonding of another 1,2,3-triazole ring with the Arg196 protein residue. The H-bonding of the amino acid residues Arg84, Leu106, Lys137 with the carbonyl oxygen atoms of the acetate protecting groups of the ribofuranosyl moiety and H-bonding of the terminal amino group of the Lys134 protein residue with the carbonyl group of the uracil moiety is a feature of the binding of compound 11c in the active site of PA-Nter. The binding of 2i-TP and 5i-TP in the PA-Nter active site is due to the interaction of their phosphate groups with the Lys134, Lys137, Gly81, Arg84 protein residues and the H-bonding of the ribofuranosyl moiety with the Val122 and Tyr23 protein residues. Moreover, one 1,2,3-triazole ring of 2i-TP is engaged in the π-cationic interaction with the protonated terminal amino group of the Lys34 residue and the 1,2,3-triazole ring of 5i-TP has the π-π stacking with the Tyr24 protein residue.
According to the molecular docking calculation, 5i and 5i-TP have the highest binding energies to the PA-Nter active site among the compounds studied (−9.3 and −9.1 kkal/mol, respectively). The binding energies of 2i and 11c are worse (−8.7 and −8.1 kkal/mol, respectively). Thus, the results of the molecular docking calculations approximately correspond to some decrease in antiviral activity against influenza virus A H1N1 in the series of compounds studied: IC50 (5i) < IC50 (11c) < IC50 (2i). Therefore, one can conclude that antiviral activity of the 1,2,3-triazolyl nucleoside analogues 2i, 5i, 11c against the H1N1 (A/PR/8/34) influenza virus can be explained by their influence on the functioning of the polymerase acidic protein of RNA-dependent RNA polymerase.
2.3.2. Coxsackievirus B3
Coxsackievirus B3 belongs to the Enteroviruses genus of the Picornaviridae family [38]. All picornaviruses have a similar structure and are small icosahedral viruses with a capsid size of about 27–30 nm. These viral particles consist of 60 copies of a complex of four different viral proteins VP1, VP2, VP3, and VP4 called coxsackievirus B3 coat proteins [39]. The surface of the capsid has a pronounced “canyon” that plays a key role in the binding of the virus to cell receptors and recognition by neutralizing antibodies [40]. Inside this “canyon” is a small endogenous lipid called the “pocket factor” [41]. Inhibitors of enterovirus infection (e.g., pleconaril) displace the “pocket factor” from the “canyon” and stabilize the viral capsid, thereby inhibiting uncoating of the virus and the release of the genome into the infected cell [42].
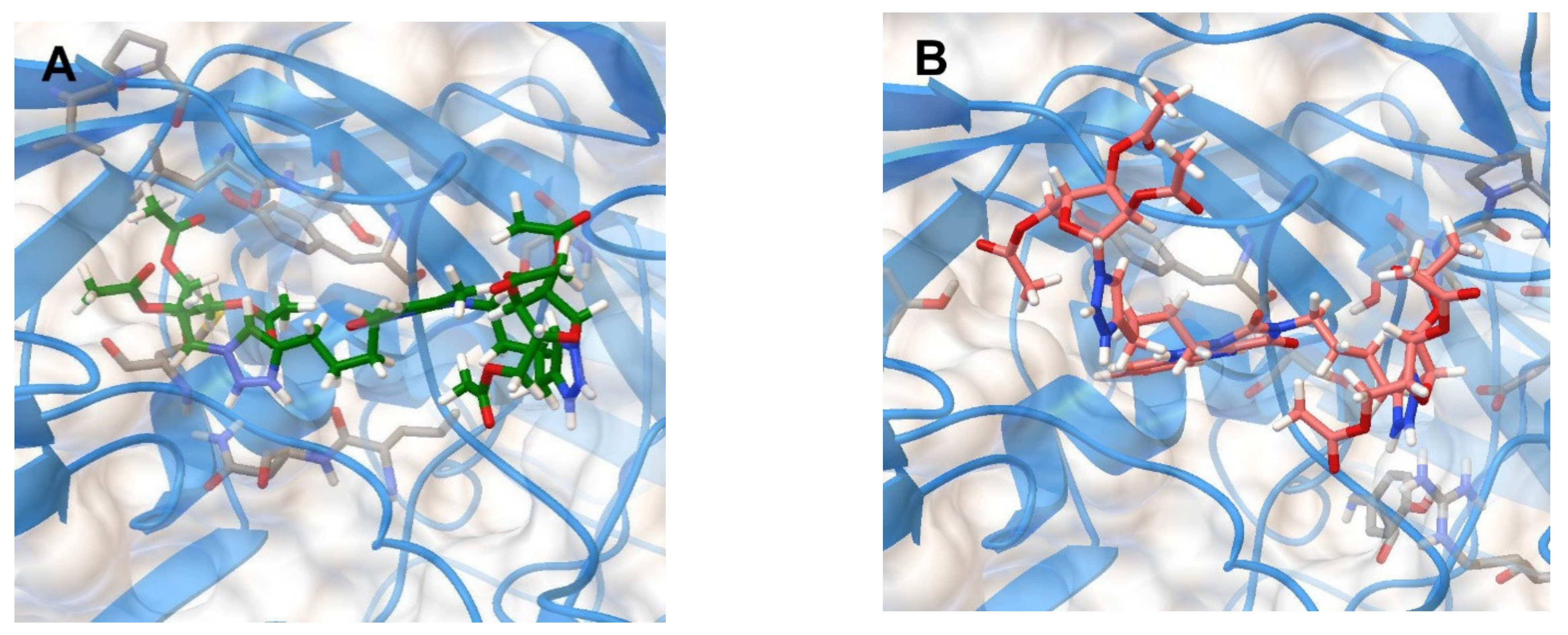
Therefore, we decided to test the ability of the lead compounds 2f and 5f to bind to the “canyon” of the coxsackievirus B3 capsid in molecular docking simulations. The binding energies of 2f and 5f with the coxsackievirus B3 coat protein (PDB code 1COV [30]) have been evaluated (Table 5). The positions of the optimized docking models of compounds 2f and 5f exhibiting the best binding energies (−8.3 and −9.2 kkal/mol, respectively) are shown in Figure 4.

Table 5.
Inhibitory activity against Coxsackievirus B3 of the lead compounds as well as their binding energies and ligand-protein interactions obtained via molecular docking simulations.
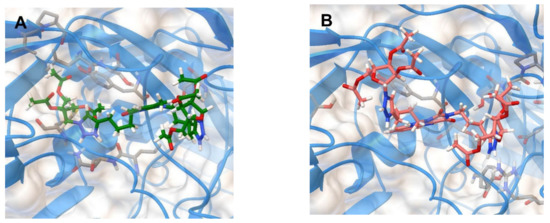
Figure 4.
Molecular docking simulations of the optimized docking model of compounds 2f (A) and 5f (B) in the “canyon” of the coxsackievirus B3 coat protein (PDB code 1COV) obtained in the lowest-energy conformations.
According to the docking simulations, compound 2f is located deep in the “canyon” due to H-bonding with the Ser209 residue of VP2, π-π stacking of 1,2,3-triazole ring with aromatic ring of the Tyr189 residue of VP1, and several hydrophobic interactions. Unlike 2f, compound 5f forms more hydrogen bonds: with the amino acid residues Tyr143 and Ser190 of VP1 and with the amino acid residue Arg196 of VP2. Perhaps because of this, 5f has the better binding energy (−9.2 kkal/mol) than 2f (−8.3 kkal/mol) that corresponds to the IC50 values shown by these nucleoside analogs (11.4 and 12.4 µM, respectively) (Table 5). The results obtained suggest that the antiviral activity of nucleoside analogs 2f and 5f against Coxsackievirus B3 is caused by their interaction with the coat proteins VP1 and VP2.
3. Conclusions
In summary, a series of novel nucleoside analogues in which two 1,2,3-triazol-4-yl-β-D-ribofuranosyl fragments are attached via polymethylene linkers to the N-1 and N-3 atoms of the pyrimidine moiety (uracil, 6-methyluracil, thymine, quinazoline-2,4-dione, alloxazine) or to the C-5 and N-3 atoms of the 6-methyluracil moiety was synthesized for the first time using the click chemistry methodology. Antiviral assays revealed three compounds, 2i, 5i, 11c, which showed moderate activity against influenza virus A/PR/8/34/(H1N1) with IC50 values of 57.5 µM, 24.3 µM, and 29.2 µM, respectively. Two nucleoside analogues 2f and 5f in which 1,2,3-triazol-4-yl-2′,3′,5′-tri-O-acetyl-β-d-ribofuranosyl fragments are attached to the C-5 and N-1 atoms of the heterocycle moiety (6-methyluracil and alloxazine, respectively) exhibited high antiviral potency against the coxsackie B3 virus with IC50 values of 12.4 and 11.3 µM, respectively, although both were inactive against the influenza virus A H1N1. It should be noted that this is the second case we have observed when nucleoside analogues with protected hydroxyl groups of ribofuranosyl residue exhibited antiviral activity. The first one was mentioned earlier [22]. According to theoretical calculations, the antiviral activity of the 1,2,3-triazolyl nucleoside analogues 2i, 5i, 11c against the H1N1 (A/PR/8/34) influenza virus can be explained by their influence on the functioning of the polymerase acidic protein of RNA-dependent RNA polymerase. As to the antiviral activity of nucleoside analogs 2f and 5f against coxsackievirus B3, it can be explained by their interaction with the coat proteins VP1 and VP2.
Supplementary Materials
The following are available online: general information (S2), procedures for the synthesis of the compounds (S2–S3, S8, S9–S10), characterization of the compounds synthesized (S3–S28) including their NMR spectra (Figures S1–S96), the molecular docking study (S78), and description of the antiviral and cytotoxicity assays (S77). References [21,23,28,29,30,31,32,33] are cited in the supplementary.
Author Contributions
Supervision, methodology, analyses of obtained results, V.E.S.; methodology, analyses of obtained results, writing the manuscript, V.E.K.; synthesis, O.V.A., M.G.B., M.A.M., L.F.S. and M.M.S.; antiviral assays, V.V.Z., A.V.S., I.L.Y. and A.S.V.; molecular docking, B.F.G.; cytotoxicity experiments, A.D.V. and A.P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation (grant no. 19-13-00003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are stored at the authors.
Acknowledgments
The authors from the Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, RAS are grateful to the Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS for technical assistance during research.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are kept amongst the authors.
References
- De Clercq, E.; Lia, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, L.A.; Efremenkova, O.V.; Andronova, V.L.; Galegov, G.A.; Solyev, P.N.; Karpenko, I.L.; Kochetkov, S.N. 5-(4-alkyl-1,2,3-triazol-1-yl)methyl derivatives of 2′-deoxyuridine as inhibitors of viral and bacterial growth. Russ. J. Bioorganic Chem. 2016, 42, 677–684. [Google Scholar] [CrossRef]
- Zhurilo, N.I.; Chudinov, M.V.; Matveev, A.V.; Smirnova, O.S.; Konstantinova, I.D.; Miroshnikov, A.I.; Prutkov, A.N.; Grebenkina, L.E.; Pulkova, N.V.; Shvets, V.I. Isosteric ribavirin analogues: Synthesis and antiviral activities. Bioorganic Med. Chem. Lett. 2018, 28, 11–14. [Google Scholar] [CrossRef]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Tirumalasetty, M.C.B.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R.; Baburao, K.; Parasa, L.S. Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives. Eur. J. Med. Chem. 2016, 123, 379–396. [Google Scholar] [CrossRef]
- Kohgo, S.; Imoto, S.; Tokuda, R.; Takamatsu, Y.; Higashi-Kuwata, N.; Aoki, M.; Amano, M.; Kansui, H.; Onitsuka, K.; Maeda, K.; et al. Synthesis of 4′-substituted purine 2′-deoxynucleosides and their activity against human immunodeficiency virus type 1 and hepatitis B virus. ChemistrySelect 2018, 3, 3313–3317. [Google Scholar] [CrossRef]
- Wang, P.; Hong, J.H.; Cooperwood, J.S.; Chu, C.K. Recent advances in l-nucleosides: Chemistry and biology. Antivir. Res. 1998, 40, 19–44. [Google Scholar] [CrossRef]
- Kim, H.O.; Shanmuganathan, K.; Alves, A.J.; Jeong, L.S.; Beach, J.W.; Schinazi, R.F.; Chang, C.N.; Cheng, Y.C.; Chu, C.K. Potent anti-HIV and anti-HBV activities of (−)-L-β-dioxolane-C and (+)-L-β-dioxolane-T and their asymmetric syntheses. Tetrahedron Lett. 1992, 33, 6899–6902. [Google Scholar] [CrossRef]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2007, 28, 278–308. [Google Scholar] [CrossRef]
- Petrova, K.T.; Potewar, T.M.; Correia-Da-Silva, P.; Barros, M.T.; Calhelha, R.C.; Ciric, A.; Sokovic, M.; Ferreira, I.C.F.R. Antimicrobial and cytotoxic activities of 1,2,3-triazole-sucrose derivatives. Carbohydr. Res. 2015, 417, 66–71. [Google Scholar] [CrossRef]
- Park, S.M.; Yang, H.; Park, S.-K.; Kim, H.M.; Kim, B.H. Design, synthesis, and anticancer activities of novel perfluoroalkyltriazole-appended 2′-deoxyuridines. Russ. J. Bioorganic Chem. Med. Chem. Lett. 2010, 20, 5831–5834. [Google Scholar] [CrossRef]
- Montagu, A.; Roy, V.; Balzarini, J.; Snoeck, R.; Andrei, G.; Agrofoglio, L.A. Synthesis of new C5-(1-substituted-1,2,3-triazol-4 or 5-yl)-2′-deoxyuridines and their antiviral evaluation. Eur. J. Med. Chem. 2011, 46, 778–786. [Google Scholar] [CrossRef]
- Shmalenyuk, E.R.; Chernousova, L.N.; Karpenko, I.L.; Kochetkov, S.N.; Smirnova, T.G.; Andreevskaya, S.N.; Chizhov, A.O.; Efremenkova, O.; Alexandrova, L.A. Inhibition of Mycobacterium tuberculosis strains H37Rv and MDR MS-115 by a new set of C5 modified pyrimidine nucleosides. Bioorganic Med. Chem. 2013, 21, 4874–4884. [Google Scholar] [CrossRef]
- Kumar, J.M.; Idris, M.M.; Srinivas, G.; Kumar, P.V.; Meghah, V.; Kavitha, M.; Reddy, C.R.; Mainkar, P.S.; Pal, B.; Chandrasekar, S.; et al. Phenyl 1,2,3-triazole-thymidine ligands stabilize g-quadruplex DNA, inhibit DNA synthesis and potentially reduce tumor cell proliferation over 3′-azido deoxythymidine. PLoS ONE 2013, 8, e70798. [Google Scholar] [CrossRef]
- Chatzileontiadou, D.S.M.; Parmenopoulou, V.; Manta, S.; Kantsadi, A.L.; Kylindri, P.; Griniezaki, M.; Kontopoulou, F.; Telopoulou, A.; Prokova, H.; Panagopoulos, D.; et al. Triazole double-headed ribonucleosides as inhibitors of eosinophil derived neurotoxin. Bioorganic Chem. 2015, 63, 152–165. [Google Scholar] [CrossRef]
- Elayadi, H.; Mesnaoui, M.; Korba, B.E.; Smietana, M.; Vasseur, J.-J.; Secrist, J.A.; Lazrek, H.B. Preparation of 1,4-disubstituted-1,2,3-triazolo ribonucleosides by Na2CuP2O7 catalyzed azide-alkyne 1,3-dipolar cycloaddition. Arkivoc 2012, 2012, 76. [Google Scholar] [CrossRef]
- Amant, A.H.S.; Bean, L.A.; Guthrie, J.P.; Hudson, R.H.E. Click fleximers: A modular approach to purine base-expanded ribonucleoside analogues. Org. Biomol. Chem. 2012, 10, 6521. [Google Scholar] [CrossRef]
- Chatzileontiadou, D.S.M.; Tsika, A.C.; Diamantopoulou, Z.; Delbé, J.; Badet, J.; Courty, J.; Skamnaki, V.T.; Parmenopoulou, V.; Komiotis, D.; Hayes, J.M.; et al. Evidence for Novel Action at the Cell-Binding Site of Human Angiogenin Revealed by Heteronuclear NMR Spectroscopy, in silico and in vivo Studies. ChemMedChem 2018, 13, 259–269. [Google Scholar] [CrossRef]
- Andreeva, O.V.; Belenok, M.G.; Saifina, L.F.; Shulaeva, M.M.; Dobrynin, A.B.; Sharipova, R.R.; Voloshina, A.D.; Saifina, A.F.; Gubaidullin, A.T.; Khairutdinov, B.I.; et al. Synthesis of novel 1,2,3-triazolyl nucleoside analogues bearing uracil, 6-methyluracil, 3,6-dimethyluracil, thymine, and quinazoline-2,4-dione moieties. Tetrahedron Lett. 2019, 60, 151276. [Google Scholar] [CrossRef]
- Sharipova, R.R.; Saifina, L.F.; Belenok, M.G.; Semenov, V.E.; Kataev, V.E. First analog of pyrimidine nucleosides with two d-ribofuranose residues. Russ. J. Org. Chem. 2020, 56, 181–184. [Google Scholar] [CrossRef]
- Andreeva, O.V.; Garifullin, B.F.; Zarubaev, V.V.; Slita, A.V.; Yesaulkova, I.L.; ·Saifina, L.F.; Shulaeva, M.M.; Belenok, M.G.; Semenov, V.E.; Kataev, V.E. Synthesis of 1,2,3-triazolyl nucleoside analogues and their antiviral activity. Mol. Divers. 2020, 25, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Belenok, M.G.; Strobykina, I.Y.; Lyubina, A.P.; Gumerova, S.K.; Kataev, V.E. Antimicrobial and cytotoxic effects of ammonium derivatives of diterpenoids steviol and isosteviol. Bioorganic Med. Chem. 2021, 32, 115974. [Google Scholar] [CrossRef] [PubMed]
- Stevaert, A.; Naesens, L. The Influenza Virus Polymerase Complex: An Update on Its Structure, Functions, and Significance for Antiviral Drug Design. Med. Res. Rev. 2016, 36, 1127–1173. [Google Scholar] [CrossRef] [PubMed]
- Jockusch, S.; Tao1, C.; Li1, X.; Anderson, T.K.; Chien, M.; Kumar, S.; Russo, J.J.; Kirchdoerfer, R.N.; Ju, J. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antivir. Res. 2020, 180, 104857. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, T.M.; Velthuis, A.J.T. The RNA-dependent RNA polymerase of the influenza A virus. Futur. Virol. 2014, 9, 863–876. [Google Scholar] [CrossRef]
- Pala, N.; Stevaert, A.; Dallocchio, R.; Dessì, A.; Rogolino, D.; Carcelli, M.; Sanna, V.; Sechi, M.; Naesens, L. Virtual Screening and Biological Validation of Novel Influenza Virus PA Endonuclease Inhibitors. ACS Med. Chem. Lett. 2015, 6, 866–871. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Fudo, S.; Yamamoto, N.; Nukaga, M.; Odagiri, T. Two Distinctive Binding Modes of Endonuclease Inhibitors to the N-Terminal Region of Influenza Virus Polymerase Acidic Subunit. Biochemistry 2016, 55, 2646–2660. [Google Scholar] [CrossRef]
- Muckelbauer, J.K.; Kremer, M.; Minor, I.; Tong, L.; Zlotnick, A.; Johnson, J.E.; Rossmann, M.G. Structure determination of coxsackievirus B3 to 3.5 Å resolution. Acta Cryst. 1995, D51, 871–887. [Google Scholar] [CrossRef]
- Bermsn, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- HyperChem Professional 8.0. Hypercube, Inc. 2007. Available online: https://www.chemits.com/en/software/molecular-modeling/hyperchem/ (accessed on 14 September 2020).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Das, K.; Aramini, J.M.; Ma, L.-C.; Krug, R.M.; Arnold, E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010, 17, 530–538. [Google Scholar] [CrossRef]
- Yan, V.C.; Muller, F.L. Advantages of the Parent Nucleoside GS-441524 over Remdesivir for Covid-19 Treatment. ACS Med. Chem. Lett. 2020, 11, 1361–1366. [Google Scholar] [CrossRef]
- Chien, M.; Anderson, T.K.; Jockusch, S.; Tao, C.; Li, X.; Kumar, S.; Russo, J.J.; Kirchdoerfer, R.N.; Ju, J. Nucleotide analogues as inhibitors of SARSCoV-2 polymerase, a key drug target for COVID-19. J. Proteome Res. 2020, 19, 4690–4697. [Google Scholar] [CrossRef]
- Wagner, C.R.; Iyer, V.V.; Mcintee, E.J. Pronucleotides: Toward thein vivo delivery of antiviral and anticancer nucleotides. Med. Res. Rev. 2000, 20, 417–451. [Google Scholar] [CrossRef]
- Adams, M.J.; King, A.M.Q.; Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2013). Arch. Virol. 2013, 158, 2023–2030. [Google Scholar] [CrossRef]
- Hogle, J.M.; Chow, M.; Filman, D.J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science 1985, 229, 1358–1365. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Fokine, A.; Meng, G.; Shin, W.-H.; Long, F.; Kuhn, R.J.; Kihara, D.; Rossmann, M.G. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015, 347, 71–74. [Google Scholar] [CrossRef]
- Smyth, M.; Tate, J.; Hoey, E.; Lyons, C.; Martin, S.; Stuart, D. Implications for viral uncoating from the structure of bovine enterovirus. Nat. Struct. Mol. Biol. 1995, 2, 224–231. [Google Scholar] [CrossRef]
- Rossmann, M.G. Viral cell recognition and entry. Protein Sci. 1994, 3, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).