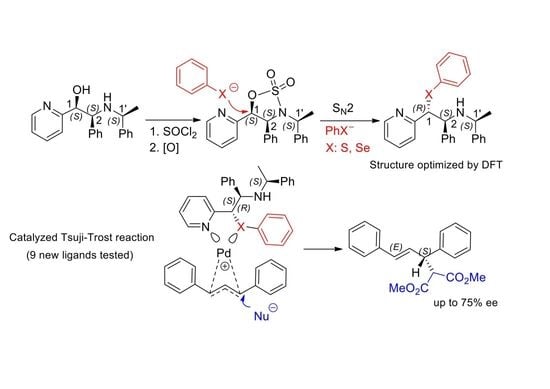
New Nitrogen, Sulfur-, and Selenium-Donating Ligands Derived from Chiral Pyridine Amino Alcohols. Synthesis and Catalytic Activity in Asymmetric Allylic Alkylation
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Sulfur and Selenium Derivatives
2.2. Synthesis of Nitrogen Derivatives (Chiral Schiff Base)
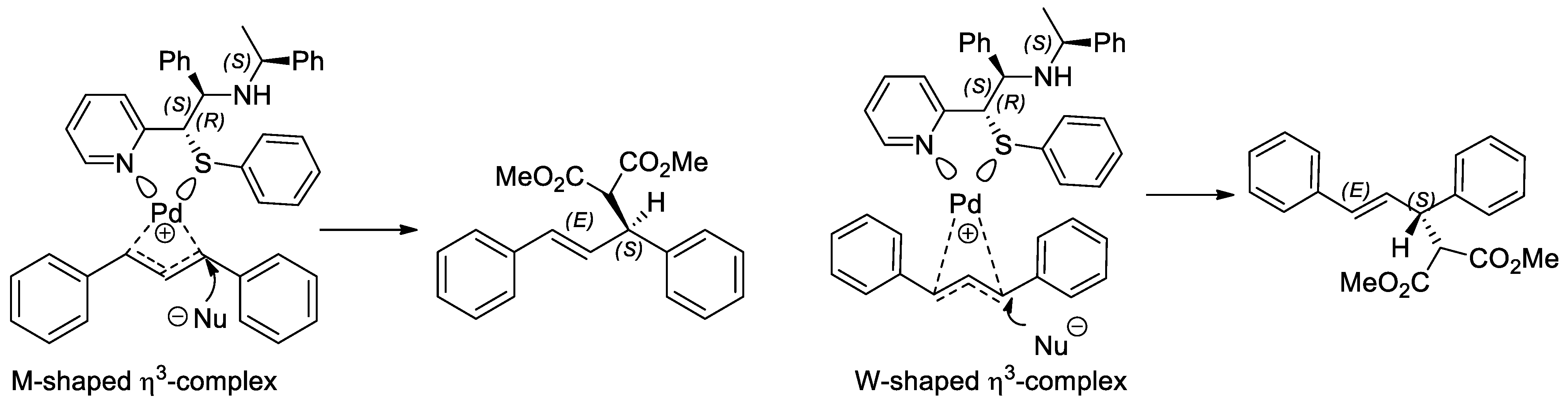
2.3. Application of Chiral S, Se, and N Derivatives in the Tsuji-Trost Reaction
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of Cyclic Sulfonamidates
3.3. General Procedure for the Synthesis of S and Se Derivatives
3.4. General Procedure for the Synthesis of Mono-Aldimine (Chiral Schiff Base)
3.5. Catalytic Reaction Procedure (Tsuji-Trost)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ojima, I. Catalysis in Asymmetric Synthesis, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Zhou, Q.-L. Privileged Chiral Ligands and Catalysts, 1st ed.; Wiley-VCH: New York, NY, USA, 2011. [Google Scholar]
- Pellissier, H. Chiral Sulfur Ligands: Asymmetric Catalysis; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chiral Organosulfur Ligands/Catalysts with a Stereogenic Sulfur Atom: Applications in Asymmetric Synthesis. Chem. Rev. 2017, 117, 4147–4181. [Google Scholar] [CrossRef]
- Masdeu-Bulto, A.M.; Dieguez, M.; Martin, E.; Gomez, M. Chiral thioether ligands: Coordination chemistry and asymmetric catalysis. Coord. Chem. Rev. 2003, 242, 159–201. [Google Scholar] [CrossRef]
- Wirth, T. Enantioselective Alkylation of Aldehydes Catalyzed by New Chiral Diselenides. Tetrahedron Lett. 1995, 36, 7849–7852. [Google Scholar] [CrossRef]
- Nishibayashi, Y.; Uemura, S. Selenium compounds as ligands and catalysts in Organoselenium Chemistry: Modern Developments in Organic Synthesis. In Topics in Current Chemistry; Wirth, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 208, pp. 235–255. [Google Scholar]
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Trost, B.M.; Van Vrankel, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef]
- Tsuji, J. Recollections of organopalladium chemistry. Pure Appl. Chem. 1999, 71, 1539–1547. [Google Scholar] [CrossRef]
- Lubbers, T.; Metz, P. Methods of Organic Chemistry (Houben Weyl), Stereoselective Synthesis; Thieme Verlag: Stuttgart, Germany, 1995; Volume E21c, pp. 2371–2473. [Google Scholar]
- Pfalz, A.; Lautens, M. Comprehensive Asymmetric Catalysis; Jacobsen, E.N., Pfaltz, A., Yamamoto, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 2, pp. 834–884. [Google Scholar]
- Oslob, J.D.; Åkermark, B.; Helquist, P.; Norrby, P.-O. Steric Influences on the Selectivity in Palladium-Catalyzed Allylation. Organometallics 1997, 16, 3015–3021. [Google Scholar] [CrossRef]
- Trost, B.M.; Crawley, M.L. Asymmetric Transition-Metal-Catalyzed Allylic Alkylations: Applications in Total Synthesis. Chem. Rev. 2003, 103, 2921–2943. [Google Scholar] [CrossRef] [PubMed]
- Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis; Kazmaier, U., Ed.; (Topics in Organometallic Chemistry); Springer: Berlin/Heidelberg, Germany, 2012; Volume 38. [Google Scholar]
- Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E.; Guiry, P.J.; Moberg, C.; Bäckvall, J.-E.; Pfaltz, A.; Pericas, M.A.; et al. Recent Advances in Enantioselective Pd-Catalyzed Allylic Substitution: From Design to Applications. Chem. Rev. 2021, 121, 4373–4505. [Google Scholar] [CrossRef]
- Noreen, S.; Zahoor, A.F.; Ahmad, S.; Shahzadi, I.; Irfan, A.; Faiz, S. Novel Chiral Ligands for Palladium-catalyzed Asymmetric Allylic Alkylation/Asymmetric Tsuji-Trost Reaction: A Review. Curr. Org. Chem. 2019, 23, 1168–1213. [Google Scholar] [CrossRef]
- Siedlecka, R.; Wojaczyńska, E.; Skarżewski, J. Chiral pyrrolidine thioethers: Effective nitrogen–sulfur donating ligands in palladium-catalyzed asymmetric allylic alkylations. Tetrahedron Asymmetry 2004, 15, 1437–1444. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Siedlecka, R.; Skarżewski, J. Chiral phenylselenyl derivatives of pyrrolidine and Cinchona alkaloids: Nitrogen-selenium donating ligands in palladium-catalyzed asymmetric allylic alkylation. Tetrahedron Asymmetry 2007, 18, 131–136. [Google Scholar] [CrossRef]
- Andrade, L.H.; Silva, A.V.; Milani, P.; Koszelewski, D.; Kroutil, W. ω-Transaminases as efficient biocatalysts to obtain novel chiral selenium-amine ligands for Pd-catalysis. Org. Biomol. Chem. 2010, 8, 2043–2051. [Google Scholar] [CrossRef]
- Ścianowski, J.; Pacuła, A.J.; Zielińska-Błajet, M.; Wojtczak, A. New diphenyl diselenides o-substituted by an O(S,Se)-caranyl skeleton—Synthesis and application in asymmetric reactions. New J. Chem. 2016, 40, 6697–6705. [Google Scholar] [CrossRef]
- Sehnem, J.A.; Vargas, F.; Milani, P.; Nascimento, V.; Braga, L.A. Modular Synthesis of Chiral N-Protected β-Seleno Amines and Amides via Cleavage of 2-Oxazolidinones and Application in Palladium-Catalyzed Asymmetric Allylic Alkylation. Synthesis 2008, 8, 1262–1268. [Google Scholar] [CrossRef]
- Margalef, J.; Borras, C.; Alegre, S.; Pamies, O.; Diéguez, M.A. readily accessible and modular carbohydrate-derived thioether/selenoether-phosphite ligand library for Pd-catalyzed asymmetric allylic substitutions. Dalton Trans. 2019, 48, 12632–12643. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Sehnem, J.A.; Galetto, F.Z.; Braga, A.L. Modular chiral β-selenium-, sulfur-, and tellurium amides: Synthesis and application in the palladium-catalyzed asymmetric allylic alkylation. Tetrahedron 2008, 64, 392–398. [Google Scholar] [CrossRef]
- Wosińska-Hyrydczuk, M.; Skarżewski, J. 2-Oxiranyl-pyridines: Synthesis and Regioselective Epoxide Ring Openings with Chiral Amines as a Route to Chiral Ligands. Heteroat. Chem. 2019. [Google Scholar] [CrossRef]
- Wosińska-Hrydczuk, M.; Boratyński, P.J.; Skarżewski, J. Regioselective and Stereodivergent Synthesis of Enantiomerically Pure Vic-Diamines from Chiral β-Amino Alcohols with 2-Pyridyl and 6-(2,2′-Bipyridyl) Moieties. Molecules 2020, 25, 727. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Sekine, M. The synthesis of S-phenyl nucleoside phosphorothioates. Chem. Lett. 1974, 15, 837–838. [Google Scholar] [CrossRef]
- Valentine, D.H.; Hillhouse, J.H. Alkyl Phosphines as Reagents and Catalysts in Organic Synthesis. Synthesis 2003, 3, 317–344. [Google Scholar] [CrossRef]
- Grieco, P.A.; Grilman, S.; Nishizawa, M. Organoselenium Chemistry. A Facile One-Step Synthesis of Alkyl Aryl Selenides from Alcohols. J. Org. Chem. 1976, 41, 1485–1486. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef]
- Liu, G.L.; Chen, W.; Jiang, Q.-Y.; Bai, X.-F.; Li, Z.; Xu, Z.; Xu, L.-W. A D-Camphor-Based Schiff Base as a Highly Efficient N, P Ligand for Enantioselective Palladium-Catalyzed Allylic Substitutions. ChemCatChem 2016, 8, 1495–1499. [Google Scholar] [CrossRef]
- Szabo, K.J. Effects of the Ancillary Ligands on Palladium−Carbon Bonding in (η3−Allyl)palladium Complexes. Implications for Nucleophilic Attack at the Allylic Carbons. Organometallics 1996, 15, 1128–1133. [Google Scholar] [CrossRef]
- Pfaltz, A. From Corrin Chemistry to Asymmetric Catalysis—A Personal Account. Synlett 1999, 835–842. [Google Scholar] [CrossRef]
- Laveson, W.; Reid, G. For the coordination of transition metals to chalcogen ethers. In Comprehensive Coordination Chemistry II; Lever, A.B.P., Ed.; Elsevier: Oxford, UK, 2004; Volume 1, pp. 395–398. [Google Scholar]
- Niu, J.-L.; Wang, M.-C.; Kong, P.-P.; Chen, Q.-T.; Zhu, Y.; Song, M.-P. Origin of enantioselectivity with heterobidentate sulfide-tertiary amine (sp3) ligands in palladium-catalyzed allylic substitution. Tetrahedron 2009, 65, 8869–8878. [Google Scholar] [CrossRef]
- Van Dort, M.E.; Jung, Y.-W.; Sherman, P.S.; Kilbourn, M.R.; Wieland, D.M. Fluorine for Hydroxy Substitution in Biogenic Amines: Asymmetric Synthesis and Biological Evaluation of Fluorine-18-Labeled -Fluorophenylalkylamines as Model Systems. J. Med. Chem. 1995, 38, 810–815. [Google Scholar] [CrossRef]
- Silva, D.R.C.; Maria, E.J.; Suárez Ordóñeza, R.M.; Thierry, J.; Cariou, K.; Dodd, R.H. Synthesis of Orthogonally N-Protected, C-4 Functionalized Cyclic Guanidines from L-Serine. Synlett 2017, 28, 815–818. [Google Scholar] [CrossRef]





| Sulfonamidates | R1 | R2 | NuH | Yield [%] a | Product 15–19 |
|---|---|---|---|---|---|
| (5S,1′S)-12 | Ph | H | PhSH | 49 | (2R,1′S)-15 |
| (5R,1′S)-12 | Ph | H | PhSH | 21 a | (2S,1′S)-15 |
| (4R,5R,1′S)-13 | Py | Ph | PhSH | 30, 58 a | (1R,2S,1′S)-16 |
| (4S,5S,1′S)-13 | Py | Ph | PhSH | 5 b | (1S,2R,1′S)-16 |
| (4R,5R,1′S)-13 | Py | Ph | PhCH2SH | 47 a | (1R,2S,1′S)-17 |
| (4R,5R,1′S)-14 | Bpy | Ph | PhSH | 56 a | (1R,2S,1′S)-18 |
| (4R,5R,1′S)-13 | Py | Ph | PhSeH | 10 a | (1R,2S,1′S)-19 |

| Chiral Diamine | R1 | R2 | R3 | Yield [%] | Product |
|---|---|---|---|---|---|
| (1S,2R,1′S)-20 | Py | Ph | Me | 70 | (1S,2R,1′S)-22 |
| (2R,1′S)-21 | Ph | H | tBu | 60 | (2R,1′S)-23 |
| (2R,1′S)-21 | Ph | H | Me | 68 | (2R,1′S)-24 |

| Chiral Ligand | Conversion [%] a | Ee [%] Configuration |
|---|---|---|
| (2R,1′S)-15 | 15 | Rac |
| (2S,1′S)-15 | 30 | 31 (R) |
| (1R,2S,1′S)-16 | 100 | 58 (S) |
| (1R,2S,1′S)-17 | 98 | 26 (S) |
| (1R,2S,1′S)-18 | 89 | 13 (R) |
| (1R,2S,1′S)-19 | 70 | 50 (S) |
| (1S,2R,1′S)-22 | 98 b | 49 (S) |
| (2R,1′S)-23 | 66 b | 38 (R) |
| (2R,1′S)-24 | 69 b | 38 (R) |
| Chiral Ligand | Temperature [°C] | Conversion [%] | Ee [%] Configuration | Time [h] |
|---|---|---|---|---|
| (1R,2S,1′S)-16 | 0–4 | 97 | 70 (S) | 48 |
| (1R,2S,1′S)-16 | −18 | 75 | 75 (S) | 96 |
| (1R,2S,1′S)-16 | −30 | 5 | 73 (S) | 10 |
| (1S,2R,1′S)-22 | −18 | 96 | 71 (S) | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wosińska-Hrydczuk, M.; Skarżewski, J. New Nitrogen, Sulfur-, and Selenium-Donating Ligands Derived from Chiral Pyridine Amino Alcohols. Synthesis and Catalytic Activity in Asymmetric Allylic Alkylation. Molecules 2021, 26, 3493. https://doi.org/10.3390/molecules26123493
Wosińska-Hrydczuk M, Skarżewski J. New Nitrogen, Sulfur-, and Selenium-Donating Ligands Derived from Chiral Pyridine Amino Alcohols. Synthesis and Catalytic Activity in Asymmetric Allylic Alkylation. Molecules. 2021; 26(12):3493. https://doi.org/10.3390/molecules26123493
Chicago/Turabian StyleWosińska-Hrydczuk, Marzena, and Jacek Skarżewski. 2021. "New Nitrogen, Sulfur-, and Selenium-Donating Ligands Derived from Chiral Pyridine Amino Alcohols. Synthesis and Catalytic Activity in Asymmetric Allylic Alkylation" Molecules 26, no. 12: 3493. https://doi.org/10.3390/molecules26123493
APA StyleWosińska-Hrydczuk, M., & Skarżewski, J. (2021). New Nitrogen, Sulfur-, and Selenium-Donating Ligands Derived from Chiral Pyridine Amino Alcohols. Synthesis and Catalytic Activity in Asymmetric Allylic Alkylation. Molecules, 26(12), 3493. https://doi.org/10.3390/molecules26123493