Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma—In Vitro Studies
Abstract
1. Introduction
2. Results
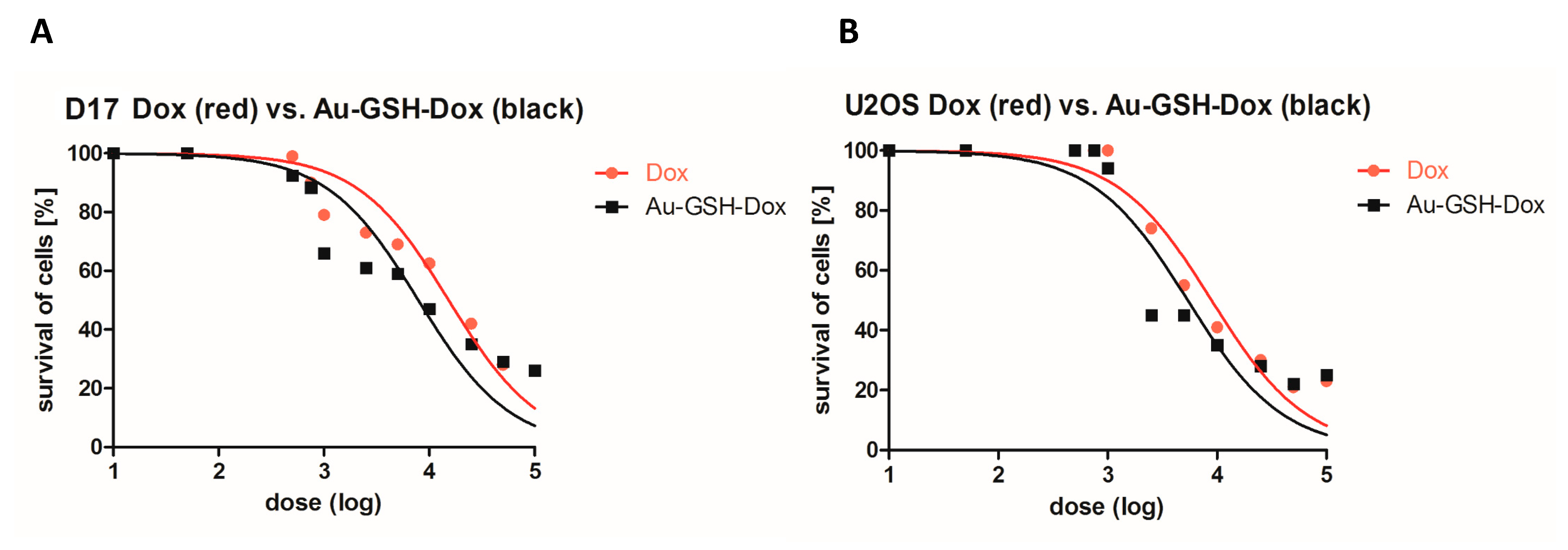
2.1. Au-GSH-Dox Treatment Increases Cytotoxicity in OSA Cell Lines
2.2. Au-GSH-Dox Acts Mainly through Apoptotic Cell Death
2.3. Au-GSH-Dox Increases Cell Mortality in the D17 Cell Line
2.4. Au-GSH-Dox but Not Au-GSH Alters OSA Cell Morphology
2.5. P-gp Inhibition with Verapamil Enhances the Intracellular Accumulation of Rhodamine 123 in the D17 Cell Line
2.6. P-gp Inhibition with Verapamil Increases the Intracellular Accumulation of Free Dox but Not Au-GSH-Dox in the D17 Cell Line
3. Discussion
4. Materials and Methods
4.1. AU-GSH-Dox and Au-GSH
4.2. Cell Culture
4.3. MTT Assay
4.4. Annexin-V and Propidium Iodide Dual Staining
4.5. Cell Mortality
4.6. Cell Morphology
4.7. Au-GSH-Dox and Dox Accumulation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 gene |
| ANS-TAT-AuNPs | Anticancer molecule 2-(9-anthracenylmethylene)-hydrazine carbothioamide conjugated to AuNPs |
| Au-GSH | Glutathione-stabilized gold nanoparticles |
| Au-GSH-Dox | Doxorubicin conjugated to glutathione-stabilized gold nanoparticles |
| AuNPs | Gold nanoparticles |
| BCRP/ABCG2 | Breast cancer resistance protein |
| BMI1 | B-cell-specific Moloney murine leukemia virus integration site 1 |
| CMC | Carboxymethyl chitosan |
| Dox/CMC-AuNPs-PEG | Doxorubicin-loaded chitosan-based gold nanoparticles coated with polyethylene glycol layer |
| DMSO | Dimethyl sulfoxide |
| Dox | Doxorubicin |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal bovine serum |
| HER2 | Human epidermal growth factor receptor 2 |
| IC50 | Half maximal inhibitory concentration |
| MDR | Multidrug-resistant |
| MRP1/ABCC1 | Multidrug resistance-associated protein 1 |
| MDR1 | Multidrug resistance 1 gene |
| MTT | Thiazolyl Blue Tetrazolium Bromide |
| OSA | Osteosarcoma |
| PBS | Phosphate-buffered saline |
| PEG | Polyethylene glycol |
| P-gp | P-glycoprotein |
| PI | Propidium iodide |
| SEM | Standard error of the mean |
| TPPS-AuNPs | Nanocomposite with porphyrin-embedded gold nanoparticles |
References
- Sapierzyński, R.; Czopowicz, M. The animal-dependent risk factors in canine osteosarcomas. Pol. J. Vet. Sci. 2017, 20, 293–298. [Google Scholar] [CrossRef]
- Egenvall, A.; Nødtvedt, A.; von Euler, H. Bone tumors in a population of 400,000 insured Swedish dogs up to 10 y of age: Incidence and survival. Can. J. Vet. Res. 2007, 71, 292–299. [Google Scholar] [PubMed]
- Wilk, S.S.; Zabielska-Koczywąs, K.A. Molecular Mechanisms of Canine Osteosarcoma Metastasis. Int. J. Mol. Sci. 2021, 22, 3639. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Selvarajah, G.T.; Kirpensteijn, J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet. J. 2010, 185, 28–35. [Google Scholar] [CrossRef]
- Boston, S.E.; Ehrhart, N.P.; Dernell, W.S.; Lafferty, M.; Withrow, S.J. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985–2004). J. Am. Vet. Med. Assoc. 2006, 228, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Selmic, L.E.; Burton, J.H.; Thamm, D.H.; Withrow, S.J.; Lana, S.E. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J. Vet. Intern. Med. 2014, 28, 554–563. [Google Scholar] [CrossRef]
- Macdonald, V. Chemotherapy: Managing side effects and safe handling. Can. Vet. J. 2009, 50, 665–668. [Google Scholar] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Jalalpure, S.; Jadhav, K.; Jagwani, S.; Chavan, R. Doxorubicin loaded gold nanoparticles: Implication of passive targeting on anticancer efficacy. Pharmacol. Res. 2016, 113, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wycislo, K.L.; Fan, T.M. The Immunotherapy of Canine Osteosarcoma: A Historical and Systematic Review. J. Vet. Intern. Med. 2015, 29, 759–769. [Google Scholar] [CrossRef]
- Zekri, J.; Mansour, M.; Karim, S.M. The anti-tumour effects of zoledronic acid. J. Bone Oncol. 2014, 3, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Koto, K.; Horie, N.; Kimura, S.; Murata, H.; Sakabe, T.; Matsui, T.; Watanabe, M.; Adachi, S.; Maekawa, T.; Fushiki, S.; et al. Clinically relevant dose of zoledronic acid inhibits spontaneous lung metastasis in a murine osteosarcoma model. Cancer Lett. 2009, 274, 271–278. [Google Scholar] [CrossRef]
- Macewen, E.G.; Kurzman, I.D.; Rosenthal, R.C.; Smith, B.W.; Manley, P.A.; Roush, J.K.; Howard, P.E. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J. Natl. Cancer Inst. 1989, 81, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.J.; Gnanandarajah, J.S.; Engiles, J.B.; Gray, F.; Laughlin, D.; Gaurnier-Hausser, A.; Wallecha, A.; Huebner, M.; Paterson, Y. Immunotherapy with a HER2-Targeting listeria induces HER2-Specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin. Cancer Res. 2016, 22, 4380–4390. [Google Scholar] [CrossRef]
- Dow, S.; Elmslie, R.; Kurzman, I.; MacEwen, G.; Pericle, F.; Liggitt, D. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum. Gene Ther. 2005, 16, 937–946. [Google Scholar] [CrossRef]
- Mayer, M.N.; Grier, C.K. Palliative radiation therapy for canine osteosarcoma. Can. Vet. J. 2006, 47, 707–709. [Google Scholar]
- Arndt, C.A.S.; Rose, P.S.; Folpe, A.L.; Laack, N.N. Common Musculoskeletal Tumors of Childhood and Adolescence. Mayo Clin. Proc. 2012, 87, 475–487. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Mealey, K.L.; Fidel, J. P-glycoprotein mediated drug interactions in animals and humans with cancer. J. Vet. Intern. Med. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Zandvliet, M.; Teske, E. Mechanisms of Drug Resistance in Veterinary Oncology—A Review with an Emphasis on Canine Lymphoma. Vet. Sci. 2015, 2, 150–184. [Google Scholar] [CrossRef]
- Gramer, I.; Kessler, M.; Geyer, J. Determination of MDR1 gene expression for prediction of chemotherapy tolerance and treatment outcome in dogs with lymphoma. Vet. Comp. Oncol. 2015, 13, 363–372. [Google Scholar] [CrossRef]
- Karita, M.; Tsuchiya, H.; Kawahara, M.; Kasaoka, S.; Tomita, K. The antitumor effect of liposome-encapsulated cisplatin on rat osteosarcoma and its enhancement by caffeine. Anticancer Res. 2008, 28, 1449–1457. [Google Scholar]
- Tang, N.; Du, G.; Wang, N.; Liu, C.; Hang, H.; Liang, W. Improving Penetration in Tumors With Nanoassemblies of Phospholipids and Doxorubicin. JNCI J. Natl. Cancer Inst. 2007, 99, 1004–1015. [Google Scholar] [CrossRef]
- Teske, E.; Rutteman, G.R.; Kirpenstein, J.; Hirschberger, J. A randomized controlled study into the efficacy and toxicity of pegylated liposome encapsulated doxorubicin as an adjuvant therapy in dogs with splenic haemangiosarcoma. Vet. Comp. Oncol. 2011, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gao, Z.G.; Lee, E.S.; Bae, Y.H. In vivo evaluation of doxorubicin-loaded polymeric micelles targeting folate receptors and early endosomal pH in drug-resistant ovarian cancer. Mol. Pharm. 2009, 6, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, T.A.; Galanou, M.C.; Paleos, C.M. Novel Amiodarone-Doxorubicin Cocktail Liposomes Enhance Doxorubicin Retention and Cytotoxicity in DU145 Human Prostate Carcinoma Cells. J. Med. Chem. 2008, 51, 6067–6074. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Lee, Y.E.K.; Ray, A.; Kopelman, R. Overcoming cancer multidrug resistance by codelivery of doxorubicin and verapamil with hydrogel nanoparticles. Macromol. Biosci. 2014, 14, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Susa, M.; Iyer, A.K.; Ryu, K.; Hornicek, F.J.; Mankin, H.; Amiji, M.M.; Duan, Z. Doxorubicin loaded polymeric nanoparticulate delivery system to overcome drug resistance in osteosarcoma. BMC Cancer 2009, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Soritau, O.; Orza, A.; Dudea, M.; Petrushev, B.; Mosteanu, O.; Susman, S.; Florea, A.; Pall, E.; Aldea, M.; et al. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J. Gastrointest. Liver Dis. 2012, 21, 188–196. [Google Scholar]
- Gu, Y.J.; Cheng, J.; Man, C.W.Y.; Wong, W.T.; Cheng, S.H. Gold-doxorubicin nanoconjugates for overcoming multidrug resistance. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 204–211. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, Y.J.; Cheng, S.H.; Wong, W.T. Surface functionalized gold nanoparticles for drug delivery. J. Biomed. Nanotechnol. 2013, 9, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Bai, J.; Deng, J.; Fang, C.J.; Chen, X. TAT-Modified Gold Nanoparticle Carrier with Enhanced Anticancer Activity and Size Effect on Overcoming Multidrug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 5828–5837. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Lewandowski, W.; Król, M.; Pawłowski, K.; Mieczkowski, J.; Lechowski, R.; Zabielska, K. Enhancing anti-tumor efficacy of Doxorubicin by non-covalent conjugation to gold nanoparticles—In vitro studies on feline fibrosarcoma cell lines. PLoS ONE 2015, 10, e0124955. [Google Scholar] [CrossRef] [PubMed]
- Zabielska-Koczywąs, K.; Wojtalewicz, A.; Użarowska, E.; Klejman, A.; Wojtkowska, A.; Dolka, I.; Wojnicki, M.; Sobczak, K.; Wójcik, M.; Shen, H.; et al. Distribution of glutathione-stabilized gold nanoparticles in feline fibrosarcomas and their role as a drug delivery system for doxorubicin—preclinical studies in a murine model. Int. J. Mol. Sci. 2018, 19, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zabielska-Koczywąs, K.; Wojtkowska, A.; Dolka, I.; Małek, A.; Walewska, M.; Wojtalewicz, A.; Żbikowski, A.; Lechowski, R. 3D chick embryo chorioallantoic membrane model as an in vivo model to study morphological and histopathological features of feline fibrosarcomas. BMC Vet. Res. 2017, 13, 201. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Pétriz, J.; García-López, J. Flow cytometric analysis of P-glycoprotein function using rhodamine 123. Leukemia 1997, 11, 1124–1130. [Google Scholar] [CrossRef]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE 2012, 7, 33253. [Google Scholar] [CrossRef]
- Aszalos, A.; Thompson, K.; Yin, J.J.; Ross, D.D. Combinations of P-glycoprotein blockers, verapamil, PSC833, and cremophor act differently on the multidrug resistance associated protein (MRP) and on P-glycoprotein (Pgp). Anticancer Res. 1999, 19, 1053–1064. [Google Scholar]
- Jouan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-glycoprotein inhibitory potential using a rhodamine 123 accumulation assay. Pharmaceutics 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of low-dose doxorubicin cytotoxicity by affecting p-glycoprotein through caryophyllane sesquiterpenes in hepg2 cells: An in vitro and in silico study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liang, X.-J. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin. J. Cancer 2012, 31, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Khdair, A.; Gerard, B.; Bachmeier, C.; Miller, D.W.; Shekhar, M.P.V.; Panyam, J. Surfactant-polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol. Pharm. 2007, 4, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Sadava, D.; Coleman, A.; Kane, S.E. Liposomal daunorubicin overcomes drug resistance in human breast, ovarian and lung carcinoma cells. J. Liposome Res. 2002, 12, 301–309. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Raposo, L.R.; Roma-Rodrigues, C.; Jesus, J.; Martins, L.M.D.R.S.; Pombeiro, A.J.; Baptista, P.V.; Fernandes, A.R. Targeting canine mammary tumours via gold nanoparticles functionalized with promising Co(II) and Zn(II) compounds. Vet. Comp. Oncol. 2017, 15, 1537–1542. [Google Scholar] [CrossRef]
- Trapani, A.; Denora, N.; Iacobellis, G.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Methotrexate-loaded chitosan- and glycolchitosan-based nanoparticles: A promising strategy for the administration of the anticancer drug to brain tumors. AAPS PharmSciTech 2011, 12, 1302–1311. [Google Scholar] [CrossRef]
- Sorenmo, K.; Samluk, M.; Clifford, C.; Baez, J.; Barrett, J.S.; Poppenga, R.; Overley, B.; Skorupski, K.; Oberthaler, K.; Van Winkle, T.; et al. Clinical and pharmacokinetic characteristics of intracavitary administration of pegylated liposomal encapsulated doxorubicin in dogs with splenic hemangiosarcoma. J. Vet. Intern. Med. 2007, 21, 1347–1354. [Google Scholar] [CrossRef]
- Reeds, K.B.; Higginbotham, M.L.; Mccaw, D.L.; Rachakatla, R.; Seo, G.M.; Pyle, M.; Goad, C.L.; Troyer, D.L. In vitro effects of canine wharton’s jelly mesenchymal stromal cells and micellar nanoparticles on canine osteosarcoma D17 cell viability. J. Vet. Sci. Technol. 2012, 3. [Google Scholar] [CrossRef]
- Ma, Y.; Manolache, S.; Denes, F.S.; Thamm, D.H.; Kurzman, I.D.; Vail, D.M. Plasma synthesis of carbon magnetic nanoparticles and immobilization of doxorubicin for targeted drug delivery. J. Biomater. Sci. Polym. Ed. 2004, 15, 1033–1049. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Steckiewicz, K.P.; Barcinska, E.; Sobczak, K.; Tomczyk, E.; Wojcik, M.; Inkielewicz-Stepniak, I. Assessment of anti-tumor potential and safety of application of glutathione stabilized gold nanoparticles conjugated with chemotherapeutics. Int. J. Med. Sci. 2020, 17, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Maiti, S.; Maity, M.; Mandal, C.; Maiti, N.C. Porphyrin-Gold Nanomaterial for Efficient Drug Delivery to Cancerous Cells. ACS Omega 2018, 3, 4602–4619. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef]
- Simpson, C.A.; Salleng, K.J.; Cliffel, D.E.; Feldheim, D.L. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 257–263. [Google Scholar] [CrossRef]
- Duan, Z.; Choy, E.; Jimeno, J.M.; Cuevas, C.D.M.; Mankin, H.J.; Hornicek, F.J. Diverse cross-resistance phenotype to ET-743 and PM00104 in multi-drug resistant cell lines. Cancer Chemother. Pharmacol. 2008, 63, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Mealey, K.L.; Barhoumi, R.; Rogers, K.; Kochevar, D.T. Doxorubicin induced expression of P-glycoprotein in a canine osteosarcoma cell line. Cancer Lett. 1998, 126, 187–192. [Google Scholar] [CrossRef]
- Cagliero, E.; Ferracini, R.; Morello, E.; Scotlandi, K.; Manara, M.C.; Buracco, P.; Comandone, A.; Baroetto Parisi, R.; Baldini, N. Reversal of multidrug-resistance using Valspodar® (PSC 833) and doxorubicin in osteosarcoma. Oncol. Rep. 2004, 12, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, A.; Sousa, E.; Fernandes, M.X.; Pinto, M.M.; Helena Vasconcelos, M. Multidrug resistance reversal effects of aminated thioxanthones and interaction with cytochrome P450 3A4. J. Pharm. Pharm. Sci. 2011, 15, 31–45. [Google Scholar] [CrossRef]
- Shahi, M.H.; York, D.; Gandour-Edwards, R.; Withers, S.S.; Holt, R.; Rebhun, R.B. BMI1 Is expressed in canine osteosarcoma and contributes to cell growth and chemotherapy resistance. PLoS ONE 2015, 10, e0131006. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Cho, H.J.; Lee, H.J.; Jin, H.E.; Maeng, H.J. Polyethylene glycol-decorated doxorubicin/carboxymethyl chitosan/gold nanocomplex for reducing drug efflux in cancer cells and extending circulation in blood stream. Int. J. Biol. Macromol. 2019, 125, 61–71. [Google Scholar] [CrossRef]
- Zabielska-Koczywąs, K.; Dolka, I.; Król, M.; Żbikowski, A.; Lewandowski, W.; Mieczkowski, J.; Wójcik, M.; Lechowski, R. Doxorubicin Conjugated to Glutathione Stabilized Gold Nanoparticles (Au-GSH-Dox) as an Effective Therapeutic Agent for Feline Injection-Site Sarcomas—Chick Embryo Chorioallantoic Membrane Study. Molecules 2017, 22, 253. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhang, J.; Shen, J.; Gao, Y.; Li, Y.; Choy, E.; Cote, G.; Harmon, D.; Mankin, H.; Gray, N.S.; et al. NVP-TAE684 reverses multidrug resistance (MDR) in human osteosarcoma by inhibiting P-glycoprotein (PGP1) function. Br. J. Pharmacol. 2016, 173, 613–626. [Google Scholar] [CrossRef]
- Yang, X.; Yang, P.; Shen, J.; Osaka, E.; Choy, E.; Cote, G.; Harmon, D.; Zhang, Z.; Mankin, H.; Hornicek, F.J.; et al. Prevention of multidrug resistance (MDR) in osteosarcoma by NSC23925. Br. J. Cancer 2014, 110, 2896–2904. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]






| Cell Line | IC50 Dox (μg/mL) | IC50 Au-GSH-Dox (μg/mL) |
|---|---|---|
| D17 | 15.2 | 7.9 |
| U2OS | 8.9 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małek, A.; Taciak, B.; Sobczak, K.; Grzelak, A.; Wójcik, M.; Mieczkowski, J.; Lechowski, R.; Zabielska-Koczywąs, K.A. Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma—In Vitro Studies. Molecules 2021, 26, 3487. https://doi.org/10.3390/molecules26123487
Małek A, Taciak B, Sobczak K, Grzelak A, Wójcik M, Mieczkowski J, Lechowski R, Zabielska-Koczywąs KA. Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma—In Vitro Studies. Molecules. 2021; 26(12):3487. https://doi.org/10.3390/molecules26123487
Chicago/Turabian StyleMałek, Anna, Bartłomiej Taciak, Katarzyna Sobczak, Agnieszka Grzelak, Michał Wójcik, Józef Mieczkowski, Roman Lechowski, and Katarzyna A. Zabielska-Koczywąs. 2021. "Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma—In Vitro Studies" Molecules 26, no. 12: 3487. https://doi.org/10.3390/molecules26123487
APA StyleMałek, A., Taciak, B., Sobczak, K., Grzelak, A., Wójcik, M., Mieczkowski, J., Lechowski, R., & Zabielska-Koczywąs, K. A. (2021). Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma—In Vitro Studies. Molecules, 26(12), 3487. https://doi.org/10.3390/molecules26123487







