Carbazole-Based Colorimetric Anion Sensors †
Abstract
1. Introduction
2. Results and Discussion
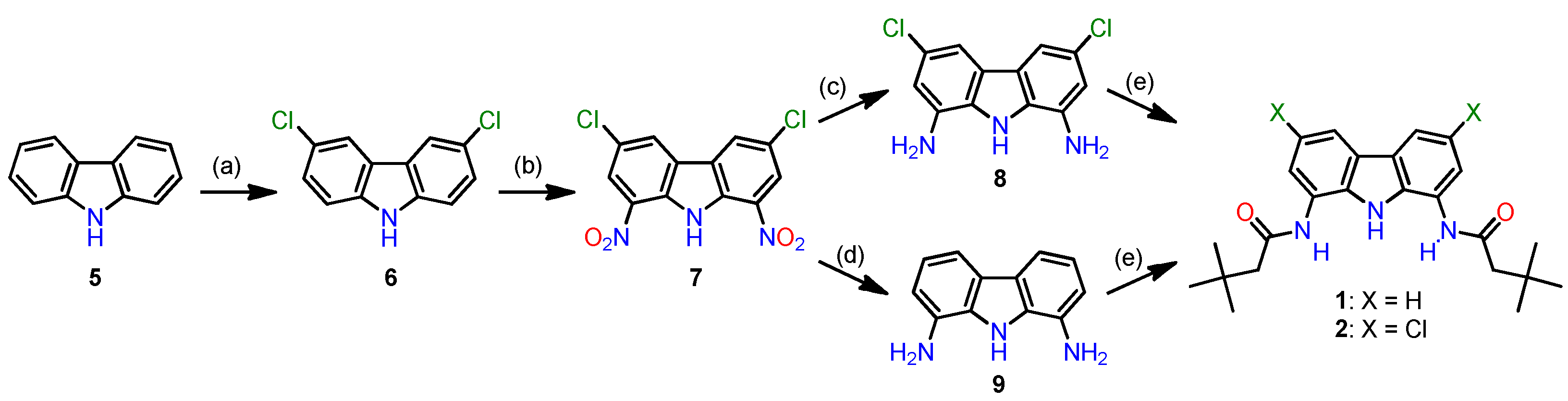
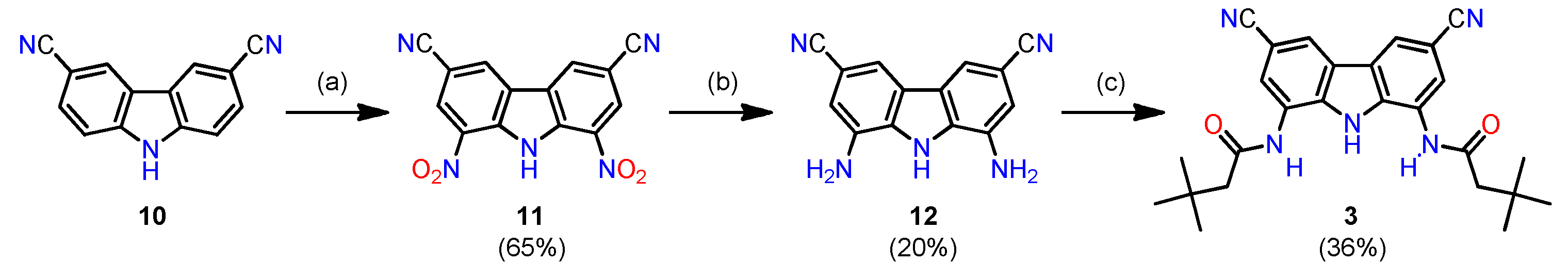
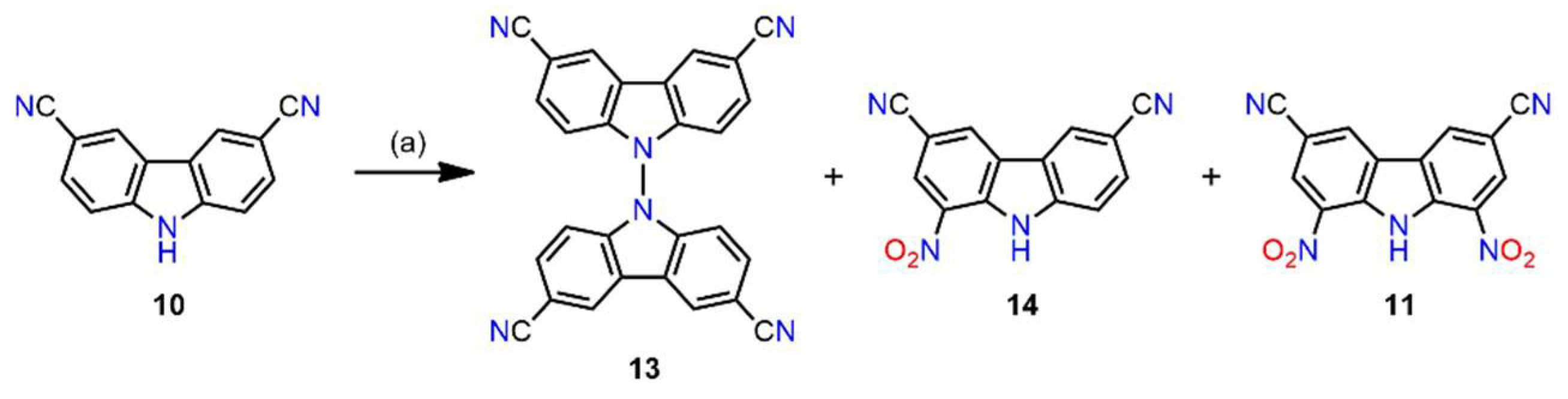
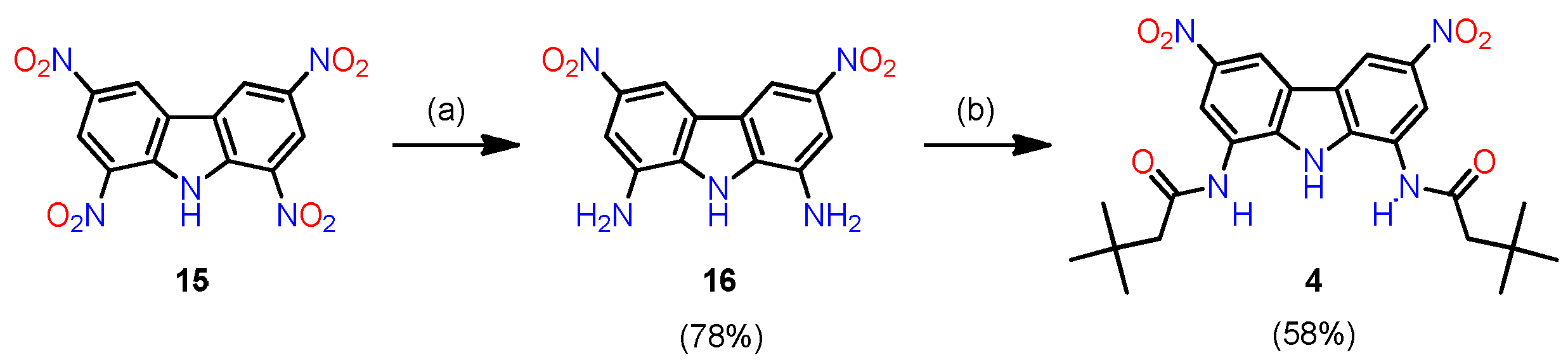
2.1. Synthesis
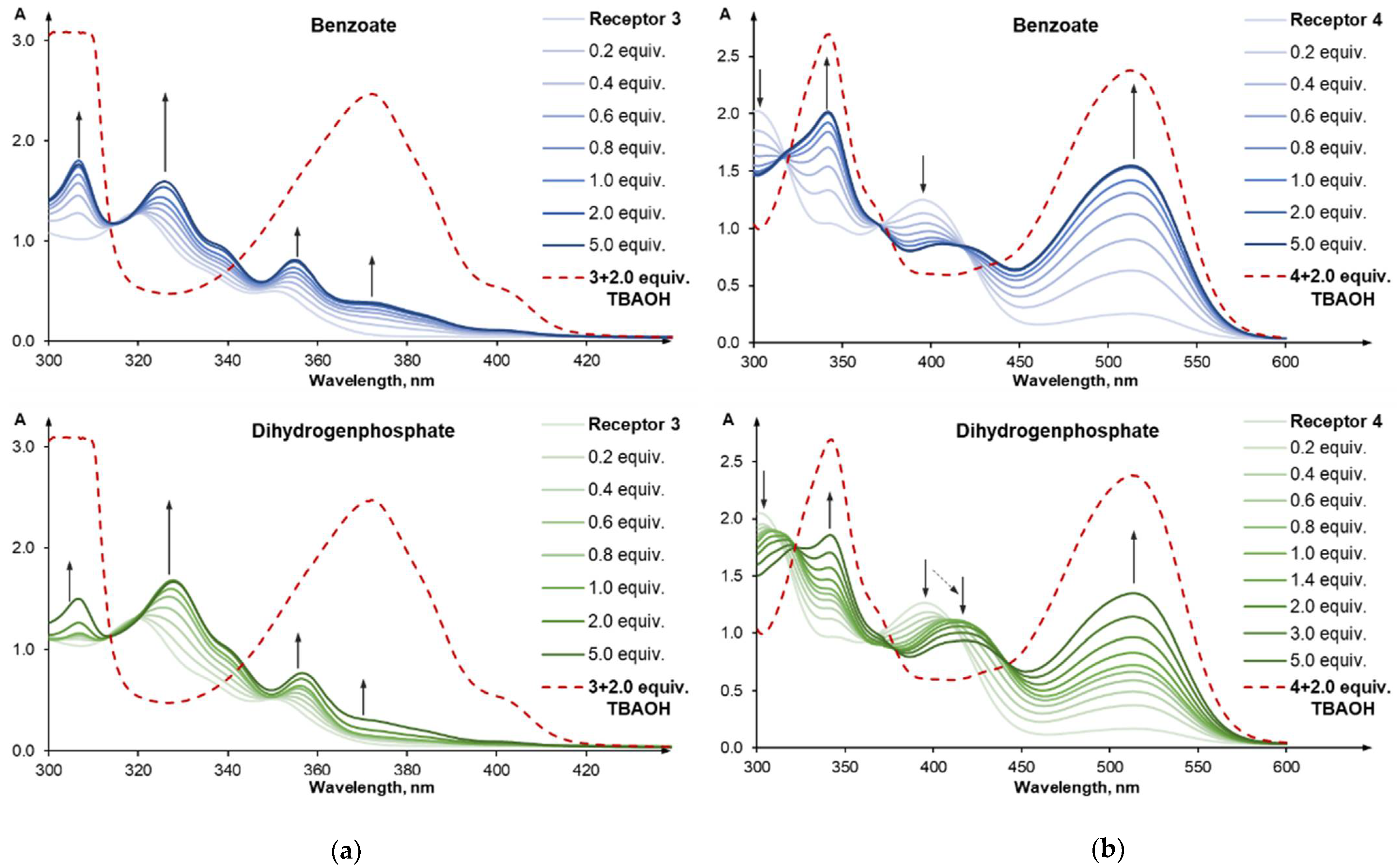
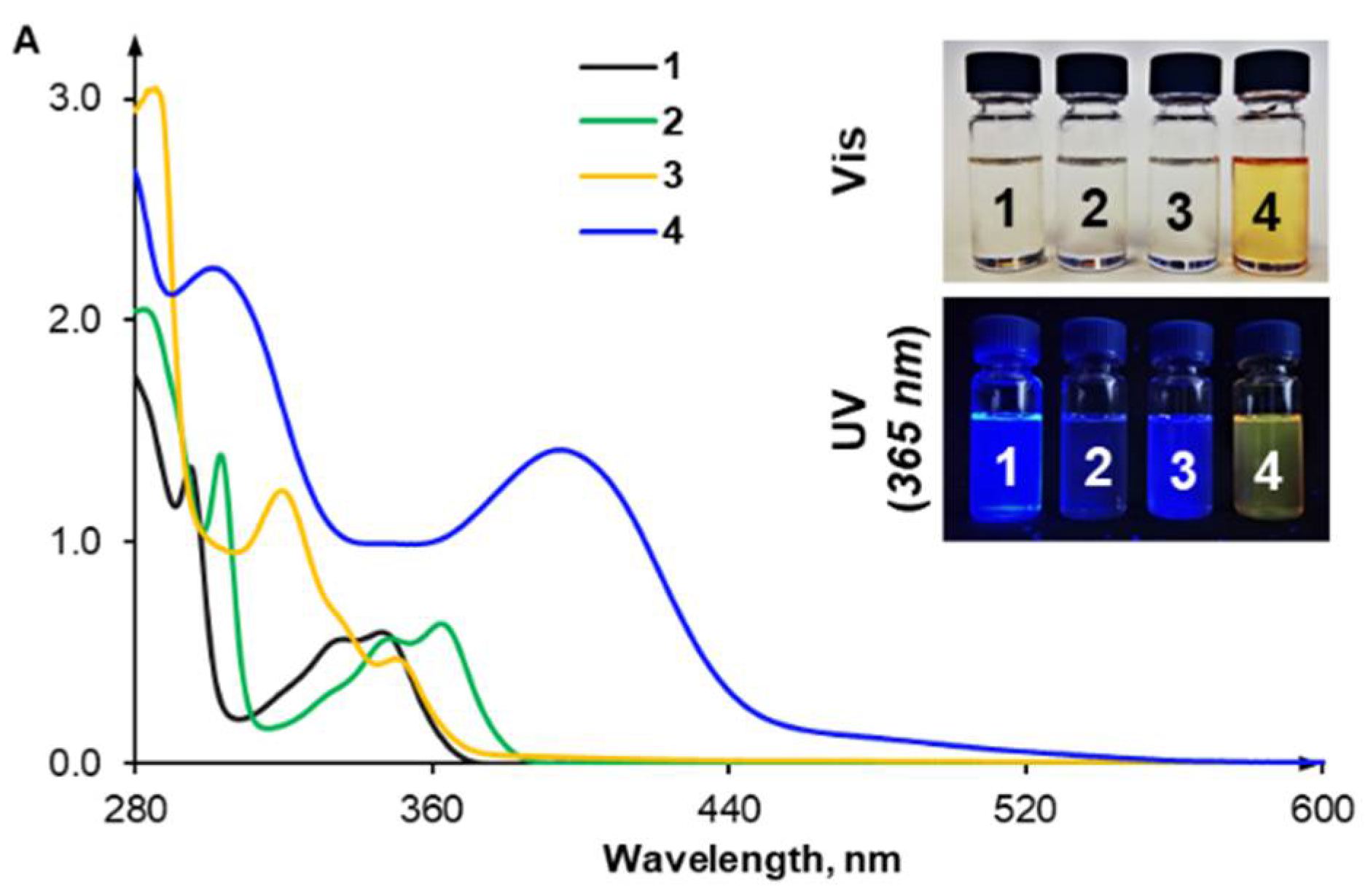
2.2. Anion Recognition Studies
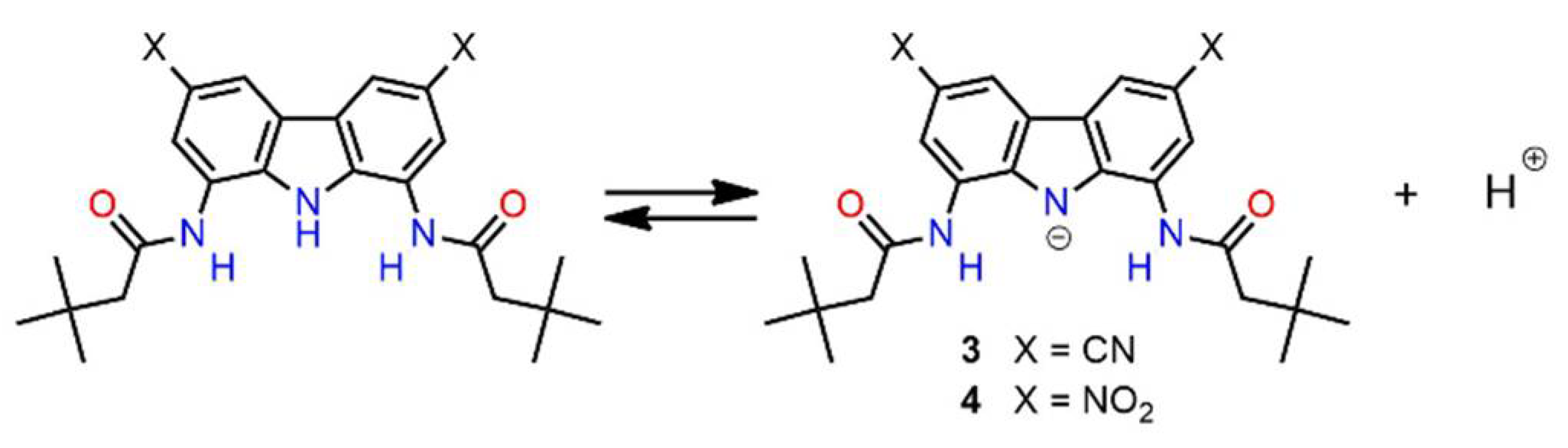
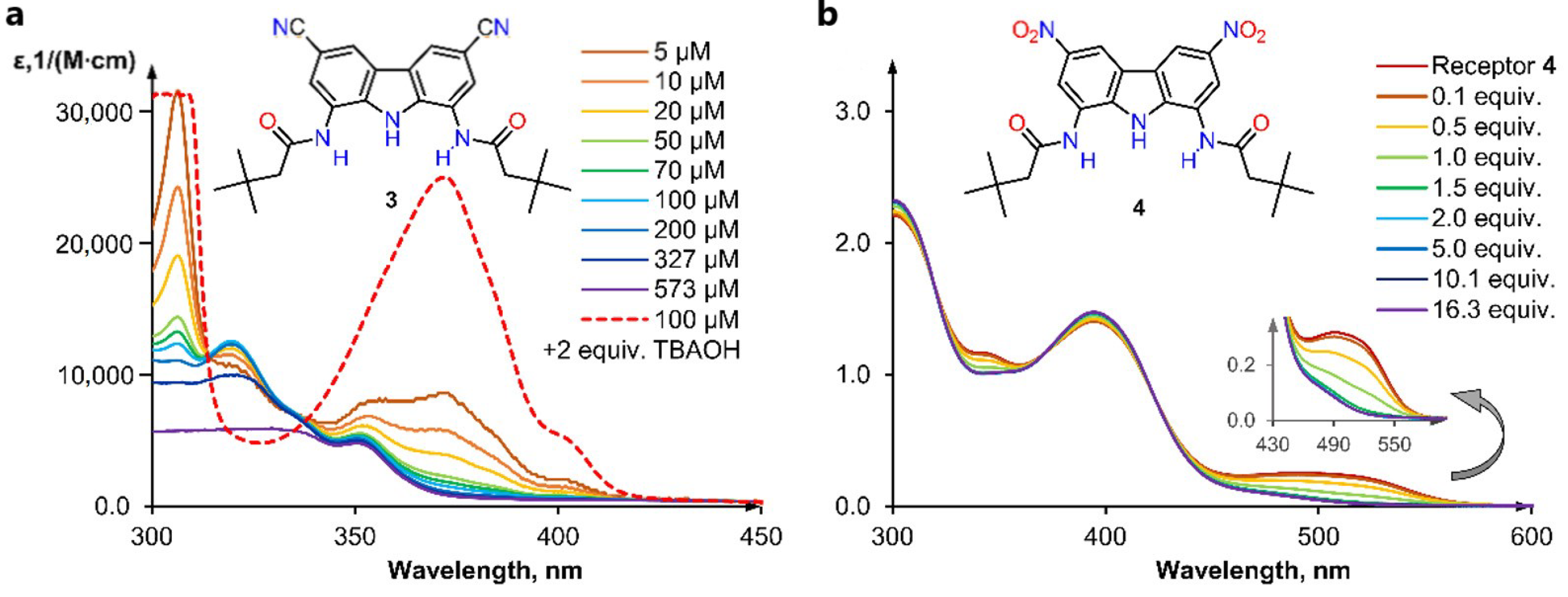
2.3. Self-Dissociation Studies
2.4. Anion Sensing Properties of 3 and 4
3. Materials and Methods
3.1. General Experimental
3.1.1. Chemicals and Consumables
- Merck-Sigma (Darmstadt, Germany): acetone (puriss. p.a., ≥99.5% (GC), 32201), acetonitrile (ACN; ≥99.9%, 34998), N,N-dimethylacetamide (DMA; anhydrous, 99.8%, 271012), 3,3-dimethylbutyryl chloride (tert-butylacetyl chloride; 99%, B88802), N,N-dimethylformamide (DMF; puriss. p.a., ACS reagent, reag. Ph. Eur., ≥99.8% (GC), 33120), dimethyl sulfoxide (DMSO; anhydrous, ≥99.9%, 276855), hydrazine monohydrate (N2H4·H2O, reagent grade, 98%, 207942), iron(II) sulfate heptahydrate (FeSO4·7H2O, ≥99.0%, 215422), methanol (MeOH; HPLC grade, ≥99.9%, 34860), nitric acid (fuming HNO3; extra pure, 100%, 1004551000), sodium hydroxide solution (2M NaOH, Titripur, 1091361000), tetrabutylammonium chloride (TBACl; ≥99.0%, 86852), tetrabutylammonium benzoate (TBAPhCOO; ≥99.0%, 86837), tetrabutylammonium phosphate monobasic (TBAH2PO4; ≥99.0% (T), 86833).
- POCH S.A. (Gliwice, Poland): acetic acid (AcOH; 99.5-99.9%, pure p.a.-basic, BA8760114), acetic anhydride (Ac2O; ACS reagent, pure p.a., 693870115), ethyl alcohol (EtOH; anhydrous, 99.8% pure p.a., 396480111), nitric acid (HNO3; 65%, pure p.a., BA9603115), hydrochloric acid (HCl; 35-38%, pure p.a., BA5283115).
- TCI (Zwijndrecht, Belgium): 1,3,6,8-tetranitrocarbazole (wetted with ca. 40% water, >70.0%, T0159).
- Alfa Aesar (Haverhill, MA, United States): trifluoromethanesulfonic acid (TfOH, anhydrous, 98%).
- Euriso-top (Saint-Aubin, France): DMSO-d6 + 0.03%TMS v/v (>99.8% D).
- Linegal Chemicals (Blizne Łaszczyńskiego, Poland): dichloromethane (DCM, pure p.a., 50-8124.4), freshly distilled over CaH2.
3.1.2. Instruments and Methods
3.2. Synthesis
3.2.1. General Methods
3.2.2. Synthesis of the previously-reported receptors 1 and 2
3.2.3. Synthesis of Receptor 3
3,6-Dicyano-1,8-dinitrocarbazole (11)
1,8-Diamino-3,6-dicyanocarbazole (12)
Receptor 3
3.2.4. Synthesis of Receptor 4
1,8-Diamino-3,6-dinitrocarbazole (16)
Receptor 4
3.3. Binding Studies
3.3.1. Typical Procedure for 1H NMR Titrations
3.3.2. Typical Procedure for UV-Vis Titrations
3.3.3. Data Fitting
3.4. Self-Dissociation Studies
3.5. Single Crystal X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busschaert, N.; Caltagirone, C.; VanRossom, W.; Gale, P.A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Kruger, P.E.; Jensen, P.; Tierney, J.; Ali, H.D.P.; Hussey, G.M. Colorimetric “Naked Eye” Sensing of Anions in Aqueous Solution. J. Org. Chem. 2005, 70, 10875–10878. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, J.R.; Caltagirone, C.; Light, M.E.; Hursthouse, M.B.; Gale, P.A. Fluorescent carbazolylurea anion receptors. Org. Biomol. Chem. 2009, 7, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Zapata, F.; Caballero, A.; White, N.G.; Claridge, T.D.W.; Costa, P.J.; Félix, V.; Beer, P.D. Fluorescent Charge-Assisted Halogen-Bonding Macrocyclic Halo-Imidazolium Receptors for Anion Recognition and Sensing in Aqueous Media. J. Am. Chem. Soc. 2012, 134, 11533–11541. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayah, M.H.; Abdalla, A.M.; Shehab, M.K. A dansyl-based optical probe for detection of singly and doubly charged anions. Supramol. Chem. 2016, 28, 224–230. [Google Scholar] [CrossRef]
- Casula, A.; Bazzicalupi, C.; Bettoschi, A.; Cadoni, E.; Coles, S.J.; Horton, P.N.; Isaia, F.; Lippolis, V.; Mapp, L.K.; Marini, G.M.; et al. Fluorescent asymmetric bis-ureas for pyrophosphate recognition in pure water. Dalton Trans. 2016, 45, 3078–3085. [Google Scholar] [CrossRef]
- Navarro-García, E.; Velasco, M.D.; Zapata, F.; Bauzá, A.; Frontera, A.; Ramírez de Arellano, C.; Caballero, A. Exploiting 1,4-naphthoquinone and 3-iodo-1,4-naphthoquinone motifs as anion binding sites by hydrogen or halogen-bonding interactions. Dalton Trans. 2019, 48, 11813–11821. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Fares, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- Chmielewski, M.J.; Charon, M.; Jurczak, J. 1,8-Diamino-3,6-dichlorocarbazole: A Promising Building Block for Anion Receptors. Org. Lett. 2004, 6, 3501–3504. [Google Scholar] [CrossRef]
- Piątek, P.; Lynch, V.M.; Sessler, L.J. Calix(4)pyrrole(2)carbazole: A New Kind of Expanded Calixpyrrole. J. Am. Chem. Soc. 2004, 126, 16073–16076. [Google Scholar] [CrossRef]
- Thangadurai, T.D.; Singh, N.J.; Hwang, I.-C.; Lee, J.W.; Chandran, R.P.; Kim, K.S. 2-Dimensional Analytic Approach for Anion Differentiation with Chromofluorogenic Receptors. J. Org. Chem. 2007, 72, 5461–5464. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.E.; Mikkilineni, V.; Lynch, V.M.; Sessler, J.L. Bis-amidopyrrolyl Receptors Based on Anthracene and Carbazole. Supramol Chem. 2010, 22, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, J.R.; Gale, P.A.; Caltagirone, C.; Hursthouse, M.B.; Light, M.E. Fluorescent carbazolylurea- and carbazolylthiourea-based anion receptors and sensors. Supramol. Chem. 2010, 22, 647–652. [Google Scholar] [CrossRef]
- Ahmed, N.; Geronimo, I.; Hwang, I.-C.; Singh, J.; Kim, K.S. cyclo-Bis(urea-3,6-dichlorocarbazole) as a Chromogenic and Fluorogenic Receptor for Anions and a Selective Sensor of Zinc and Copper Cations. Chem. Eur. J. 2011, 17, 8542–8548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, M.; Marshall, L.J.; de Mendoza, J. Hydrogen-Bonded Cyclic Tetramers Based on Ureidopyrimidinones Attached to a 3,6-Carbazolyl Spacer. Org. Lett. 2011, 13, 3186–3189. [Google Scholar] [CrossRef] [PubMed]
- Fuentes de Arriba, A.L.; Turiel, M.G.; Simón, L.; Sanz, F.; Boyero, J.F.; Muñiz, F.M.; Morána, J.R.; Alcázar, V. Sulfonamide carbazole receptors for anion recognition. Org. Biomol. Chem. 2011, 9, 8321–8327. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Espinosa, A.; Curiel, D.; Tarraga, A.; Molina, P. Bis(carbazolyl)ureas as Selective Receptors for the Recognition of Hydrogenpyrophosphate in Aqueous Media. J. Org. Chem. 2013, 78, 9725–9737. [Google Scholar] [CrossRef] [PubMed]
- Bąk, K.M.; Chmielewski, M.J. Sulfate templated assembly of neutral receptors in aqueous DMSO – orthogonal versus biplane structures. Chem. Commun. 2014, 50, 1305–1308. [Google Scholar] [CrossRef]
- Bąk, K.M.; Masłowska, K.; Chmielewski, M.J. Selective turn-on fluorescence sensing of sulfate in aqueous–organic mixtures by an uncharged bis(diamidocarbazole) receptor. Org. Biomol. Chem. 2017, 15, 5968–5975. [Google Scholar] [CrossRef]
- Martin, K.; Nõges, J.; Haav, K.; Kadam, S.A.; Pung, A.; Leito, I. Exploring Selectivity of 22 Acyclic Urea-, Carbazole- and Indolocarbazole- Based Receptors towards 11 Monocarboxylates. Eur. J. Org. Chem. 2017, 35, 5231–5237. [Google Scholar] [CrossRef]
- Bąk, K.M.; Chabuda, K.; Montes, H.; Quesada, R.; Chmielewski, M.J. 1,8-Diamidocarbazoles: An easily tuneable family of fluorescent anion sensors and transporters. Org. Biomol. Chem. 2018, 16, 5188–5196. [Google Scholar] [CrossRef]
- Rüütel, A.; Yrjänä, V.; Kadam, S.A.; Saar, I.; Ilisson, M.; Darnell, A.; Haav, K.; Haljasorg, T.; Toom, L.; Bobacka, J.; et al. Design, synthesis and application of carbazole macrocycles in anion sensors. Beilstein J. Org. Chem. 2020, 16, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Yrjänä, V.; Saar, I.; Ilisson, M.; Kadam, S.A.; Leito, I.; Bobacka, J. Potentiometric Carboxylate Sensors Based on Carbazole-Derived Acyclic and Macrocyclic Ionophores. Chemosensors 2021, 9, 4. [Google Scholar] [CrossRef]
- Bąk, K.M.; Kolck, B.v.; Maslowska-Jarzyna, K.; Papadopoulou, P.; Kros, A.; Chmielewski, M.J. Oxyanion transport across lipid bilayers: Direct measurements in large and giant unilamellar vesicles. Chem. Commun. 2020, 56, 4910–4913. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, R.; García-Valverde, M.; Quesada, R.; Chmielewski, M.J. Transmembrane anion transport promoted by thioamides. RSC Advances 2021, in press. [Google Scholar] [CrossRef]
- Muzık, F.; Allan, Z.; Poskocil, J. Derivate des Carbazols IV. Herstellung von 3,6-Dichlor-1,8-diaminocarbazol. Collect. Czech. Chem. Commun. 1958, 23, 770–772. [Google Scholar] [CrossRef]
- Fedorczyk, A.; Pomorski, R.; Chmielewski, M.J.; Ratajczak, J.; Kaszkur, Z.; Skompska, M. Bimetallic Au@Pt nanoparticles dispersed in conducting polymer—A catalyst of enhanced activity towards formic acid electrooxidation. Electrochim. Acta 2017, 246, 1029–1041. [Google Scholar] [CrossRef]
- Weselinski, L.J.; Luebke, R.; Eddaoudi, M. A Convenient Preparation of 9H-Carbazole-3,6-dicarbonitrile and 9H-Carbazole-3,6-dicarboxylic Acid. Synthesis 2014, 46, 596–599. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Sanchirico, R. Thermal decomposition of acetic anhydride–nitric acid mixtures. J. Hazard. Mater. 2002, 90, 111–121. [Google Scholar] [CrossRef]
- Sharma, U.; Verma, P.K.; Kumar, N.; Kumar, V.; Bala, M.; Singh, B. Phosphane-Free Green Protocol for Selective Nitro Reduction with an Iron-Based Catalyst. Chem. Eur. J. 2011, 17, 5903–5907. [Google Scholar] [CrossRef]
- Bindfit-Fit Data to 1:1 Host-Guest Equilibria. Available online: http://supramolecular.org (accessed on 16 March 2021).
- CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Yarnton, UK, 2008.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Zhanga, Z.; Schreiner, P.R. (Thio)urea organocatalysis—What can be learnt from anion recognition? Chem. Soc. Rev. 2009, 38, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Elmes, R.B.P.; Busschaert, N.; Czech, D.D.; Gale, P.A.; Jolliffe, K.A. pH switchable anion transport by an oxothiosquaramide. Chem. Commun. 2015, 51, 10107–10110. [Google Scholar] [CrossRef]
- Saha, A.; Akhtar, N.; Kumar, V.; Kumar, S.; Srivastava, H.K.; Kumarb, S.; Manna, D. pH-Regulated anion transport activities of bis (iminourea) derivatives across the cell and vesicle membrane. Org. Biomol. Chem. 2019, 17, 5779–5788. [Google Scholar] [CrossRef]
- Howe, E.N.W.; Busschaert, N.; Wu, X.; Berry, S.N.; Ho, J.; Light, M.E.; Czech, D.D.; Klein, H.A.; Kitchen, J.A.; Gale, P.A. pH-Regulated Nonelectrogenic Anion Transport by Phenylthiosemicarbazones. J. Am. Chem. Soc. 2016, 138, 8301–8308. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Elmes, R.B.P.; Czech, D.D.; Wu, X.; Kirby, I.L.; Peck, E.M.; Hendzel, K.D.; Shaw, S.K.; Chan, B.; Smith, B.D.; et al. Thiosquaramides: pH switchable anion transporters. Chem. Sci. 2014, 5, 3617–3626. [Google Scholar] [CrossRef]
- Maslowska-Jarzyna, K.; Korczak, M.L.; Chmielewski, M.J. Boosting anion transport activity of diamidocarbazoles by electron withdrawing substituents. Front. Chem. 2021, 9, 690035. [Google Scholar] [CrossRef]
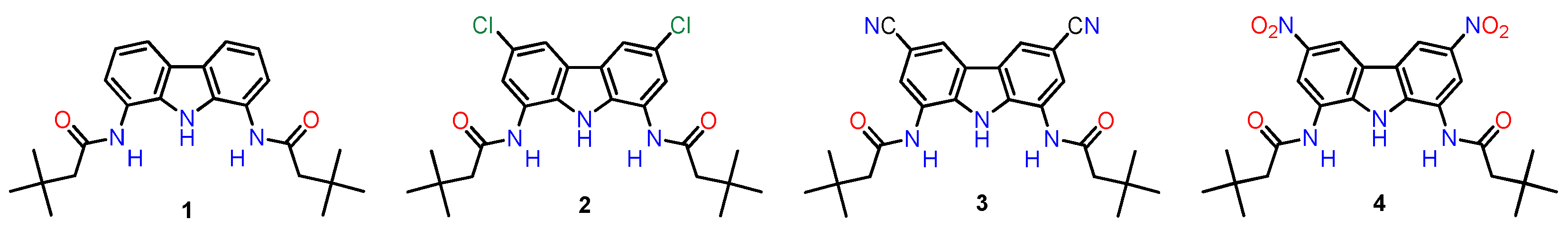




| Parameter | Atom | 2 × Cl− | 3 × Cl− | 4 × Cl− |
|---|---|---|---|---|
| Bond length, Å | Cl−∙∙∙NHcarb. | 2.223 | 2.198 | 2.170 |
| Cl−∙∙∙NHamide1 | 2.567 | 2.563 | 2.533 | |
| Cl−∙∙∙NHamide2 | 2.620 | 2.569 | 2.595 | |
| Cl−∙∙∙CHt-Bu | 2.850 | 2.910 | 2.878 | |
| Cl−∙∙∙Ph4P+ | 2.771 | 2.835 | 2.778 | |
| Torsion angle φ, ˚ | C2-C1-Namide1-Camide1 | 6.83 | 3.80 | 5.00 |
| C7-C8-Namide2-Camide2 | 0.23 | −5.22 | 0.38 | |
| Torsion angle φ, ˚ | C9a-C1-Namide1-Camide1 | −175.26 | −178.32 | −176.94 |
| C8a-C8-Namide2-Camide2 | −178.76 | 177.43 | 174.60 |
| Receptor | Ka Cl− | Ka PhCOO− | Ka H2PO4− | Ka CH3COO− |
|---|---|---|---|---|
| 1 | 48 [a] | 103.67 [a] | 104.01 [a] | 104.07 [a] |
| 2 | 159 [a] | 104.47 [a] | 104.91 [a] | 104.95 [a] |
| 3 | 309 | deprotonation | 105.4 * | n.d. |
| 4 | 347 | deprotonation | deprotonation | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslowska-Jarzyna, K.; Korczak, M.L.; Wagner, J.A.; Chmielewski, M.J. Carbazole-Based Colorimetric Anion Sensors. Molecules 2021, 26, 3205. https://doi.org/10.3390/molecules26113205
Maslowska-Jarzyna K, Korczak ML, Wagner JA, Chmielewski MJ. Carbazole-Based Colorimetric Anion Sensors. Molecules. 2021; 26(11):3205. https://doi.org/10.3390/molecules26113205
Chicago/Turabian StyleMaslowska-Jarzyna, Krystyna, Maria L. Korczak, Jakub A. Wagner, and Michał J. Chmielewski. 2021. "Carbazole-Based Colorimetric Anion Sensors" Molecules 26, no. 11: 3205. https://doi.org/10.3390/molecules26113205
APA StyleMaslowska-Jarzyna, K., Korczak, M. L., Wagner, J. A., & Chmielewski, M. J. (2021). Carbazole-Based Colorimetric Anion Sensors. Molecules, 26(11), 3205. https://doi.org/10.3390/molecules26113205






