Ameliorative Potential of Hydroethanolic Leaf Extract of Parquetina nigrescens on d-Galactose-Induced Testicular Injury
Abstract
1. Introduction
2. Results
2.1. Chemical Constituents of HELEPN
2.2. Effect of Graded Doses of HELEPN on Body Weight Gain, Paired Testicular Weight, and Relative Testicular Weight
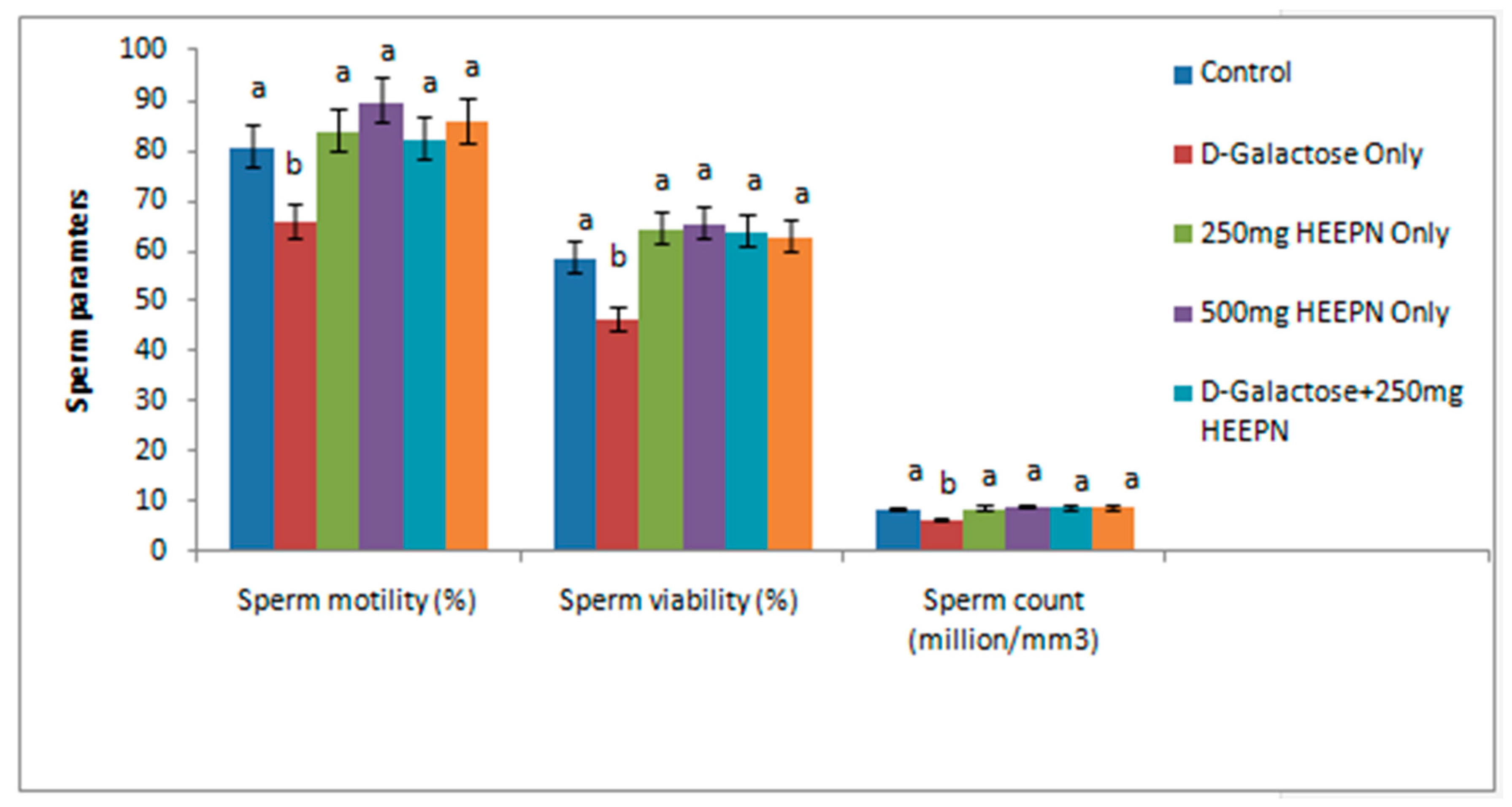
2.3. Effect of Graded Doses of HELEPN on Sperm Analysis and Morphology
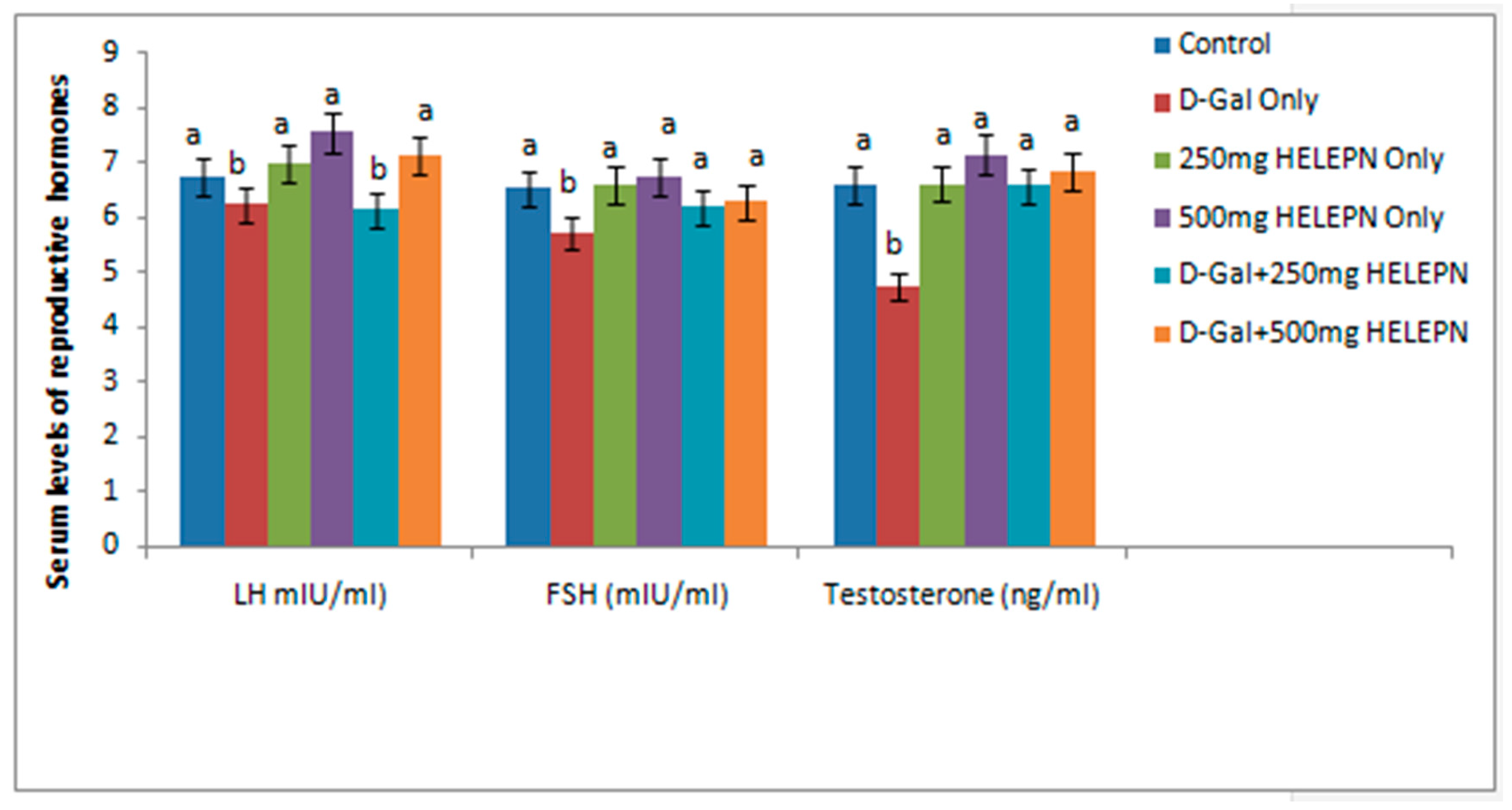
2.4. Effect of Graded Doses of HELEPN on Reproductive Hormones
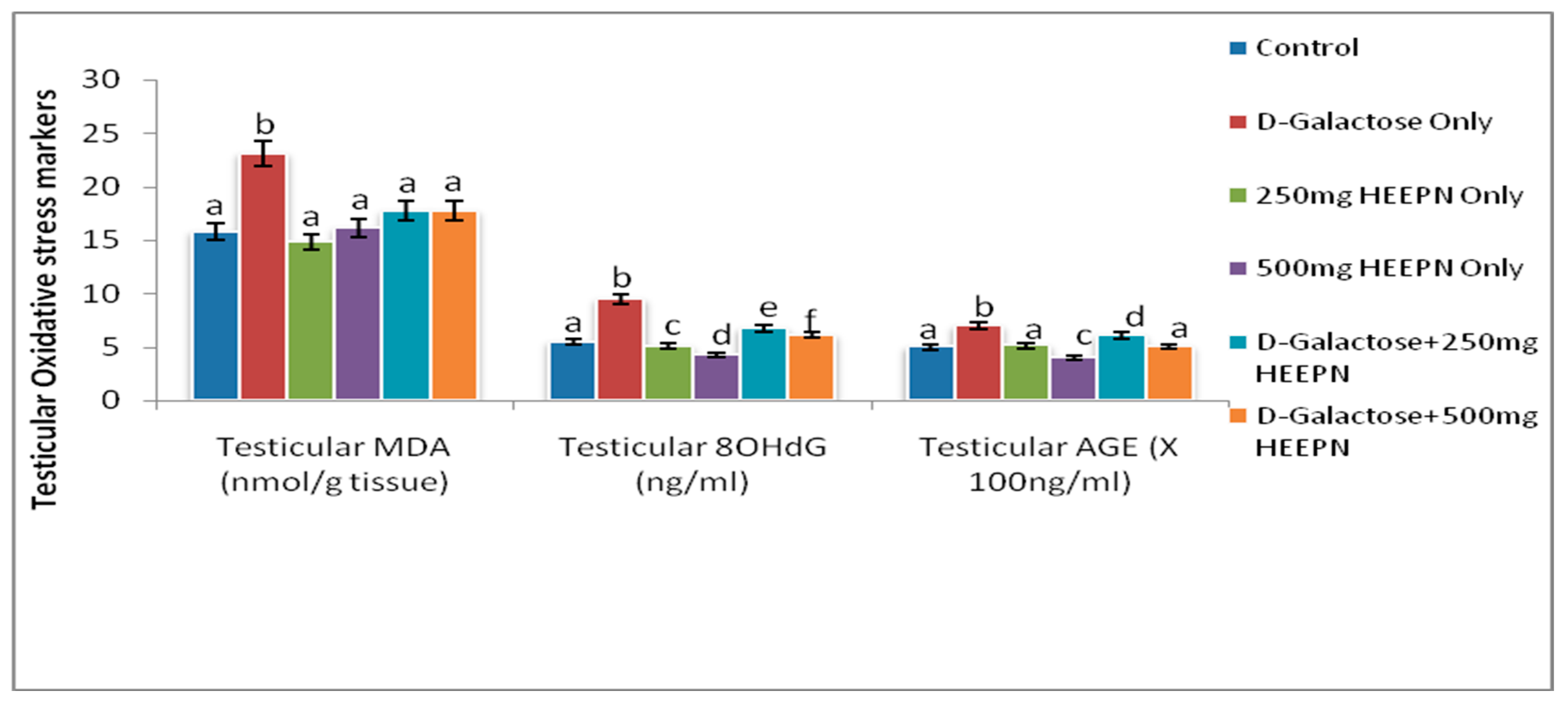
2.5. Effect of Graded Doses of HELEPN on Oxidative Stress Biomarkers
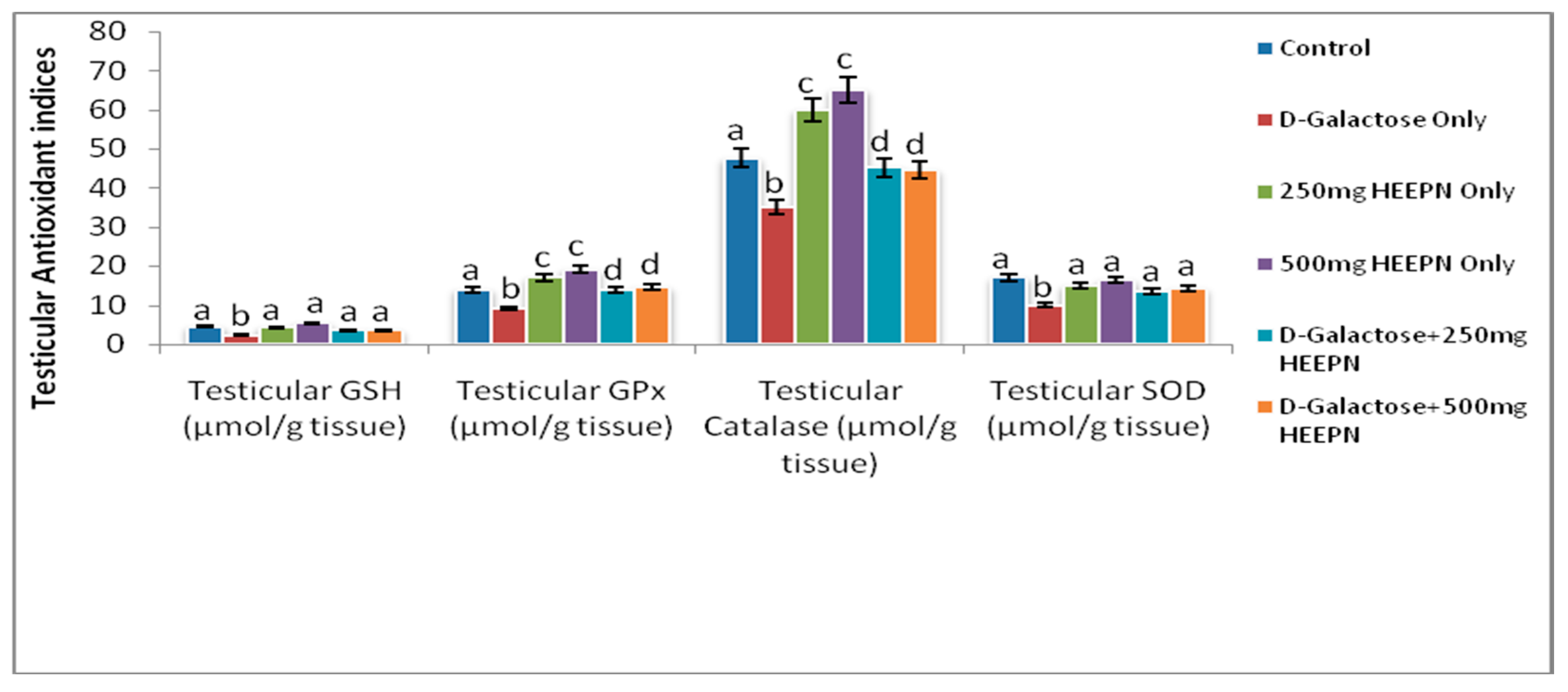
2.6. Effect of Graded Doses of HELEPN on Antioxidant Indices
2.7. Effect of Graded Doses of HELEPN on Inflammatory Biomarkers
2.8. Effect of Graded Doses of HELEPN on Apoptotic Biomarkers
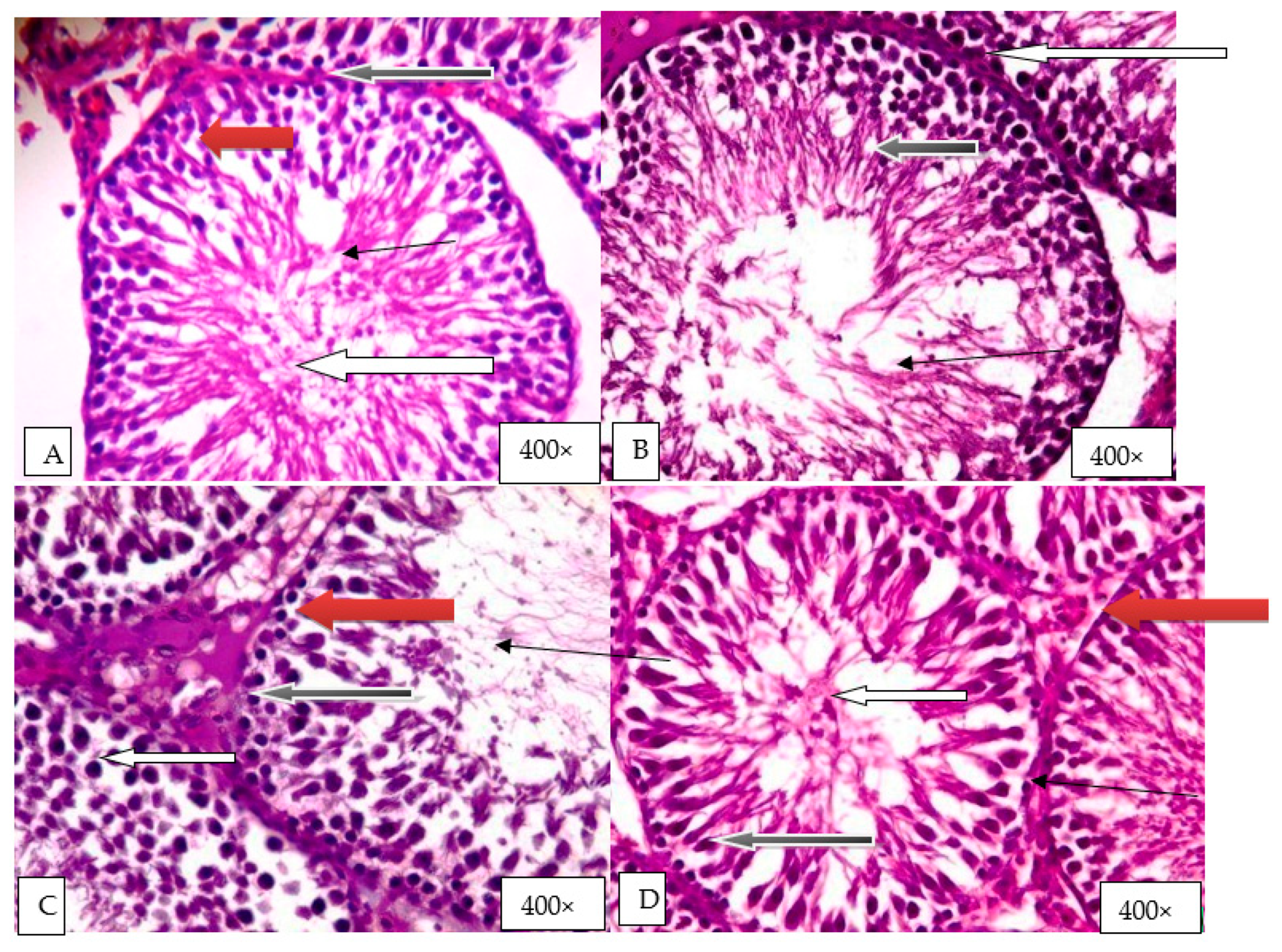
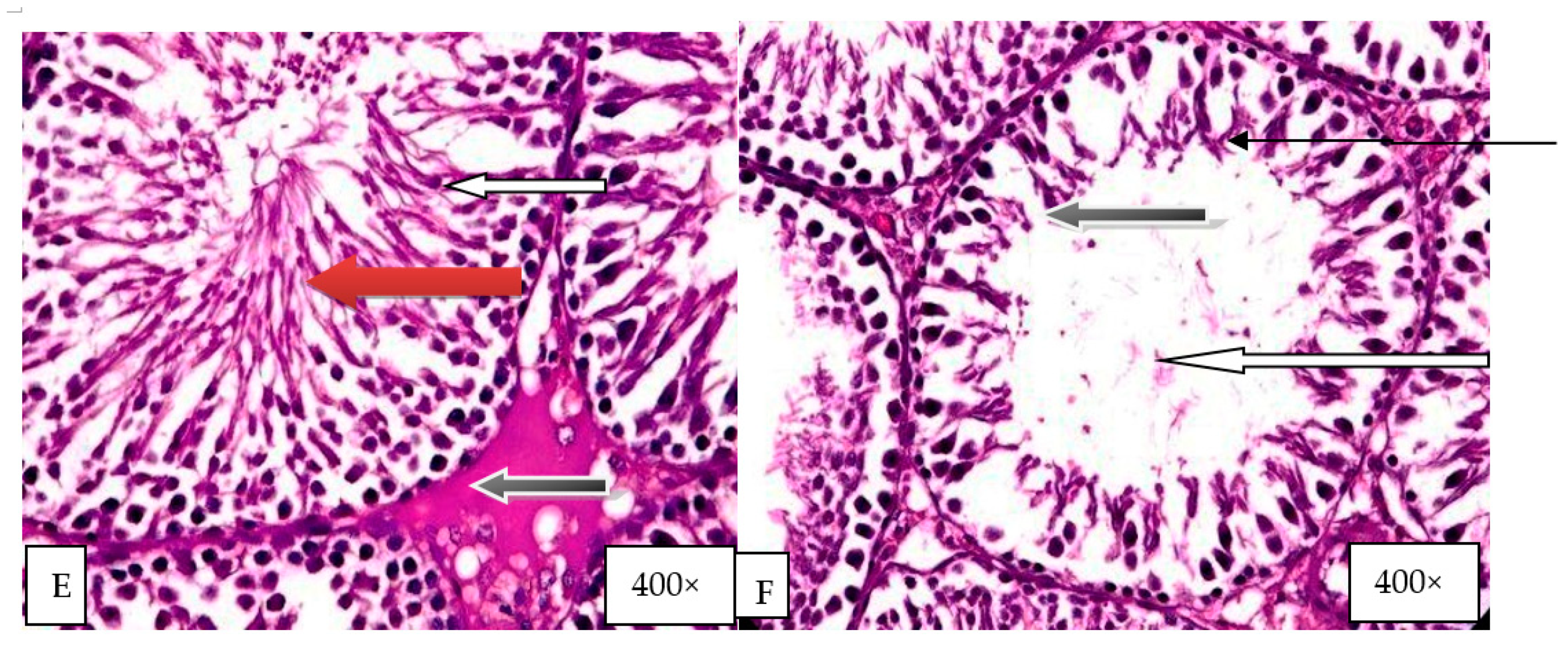
2.9. Histological Evaluation of the Effect of Graded Doses of HELEPN on the Testis
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Preparation of Plant Extract
4.3. Phytochemical Analysis
4.4. Experimental Animals
4.5. Experimental Design
4.6. Blood and Tissue Sample Collection
4.7. Antioxidant Indices
4.8. Oxidative Stress Biomarkers
4.9. Apoptotic Biomarkers
4.10. Inflammatory Biomarkers
4.11. DNA Fragmentation Index Assay (DFI)
4.12. Reproductive Hormones
4.13. Sperm Parameters
4.14. Histology
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| ANOVA | analysis of variance |
| SOD | superoxide Dismutase |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| GPx | glutathione peroxidase |
| HELEPN | hydroethanolic extract of Parquetina nigrescens |
| CAT | Catalase |
| DNA | deoxyribonucleic acid |
| LH | leutinizing hormone |
| FSH | follicle-stimulating hormone |
| ROS | reactive oxygen specie |
| AGEs | advanced glycation end products |
| 8-OHdG | 8 hydroxydeoxyguanosine |
| SPSS | Statistical Package for Social Sciences |
| NO | nitric oxide |
| DFI | DNA fragmentation index |
| TNF | tumor necrosis factor |
| HPLC | high-performance liquid chromatography |
| ELISA | enzyme-linked immunosorbent assay |
References
- Saba, A.B.; Oyagbemi, A.A.; Azeez, O.I. Antidiabetic and haematinic effects of Parquetina nigrescenson alloxan induced type-1 diabetes and normocytic normochromic anaemia in Wistar rats. Afr. Health Sci. 2010, 10, 276–282. [Google Scholar]
- Erinoso, S.M.; Aworinde, D.O. Ethnobotanical survey of some medicinal plants used in traditional health care in Abeokuta areas of Ogun State, Nigeria. Afr. J. Pharm. Pharmacol. 2012, 6, 1352–1362. [Google Scholar] [CrossRef]
- Kayode, O.T.; Yakubu, M.T. Parquetina nigrescens leaves: Chemical profile and influence on the physical and biochemical indices of sexual activity of male Wistar rats. J. Integr. Med. 2017, 15, 64–76. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Oyelowo, O.T.; Biliaminu, S.A.; Alaran, O.N.; Alimi, S.A.; Saliu, R.S. Hematological and biochemical studies on Parquetina nigrescens root extract in albino rats. J. Appl. Pharm. Sci. 2011, 1, 176. [Google Scholar]
- Kokwaro, J.O. Medicinal Plants of East Africa; East African literature Bureau: Nairobi, Kenya, 1976; p. 45. [Google Scholar]
- Odetola, A.A.; Oluwole, F.S.; Adeniyi, B.A.; Olatiregun, A.M.; Ikupolowo, O.R.; Busari, K.O.; Shorinola, J.A. Antimicrobial and gastrointestinal protective properties of Parquetina nigrescens (Afzel.) Bullock. J. Biol. Sci. 2006, 6, 701–705. [Google Scholar]
- Adu-Amoah, L. Antimicrobial and Toxicity Studies of Erythrophleum ivorense (Leguminoseae) and Parquetina nigrescens (Ascelpiadaceae). Ph.D. Thesis, Kwame Nkrumah University of science and Technology, Kumasi, Ghana, 2014. [Google Scholar]
- Ayeleso, A.O.; Oguntibeju, O.O.; Brooks, N.L. In vitro study on the antioxidant potentials of the leaves and fruits of Nauclea latifolia. Sci. World J. 2014, 2014, 437081. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, A.O.; Akinloye, O.; Oguntibeju, O.O.; Oke, J.M.; Odetola, A.A. Antioxidant activities of Parquetina nigrescens. Afr. J. Biotechnol. 2011, 10, 4920–4925. [Google Scholar]
- Cudicini, C.; Lejeune, H.; Gomez, E.; Bosmans, E.; Ballet, F.; Sáez, J.; Jégou, B. Human Leydig Cells and Sertoli Cells Are Producers of Interleukins-1 and -6 1. J. Clin. Endocrinol. Metab. 1997, 82, 1426–1433. [Google Scholar] [CrossRef]
- García-Ferreyra, J. New Discovery in Embryology (Sperm DNA Fragmentation and Its Relation with Fertility); IntechOpen Limited: London, UK, 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Bennetts, L.E.; Aitken, R.J. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol. Reprod. Dev. 2005, 71, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Lysiak, J.J. Oxidative Stress: A Common Factor in Testicular Dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, R.E.; Ajayi, A.F. Antispermatogenic mechanism of trona is associated with lipid peroxidation but not testosterone suppression. J. Hum. Reprod. Sci. 2017, 10, 124–127. [Google Scholar] [CrossRef]
- Said, T.M.; Paasch, U.; Glander, H.; Agarwal, A. Role of caspases in male infertility. Hum. Reprod. Update 2004, 10, 39–51. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins Reversed d-galactose-Induced Oxidative Stressand Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hou, C.-L.; Mu, X.-P.; Sun, C.; Zhu, Y.-C.; Wang, M.-J.; Lv, Q.-Z. H2S Donor NaHS Changes the Production of Endogenous H2S and NO in d-galactose-Induced Accelerated Ageing. Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bo-Htay, C.; Palee, S.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Effects of d-galactose-induced ageing on the heart and its potential interventions. J. Cell. Mol. Med. 2018, 22, 1392–1410. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end productsin cardiovascular disease. World J. Cardiol. 2012, 4, 90. [Google Scholar] [CrossRef]
- Nsiah, k.; Terlabi, E.O.; Woode, E.; Obiri, D.D.; Ansah, C.; Duwiejua, M. Toxicological assessment of Parquetina nigrescence extracts in rats. J. Sci. Technol. 2003, 26, 24–31. [Google Scholar]
- Cardoso, A.; Magano, S.; Marrana, F.; Andrade, J.P. d-galactose High-Dose Administration Failed to Induce Accelerated Aging Changes in Neurogenesis, Anxiety, and Spatial Memory on Young Male Wistar Rats. Rejuvenation Res. 2015, 18, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, M.; Kamada, M.; Irahara, M.; Yamamoto, S.; Yoshikawa, S.; Kasai, Y.; Ohmoto, Y.; Gima, H.; Thaler, C.J.; Aono, T. A repertoire of cytokines in human seminal plasma. J. Reprod. Immunol. 2002, 54, 33–42. [Google Scholar] [CrossRef]
- Hassan, H.S.; Sule, M.I.; Musa, A.M.; Musa, K.Y.; Abubakar, M.S.; Hassan, A.S. Anti-Inflammatory Activity of Crude Saponin Extracts from Five Nigerian Medicinal Plants. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 250–255. [Google Scholar] [CrossRef]
- Fakurazi, S.; Adewoyin, M.; Mohsin, S.M.N.; Arulselvan, P.; Hussein, M.Z. Enhanced anti-inflammatory potential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. Drug Des. Dev. Ther. 2015, 9, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.P.; Huang, B.M.; Tsai, H.C.; Lui, M.C.; Liu, M.Y. Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryonic development. Arch. Androl. 2004, 50, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Hamada, A.; Esteves, S.C. Insight into oxidative stress in varicocele-associated male infertility: Part 1. Nat. Rev. Urol. 2012, 9, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Kutchukian, P.S.; Musah, R.A.; Wink, D.A.; Grisham, M.B. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem. 2001, 276, 28799–28805. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.B.; De Lamirande, E.; Gagnon, C. Tyrosine nitration in human spermatozoa: A physiological function of peroxynitrite, the reaction product of nitric oxide and superoxide. Mol. Hum. Reprod. 2001, 7, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, T.; Wink, M. Utilization of Alkaloids in Modern Medicine. In Alkaloids; Springer: Berlin/Heidelberg, Germany, 1998; pp. 435–459. [Google Scholar]
- Szczawinska, K.; Bobkiewicz, K.T.; Kozaryn, K.; Peretiatkowicz, M.; Gulewicz, K. Some pharmacological properties of an extract from bitter Lupin (L. angustifolius) seeds. In Advances in Lupin Research, Proceeding of the VIIth International Lupin Conference, Evora, Portugal, 18–23 April 1993; Neves-Martin, J.M., Beirão da Costa, M.L., Eds.; Instituto Superior de Agronomia: Lisbon, Portugal, 1994; pp. 297–300. [Google Scholar]
- Akhigbe, R.; Ajayi, A. Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities. PLoS ONE 2020, 15, e0224052. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2016, 16, 123–129. [Google Scholar]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yuan, Y.; Sun, Y.; Qin, Y.; Deng, Z.; Li, H. Protective Effects of Selenium, Vitamin E, and Purple Carrot Anthocyanins on d-galactose-Induced Oxidative Damage in Blood, Liver, Heart and Kidney Rats. Biol. Trace Elem. Res. 2016, 173, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Vernet, P.; Aitken, R.; Drevet, J. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Pant, N.; Srivastava, S.C.; Kumar, R. Effect of dermal application of hexachlorocyclohexane (HCH) on male reproductive system of rat. Hum. Exp. Toxicol. 1995, 14, 484–488. [Google Scholar] [CrossRef]
- Peltola, V.; Huhtaniemi, I.; Ahotupa, M. Antioxidant enzyme activity in the maturing rat testis. J. Androl. 1992, 13, 450–455. [Google Scholar] [PubMed]
- Akinrinmade, F.J.; Akinleye, S.A.; Olubisi, O.S. Ademola, A.O. Antioxidant Potential of the Methanol Extract of Parquetina nigrescens Mediates Protection against Intestinal Ischemia-Reperfusion Injury in Rats. J.Diet. Suppl. 2015, 13, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Ain, Q.U.; Ullah, H. Therapeutic effects of quercetin against bisphenol A induced testicular damage in male Sprague Dawley rats. Syst. Biol. Reprod. Med. 2016, 62, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Afsar, T.; Razak, S.; Khan, M.R.; Almajwal, A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Oehniger, S.; Franken, D.R.; Ombelet, W. Sperm functional test. FertilSteril. 2014, 102, 1528–1533. [Google Scholar]
- Oeniger, S. Clinical management of male infertility in assisted reproducrion: ICSI and beyond. Int. J. Androl. 2011, 34, e319–e329. [Google Scholar] [CrossRef]
- Elangovan, N.; Chiou, T.-J.; Tzeng, W.-F.; Chu, S.-T. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology 2006, 222, 60–70. [Google Scholar] [CrossRef]
- Cherry, B.; Siripong, P.; Nattayaporn, A.; Siriporn, C.; Nipon, C. Effects of d-galactose induced ageing on the heart and its potential interventions. J. Cell Mol. Med. 2018, 22, 1392–1410. [Google Scholar]
- Gupta, R.S.; Kim, J.; Gomes, C.; Oh, S.; Park, J.; Im, W.-B.; Seong, J.Y.; Ahn, R.S.; Kwon, H.-B.; Soh, J. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol. Cell. Endocrinol. 2004, 221, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Creagh, E.M.; Martin, S.J. Caspases: Cellular demolition experts. Biochem. Soc. Trans. 2001, 29, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-mediated apoptosis. Ann. Rev. Biochem. 2004, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Zribi, N.; Chakroun, N.F.; Elleuch, H.; Ben Abdallah, F.; Ben Hamida, A.S.; Gargouri, J.; Fakhfakh, F.; Keskes, L.A. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod. Biol. Endocrinol. 2011, 9, 47. [Google Scholar] [CrossRef]
- Cui, J.; Holmes, E.H.; Greene, T.G.; Liu, P.K. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000, 14, 955–967. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Sharma, V.; Dixit, V.K.; Thakur, M. A Review on Plants Used for Improvement of Sexual Performance and Virility. BioMed. Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T.; Adesokan, A.A. Androgenic potentials of aqeous extract of Massularia acuminate (G.Don) Bullock ex Holy. Stem in male wistar rats. J. Ethnopharmacol. 2008, 118, 508–513. [Google Scholar] [CrossRef]
- Dua, A.; Gaurav, G.; Balkar, S.; Mahajan, R. Antimicrobial properties of methanolic extract of cumin (Cuminum cyminum) seeds. Int. J. Res. Ayurveda Pharm. 2013, 4, 104–107. [Google Scholar] [CrossRef]
- Sofowora, A. Medicinal Plant and Traditional Medcine in Africa; Chichester John Willey and Sons: New York, NY, USA, 1993; p. 256. [Google Scholar]
- Trease, G.E.; Evans, W.C. A Textbook of Pharmacognosy; Bailliere Tindall Ltd.: London, UK, 1989; p. 53. [Google Scholar]
- Richardson, P.M.; Harborne, J.B. Phytochemical Methods. Brittonia 1985, 37, 309. [Google Scholar] [CrossRef]
- Obadoni, B.O.; Ochuko, P.O. Phytochemical Studies and Comparative Efficacy of the Crude Extracts of Some Haemostatic Plants in Edo and Delta States of Nigeria. Glob. J. Pure Appl. Sci. 2002, 8, 203–208. [Google Scholar] [CrossRef]
- Okwu, D.E. Phytochemical and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain. Agric. Environ. 2004, 6, 30–34. [Google Scholar]
- Van Buren, J.P.; Robinson, W.B. Formation of complexes between protein and tannic acid. J. Agric. Food Chem. 1969, 17, 772–777. [Google Scholar] [CrossRef]
- Bonnie, T.C. The wistar rat as a right choice: Establishing mammalian standards and the ideal of a standard mammal. J. History Biol. 1993, 26, 329–349. [Google Scholar]
- Justo, O.R.; Simioni, P.U.; Gabriel, D.L.; Tamashiro, W.M.D.S.C.; Rosa, P.D.T.V.; Moraes, A.M. Evaluation of in vitro anti-inflammatory effects of crude ginger and rosemary extracts obtained through supercritical CO2 extraction on macrophage and tumor cell line: The influence of vehicle type. BMC Complement. Altern. Med. 2015, 15, 390. [Google Scholar] [CrossRef]
- Çoban, J.; Betül-Kalaz, E.; Küçükgergin, C.; Aydın, A.F.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Blueberry treatment attenuates d-galactose-induced oxidative stress and tissue damage in rat liver. Geriatr. Gerontol. Int. 2014, 14, 490–497. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Aniviye, B.O.; Kehinde, B.D.; Akintola, A.O. Age-related changes in the expression of heat shock protein 70 and 90 on the gastric mucosa during gastric ulcer healing. UK J. Pharm Biosci. 2018, 6, 1–10. [Google Scholar]
- Ajayi, A.F.; Akhigbe, R.E. Codeine-induced sperm DNA damage is mediated predominantly by oxidative stress rather than apoptosis. Redox Rep. 2020, 25, 33–40. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Moron, M.; Depierre, J.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta BBA Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Iqbal, M.; Sharma, S.D.; Rezazadeh, H.; Hasan, N.; Abdulla, M.; Athar, M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep. 1996, 2, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Perandones, C.E.; Illera, V.A.; Peckham, D.; Stunz, L.L.; Ashman, R.F. Regulation of apoptosis in vitro in mature murine spleen T cells. J. Immunol. 1993, 151, 3521–3529. [Google Scholar]
- Woode, E.; Alhassan, A.; Abaidoo, C.S. Effect of ethanolic fruit extract of Xylopiaaethiopica on reproductive function of male rats. Int. J. Pharm. Biomed. Res. 2011, 2, 161–165. [Google Scholar]


| Pytochemicals | Values |
|---|---|
| Alkaloids (mg/g) | 72.00 ± 0.45 |
| Saponins (mg/g) | 18.00 ± 0.68 |
| Tannins (mg/L) | 4.12 ± 0.054 |
| Cardiac glycosides (mg/L) | 12.70 ± 0.12 |
| Anthraquinones (mg/100 g) | 15.20 ± 0.34 |
| Group | Initial BW (g) | Final BW (g) | Difference (g) | PTW (g) | PTW/BW × 100 |
|---|---|---|---|---|---|
| Control | 188.00 ± 2.63 a | 248.05 ± 1.92 a | 60.05 ± 1.93 a | 1.04 ± 0.3 a | 0.42 ± 0.01 a |
| d-galactose | 192.62 ± 2.21 a | 213.80 ± 1.95 b | 21.18 ± 2.11 b | 0.52 ± 0.33 b | 0.24 ± 0.04 b |
| 250 mg/kg HELEPN | 193.91 ± 2.19 a | 215.09 ± 1.86 b | 36.32 ± 2.09 b | 1.06 ± 0.42 a | 0.49 ± 0.03 a |
| 500 mg/kg HELEPN | 189.89 ± 2.11 a | 217.21 ± 2.01 b | 27.32 ± 1.09 b | 1.09 ± 0.36 a | 0.50 ± 0.01 a |
| d-galactose + 250 mg/kg HELEPN | 192.34 ± 1.91 a | 217.52 ± 1.22 b | 25.18 ± 2.13 b | 0.99 ± 0.53 a | 0.46 ± 0.01 a |
| d-galactose + 500 mg/kg HELEPN | 189.00 ± 1.87 a | 213.32 ± 1.67 b | 24.32 ± 1.19 b | 0.90 ± 0.43 a | 0.43 ± 0.01 a |
| Parameters | Tailless Head | Headless Tail | Rudiment Tail | Bent Tail | Bentmid Piece | Curved Tail | Curved Midpiece | Looped Tail |
|---|---|---|---|---|---|---|---|---|
| Control | 4.4 ± 0.8 a | 4.6 ± 0.8 a | 2.0 ± 1.0 a | 9.8 ± 1.0 a | 10.0 ± 0.7 a | 10.8 ± 0.8 a | 10.2 ± 0.4 a | 1.8 ± 0.8 a |
| d-galactose | 5.8 ± 0.4 b | 5.6 ± 0.5 b | 2.5 ± 0.8 b | 11.6 ± 1.1 b | 11.8 ± 1.5 b | 11.6 ± 1.3 b | 10.8 ± 0.4 b | 2.8 ± 0.4 b |
| 250 mg/kg HELEPN | 4.6 ± 1.1 a | 2.5 ± 0.8 b | 2.0 ± 1.0 a | 8.6 ± 1.8 a | 9.2 ± 1.4 a | 9.0 ± 1.2 a | 9.0 ± 1.2 a | 1.8 ± 0.8 a |
| 500 mg /kg HELEPN | 4.6 ± 0.5 a | 4.0 ± 0.7 a | 2.0 ± 1.0 a | 9.2 ± 1.0 a | 9.6 ± 1.1 a | 9.6 ± 1.1 a | 9.2 ± 0.4 a | 1.6 ± 0.5 a |
| d-galactose + 250 mg/kg HELEPN | 4.8 ± 0.8 a | 3.8 ± 0.8 a | 2.2 ± 0.8 a | 9.7 ± 1.3 a | 9.6 ± 1.1 a | 9.8 ± 0.8 a | 7.8 ± 0.8 a | 2.0 ± 1.0 a |
| d-galactose + 500 mg/kg HELEPN | 4.6 ± 0.8 a | 3.8 ± 0.8 a | 2.0 ± 1.0 a | 9.0 ± 1.2 a | 9.8 ± 1.0 a | 9.2 ± 0.4 a | 9.4 ± 1.1 a | 2.0 ± 1.0 a |
| Group | Testicular DFI (%) | Caspase 3 (ng/mL) |
|---|---|---|
| Control | 21.8 ± 0.72 a | 0.54 ± 0.02 a |
| d-galactose only | 36.2 ± 0.90 b | 0.9 ± 0.02 b |
| 250 mg/kg HELEPN Only | 18.9 ± 0.20 a | 0.46 ± 0.02 a |
| 500 mg/kg HELEPN Only | 17.4 ± 0.30 a | 0.42 ± 0.07 a |
| d-galactose + 250 mg/kg HELEPN | 28.2 ± 0.83 c | 0.52 ± 0.04 a |
| d-galactose + 500 mg/kg HELEPN | 24.4 ± 0.92 c | 0.49 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajayi, L.; Ayeleso, A.; Oyedepo, T.; Mukwevho, E. Ameliorative Potential of Hydroethanolic Leaf Extract of Parquetina nigrescens on d-Galactose-Induced Testicular Injury. Molecules 2021, 26, 3424. https://doi.org/10.3390/molecules26113424
Ajayi L, Ayeleso A, Oyedepo T, Mukwevho E. Ameliorative Potential of Hydroethanolic Leaf Extract of Parquetina nigrescens on d-Galactose-Induced Testicular Injury. Molecules. 2021; 26(11):3424. https://doi.org/10.3390/molecules26113424
Chicago/Turabian StyleAjayi, Lydia, Ademola Ayeleso, Temitope Oyedepo, and Emmanuel Mukwevho. 2021. "Ameliorative Potential of Hydroethanolic Leaf Extract of Parquetina nigrescens on d-Galactose-Induced Testicular Injury" Molecules 26, no. 11: 3424. https://doi.org/10.3390/molecules26113424
APA StyleAjayi, L., Ayeleso, A., Oyedepo, T., & Mukwevho, E. (2021). Ameliorative Potential of Hydroethanolic Leaf Extract of Parquetina nigrescens on d-Galactose-Induced Testicular Injury. Molecules, 26(11), 3424. https://doi.org/10.3390/molecules26113424






