Antimutagenic, Cytoprotective and Antioxidant Properties of Ficus deltoidea Aqueous Extract In Vitro
Abstract
1. Introduction
2. Results
2.1. Mutagenicity of FDD
2.2. Antimutagenicity of FDD
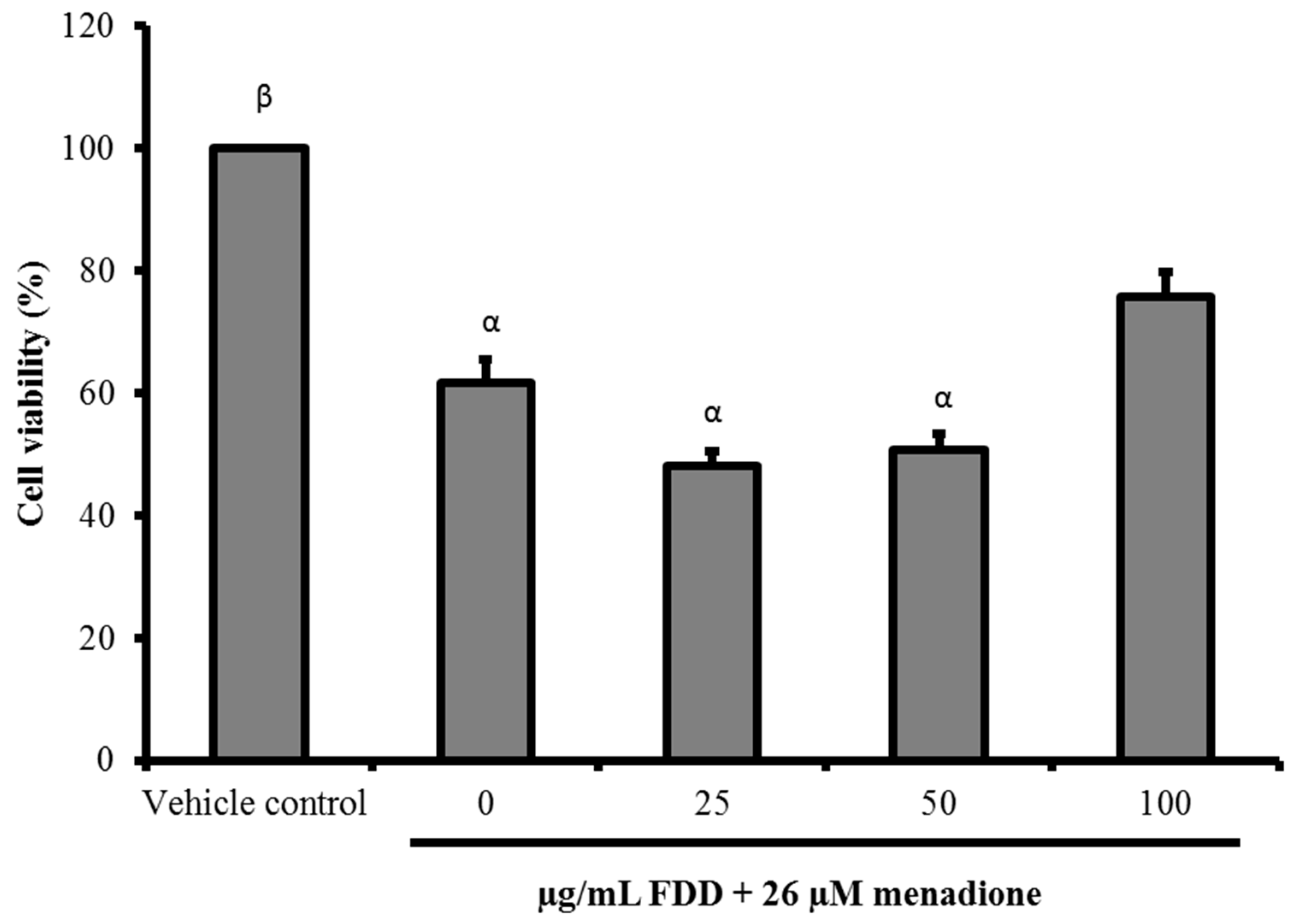
2.3. Cytotoxicity of FDD
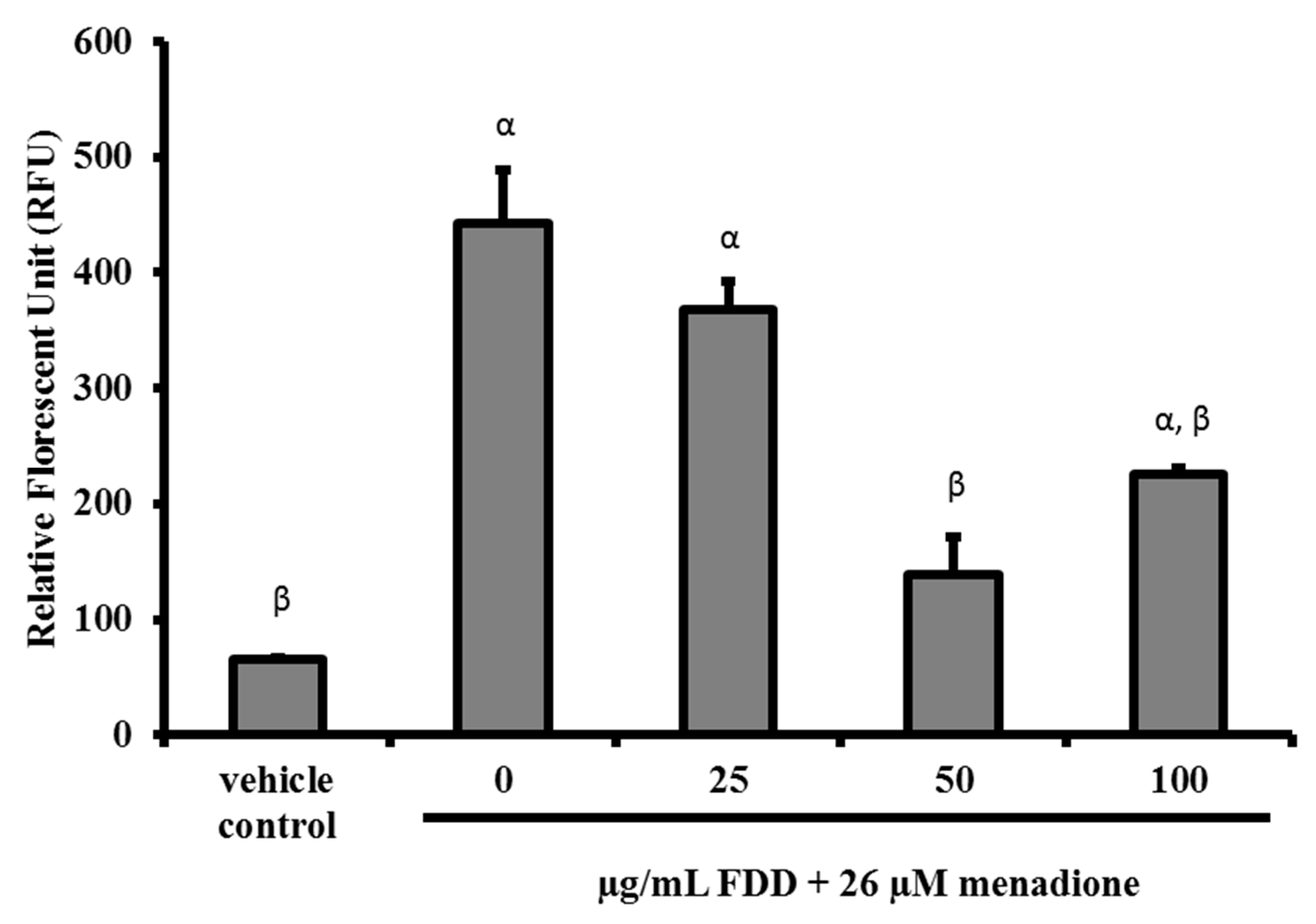
2.4. Cytoprotective Effect of FDD
2.5. FDD Modulation on GSH Level
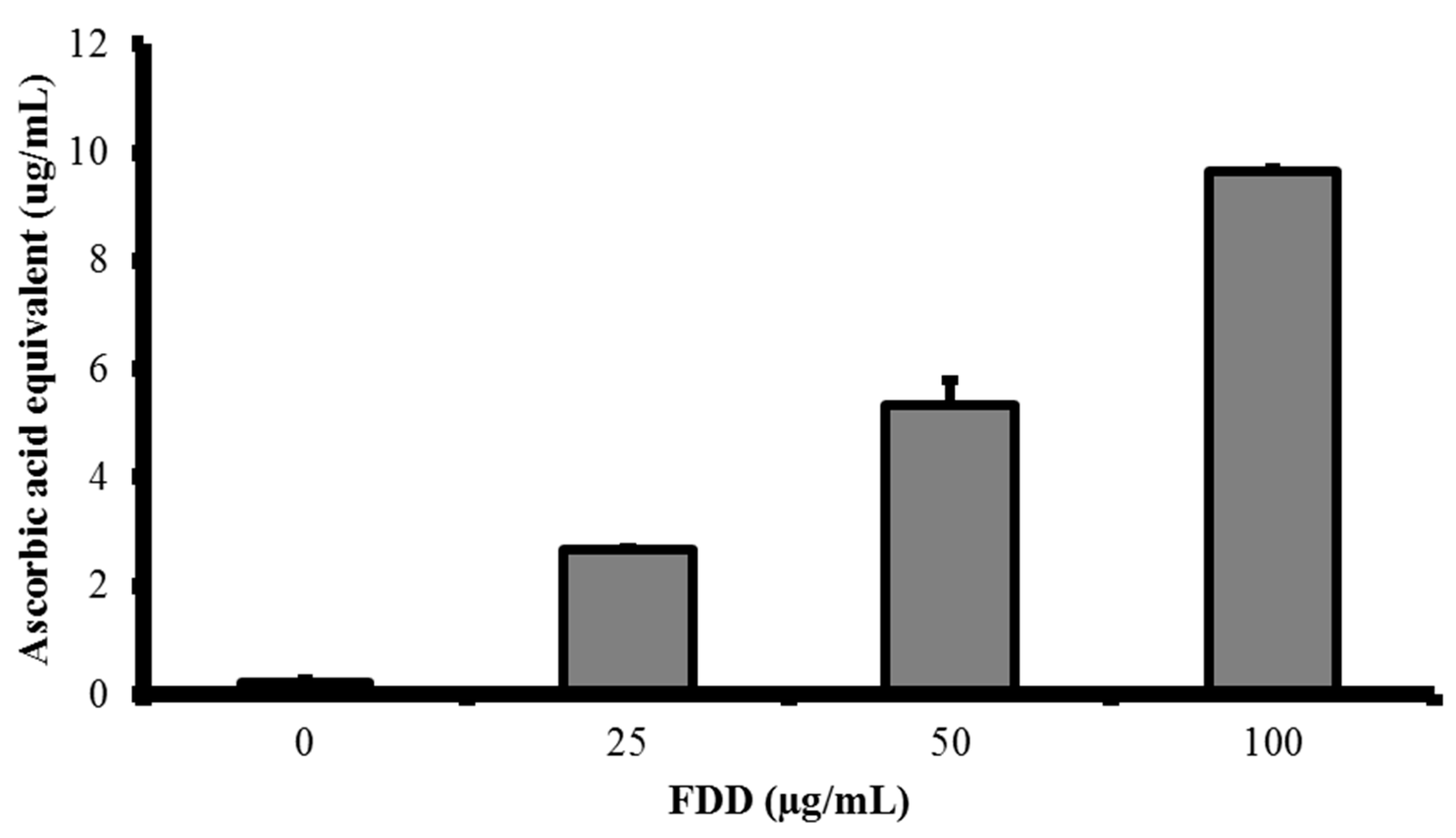
2.6. Ferric-Reducing Antioxidant Power of FDD
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Salmonella Mutagenicity Assay
4.3. Antimutagenicity Assay
4.4. Cell Culture
4.5. MTT Assay
4.6. Superoxide Anion Assessment
4.7. Reduced Glutathione Quantification
4.8. Ferric-reducing Antioxidant Power (FRAP) Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Van Cutsem, E.; Borràs, J.M.; Castells, A.; Ciardiello, F.; Ducreux, M.; Haq, A.; Schmoll, H.-J.; Tabernero, J. Improving outcomes in colorectal cancer: Where do we go from here? Eur. J. Cancer 2013, 49, 2476–2485. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases Country Profile 2014. 2014. Available online: http://apps.who.int/iris/bitstream/10665/128038/1/9789241507509_eng.pdf?ua=1 (accessed on 20 January 2021).
- Hagland, H.R.; Søreide, K. Cellular metabolism in colorectal carcinogenesis: Influence of lifestyle, gut microbiome and metabolic pathways. Cancer Lett. 2015, 356, 273–280. [Google Scholar] [CrossRef]
- Słoczyńska, K.; Powroźnik, B.; Pękala, E.; Waszkielewicz, A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014, 55, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Castiglia, D.; Zambruno, G. Mutation Mechanisms. Dermatol. Clin. 2010, 28, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Saxena, S.; Kumar, S. Fruits and Vegetables as Dietary Sources of Antimutagens. J. Food Chem. Nanotechnol. 2016, 2, 97–114. [Google Scholar] [CrossRef]
- Akram, M.; Riaz, M.; Wadood, A.W.C.; Hazrat, A.; Mukhtiar, M.; Ahmad Zakki, S.; Daniyal, M.; Shariati, M.A.; Said Khan, F.; Zainab, R. Medicinal plants with anti-mutagenic potential. Biotechnol. Biotechnol. Equip. 2020, 34, 309–318. [Google Scholar] [CrossRef]
- Chatti, I.B.; Boubaker, J.; Skandrani, I.; Bhouri, W.; Ghedira, K.; Chekir Ghedira, L. Antioxidant and antigenotoxic activities in Acacia salicina extracts and its protective role against DNA strand scission induced by hydroxyl radical. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1753–1758. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Bautista-Bautista, N.; Blasco-Cabal, J.L.; Gonzalez-Ávila, M.; Gutiérrez-Lomelí, M.; Arriaga-Alba, M. Antimutagenicity of Methanolic Extracts from Anemopsis californica in Relation to Their Antioxidant Activity. Evid. Based Complement. Altern. Med. 2014, 2014, 273878. [Google Scholar] [CrossRef]
- Ghazali, A.R.; Abdullah, R.; Ramli, N.; Rajab, N.F.; Ahmad-Kamal, M.S.; Yahya, N.A. Mutagenic and antimutagenic activities of mitragyna speciosa korth extract using Ames test. J. Med. Plants Res. 2011, 5, 1345–1348. [Google Scholar]
- Bouguellid, G.; Russo, C.; Lavorgna, M.; Piscitelli, C.; Ayouni, K.; Wilson, E.; Kim, H.K.; Verpoorte, R.; Choi, Y.H.; Kilani-Atmani, D.; et al. Antimutagenic, antigenotoxic and antiproliferative activities of Fraxinus angustifolia Vahl. leaves and stem bark extracts and their phytochemical composition. PLoS ONE 2020, 15, e0230690. [Google Scholar] [CrossRef]
- Cherdshewasart, W.; Sutjit, W.; Pulcharoen, K.; Chulasiri, M. The mutagenic and antimutagenic effects of the traditional phytoestrogen-rich herbs, Pueraria mirifica and Pueraria lobata. Braz. J. Med. Biol. Res. 2009, 42, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Loh, D.S.Y.; Er, H.M.; Chen, Y.S. Mutagenic and antimutagenic activities of aqueous and methanol extracts of Euphorbia hirta. J. Ethnopharmacol. 2009, 126, 406–414. [Google Scholar] [CrossRef]
- Rathnasamy, S.; Mohamed, K.B.; Sulaiman, S.F.; Akinboro, A. Evaluation of cytotoxic, mutagenic and antimutagenic potential of leaf extracts of three medicinal plants using Allium cepa chromosome assay. Int. Curr. Pharm. J. 2013, 2, 131–140. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Peng, X.; Wei, Z.-F.; Wei, F.-Y.; Wang, W.; Ma, W.-D.; Yao, L.-P.; Fu, Y.-J.; Zu, Y.-G. Geraniin exerts cytoprotective effect against cellular oxidative stress by upregulation of Nrf2-mediated antioxidant enzyme expression via PI3K/AKT and ERK1/2 pathway. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Cho, S.S.; Yang, J.H.; Kim, K.M.; Jang, C.H.; Park, D.E.; Bang, J.S.; Jung, Y.S.; Ki, S.H. The chalcone compound isosalipurposide (ISPP) exerts a cytoprotective effect against oxidative injury via Nrf2 activation. Toxicol. Appl. Pharmacol. 2015, 287, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signalling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Bunawan, H.; Amin, N.M.; Bunawan, S.N.; Baharum, S.N.; Mohd Noor, N. Ficus deltoidea Jack: A Review on Its Phytochemical and Pharmacological Importance. Evid. Based. Complement. Alternat. Med. 2014, 2014, 902734. [Google Scholar] [CrossRef]
- Ibrahim, F.W.; Derased, N.I.; Zainudin, U.R.A.; Rajab, N.F. Mechanism Identification of Ficus Deltoidea Aqueous Extract in Rat Uterine Contractions. J. Sains Kesihat. Malaysia 2018, 16, 75–81. [Google Scholar] [CrossRef]
- Amiera, Z.U.R.; Nihayah, M.; Wahida, I.F.; Rajab, N.F. Phytochemical characteristic and uterotonic effect of aqueous extract of Ficus deltoidea leaves in rats uterus. Pak. J. Biol. Sci. PJBS 2014, 17, 1046–1051. [Google Scholar] [CrossRef]
- Farsi, E.; Shafaei, A.; Hor, S.Y.; Ahamed, M.B.K.; Yam, M.F.; Asmawi, M.Z.; Ismail, Z. Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of Ficus deltoidea leaves. Clinics 2013, 68, 865–875. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-naphthoquinones: From oxidative damage to cellular and inter-cellular signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef]
- Hassan, G.S. Menadione. Profiles Drug Subst. Excip. Relat. Methodol. 2013, 38, 227–313. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Klotz, L.-O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Phuneerub, P.; Limpanasithikul, W.; Palanuvej, C.; Ruangrungsi, N. In vitro anti-inflammatory, mutagenic and antimutagenic activities of ethanolic extract of Clerodendrum paniculatum root. J. Adv. Pharm. Technol. Res. 2015, 6, 48–52. [Google Scholar] [CrossRef]
- Siew, E.L.; Farris, A.F.; Rashid, N.; Chan, K.M.; Rajab, N.F. In vitro toxicological assessment of gadolinium (III) chloride in V79-4 fibroblasts. Genes Environ. 2020, 42, 22. [Google Scholar] [CrossRef] [PubMed]
- Inayat-Hussain, S.H.; Chan, K.M.; Rajab, N.F.; Din, L.B.; Chow, S.C.; Kizilors, A.; Farzaneh, F.; Williams, G.T. Goniothalamin-induced oxidative stress, DNA damage and apoptosis via caspase-2 independent and Bcl-2 independent pathways in Jurkat T-cells. Toxicol. Lett. 2010, 193, 108–114. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hasiah, A.H.; Ghazali, A.R.; Weber, J.F.F.; Velu, S.; Thomas, N.F.; Inayat Hussain, S.H. Cytotoxic and antioxidant effects of methoxylated stilbene analogues on HepG2 hepatoma and Chang liver cells: Implications for structure activity relationship. Hum. Exp. Toxicol. 2011, 30, 138–144. [Google Scholar] [CrossRef] [PubMed]


| S. typhimurium Strains | Concentration (mg/mL) | No. of Revertant Colony (Mean ± SD) | |
|---|---|---|---|
| Without S9 | With S9 | ||
| TA 98 | PC | 444 ± 12.9 a, α | 531 ± 19.86 b, α |
| NC | 14 ± 3.61 | 19 ± 2.65 | |
| 3.125 | 14 ± 1.00 | 16 ± 0.58 | |
| 6.25 | 9 ± 0.58 | ND | |
| 12.5 | 10 ± 1.00 | 20 ± 0.00 | |
| 25 | 14 ± 0.58 | ND | |
| 50 | 17 ± 2.00 | 19 ± 0.00 | |
| TA 100 | PC | 932 ± 32.72 c, α | 1209 ± 50.06 b, α |
| NC | 84 ± 14.18 | 83 ± 3.21 | |
| 3.125 | 83 ± 4.93 | 80 ± 6.81 | |
| 6.25 | 63 ± 2.65 | ND | |
| 12.5 | 76 ± 3.06 | 78 ± 4.00 | |
| 25 | 63 ± 1.73 | ND | |
| 50 | 74 ± 0.58 | 84 ± 1.00 | |
| Strains | Concentration (mg/mL) | Percentage Inhibition (%) | |||||
|---|---|---|---|---|---|---|---|
| 2-Nitrofluorene | 2-Aminoanthracene | Sodium Azide | |||||
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | ||
| TA 98 | 12.5 | 11.27 α | − | − | 60.25 α | − | − |
| 50 | 28.74 α | − | − | 74.75 α | − | − | |
| TA 100 | 12.5 | − | − | − | 17.91 α | 11.27 | − |
| 50 | − | − | − | 79.73 α | 13.9 | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, T.C.; Ibrahim, F.W.; Ahmad, S.; Chan, K.M.; Leong, L.M.; Mohammad, N.; Siew, E.L.; Rajab, N.F. Antimutagenic, Cytoprotective and Antioxidant Properties of Ficus deltoidea Aqueous Extract In Vitro. Molecules 2021, 26, 3287. https://doi.org/10.3390/molecules26113287
Ooi TC, Ibrahim FW, Ahmad S, Chan KM, Leong LM, Mohammad N, Siew EL, Rajab NF. Antimutagenic, Cytoprotective and Antioxidant Properties of Ficus deltoidea Aqueous Extract In Vitro. Molecules. 2021; 26(11):3287. https://doi.org/10.3390/molecules26113287
Chicago/Turabian StyleOoi, Theng Choon, Farah Wahida Ibrahim, Shakirah Ahmad, Kok Meng Chan, Lek Mun Leong, Nihayah Mohammad, Ee Ling Siew, and Nor Fadilah Rajab. 2021. "Antimutagenic, Cytoprotective and Antioxidant Properties of Ficus deltoidea Aqueous Extract In Vitro" Molecules 26, no. 11: 3287. https://doi.org/10.3390/molecules26113287
APA StyleOoi, T. C., Ibrahim, F. W., Ahmad, S., Chan, K. M., Leong, L. M., Mohammad, N., Siew, E. L., & Rajab, N. F. (2021). Antimutagenic, Cytoprotective and Antioxidant Properties of Ficus deltoidea Aqueous Extract In Vitro. Molecules, 26(11), 3287. https://doi.org/10.3390/molecules26113287







