Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles
Abstract
1. Introduction
2. Results and Discussion
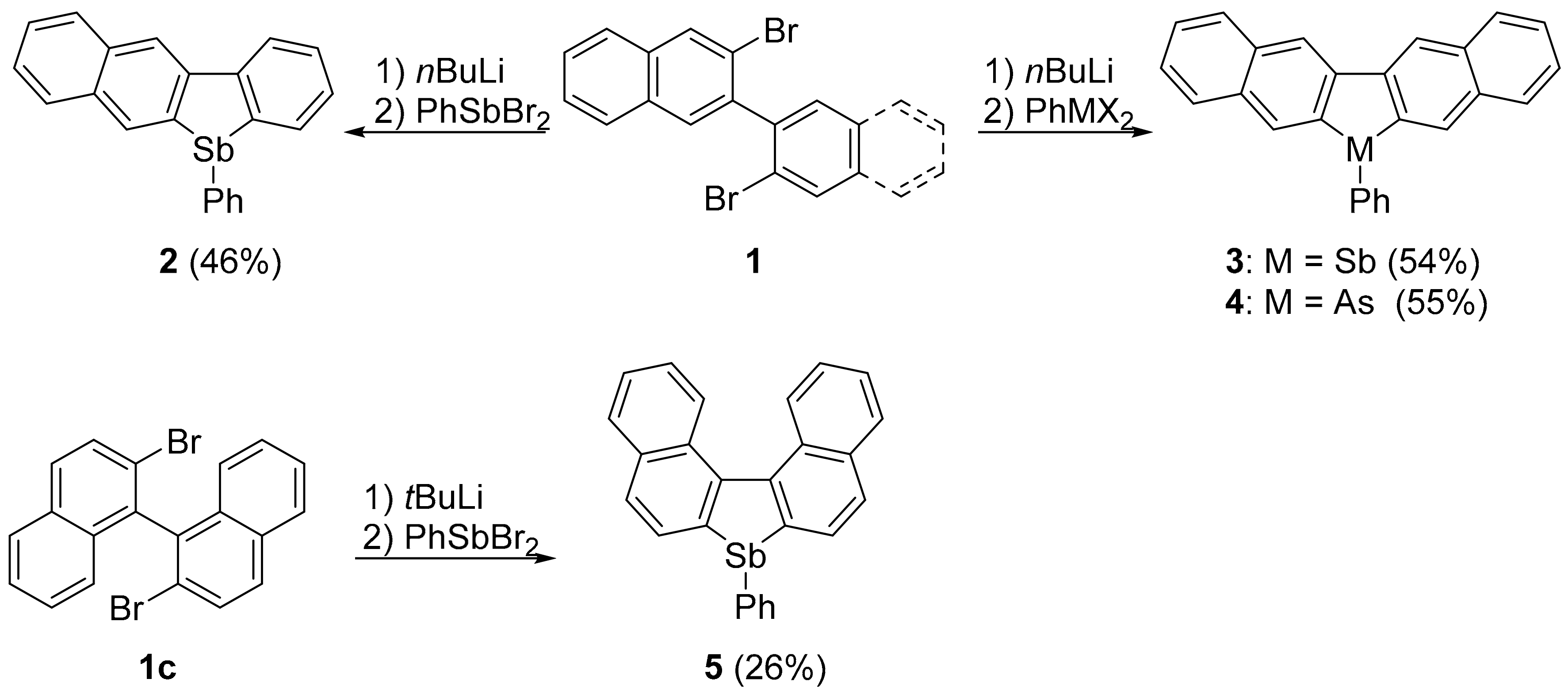
2.1. Synthesis
2.2. Structure Analysis
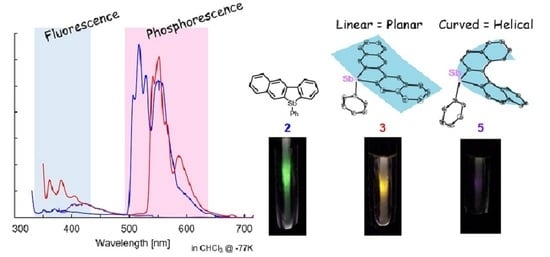
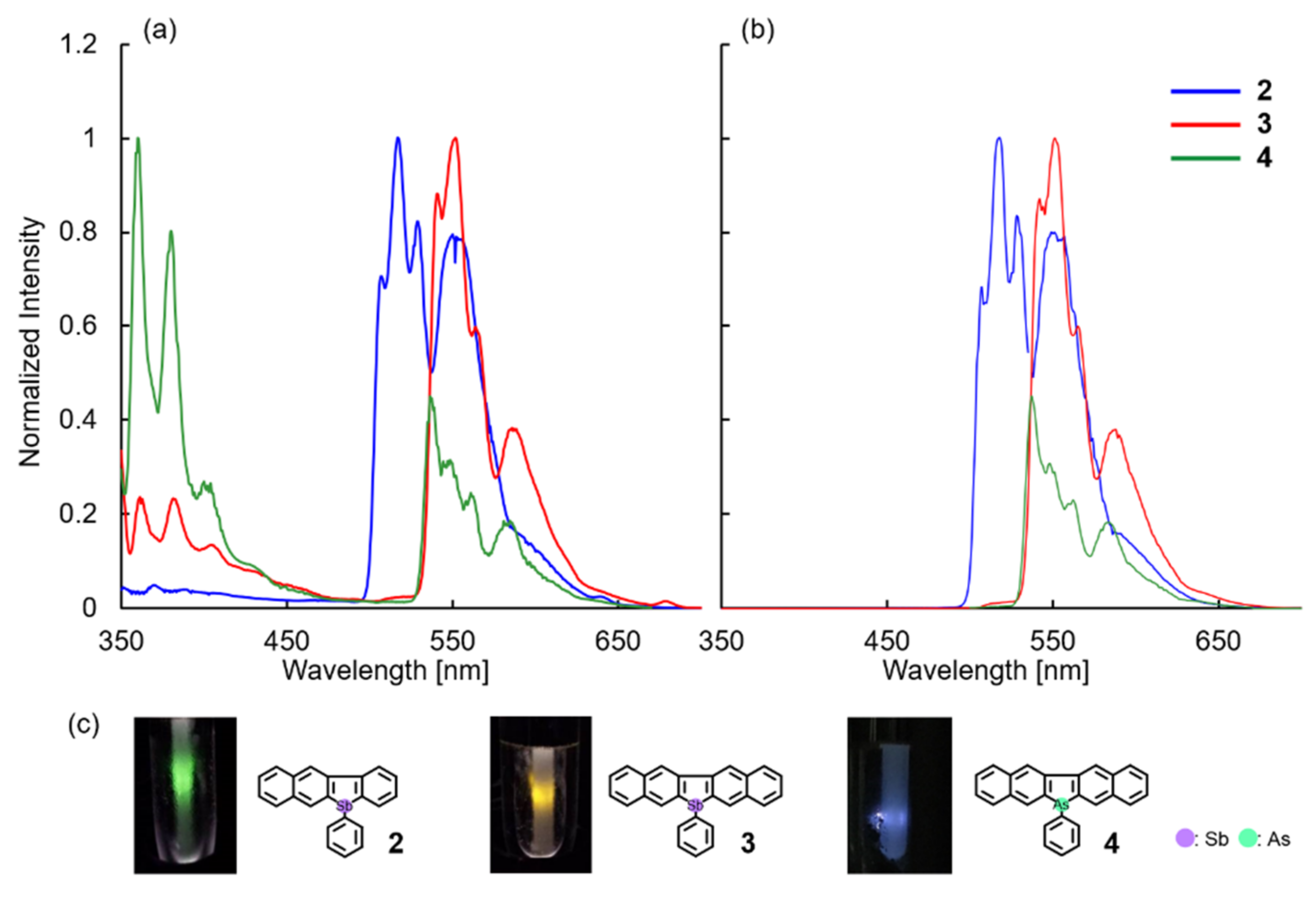
2.3. Optical Properties
3. Materials and Methods
3.1. General Information
3.2. Preparation and Characterization of Novel Compounds
3.2.1. 2-Bromo-3-(2-bromophenyl)naphthalene (1a)
3.2.2. 5-Phenylbenzo[b]naphtho[2,3-d]stibole (2)
3.2.3. 6-Phenyldinaphtho[2,3-b,2′,3′-d]stibole (3)
3.2.4. 6-Phenyldinaphtho[2,3-b,2′,3′-d]arsole (4)
3.2.5. 7-Phenyldinaphtho[2,1-b,1′,2′-d]stibole (5)
3.3. Single Crystal X-ray Diffraction Experiment
3.3.1. Compound 3
3.3.2. Compound 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baumgartner, T.; Jäkle, F. (Eds.) Main Group Strategies towards Functional Hybrid Materials; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- Hissler, M.; Dyer, P.W.; Réau, R. Linear organic π-conjugated systems featuring the heavy Group 14 and 15 elements. Coord. Chem. Rev. 2003, 244, 1–44. [Google Scholar] [CrossRef]
- Baumgartner, T.; Réau, R. Organophosphorus π-conjugated materials. Chem. Rev. 2006, 106, 4681–4727. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.G.; Baumgartner, T. Recent developments in phosphole-containing oligo- and polythiophene materials. Eur. J. Inorg. Chem. 2007, 3611–3628. [Google Scholar] [CrossRef]
- Zagidullin, A.A.; Bezkishko, I.A.; Miluykov, V.A.; Sinyashin, O.G. Phospholes-development and recent advances. Mendeleev Commun. 2013, 23, 117–130. [Google Scholar] [CrossRef]
- Duffy, M.P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. π-Conjugated phospholes and their incorporation into devices: Components with a great deal of potential. Chem. Soc. Rev. 2016, 45, 5296–5310. [Google Scholar] [CrossRef]
- Ishijima, K.; Tanaka, S.; Imoto, H.; Naka, K. 2-Arylbenzo[b]arsoles: An experimental and computational study on the relationship between structural and photophysical properties. Dalton Trans. 2020, 49, 15612–15621. [Google Scholar] [CrossRef]
- Fujii, T.; Tanaka, S.; Hayashi, S.; Imoto, H.; Naka, K. Dipyridinoarsole: A new class of stable and modifiable heteroatom-bridged bipyridines. Chem. Commun. 2020, 56, 6035–6038. [Google Scholar] [CrossRef]
- Urushizaki, A.; Yumura, T.; Kitagawa, Y.; Hasegawa, Y.; Imoto, H.; Naka, K. Dithieno[3,4-b:3′,4′-d]arsole: A novel class of hetero[5]radialenes. Eur. J. Org. Chem. 2020, 2020, 3965–3970. [Google Scholar] [CrossRef]
- Tanaka, S.; Enoki, T.; Imoto, H.; Ooyama, Y.; Ohshita, J.; Kato, T.; Naka, K. Highly efficient singlet oxygen generation and high oxidation resistance enhanced by arsole-polymer-based photosensitizer: Application as a recyclable photooxidation catalyst. Macromolecules 2020, 53, 2006–2013. [Google Scholar] [CrossRef]
- Imoto, H.; Naka, K. The Dawn of functional organoarsenic chemistry. Chem. Eur. J. 2019, 25, 1883–1894. [Google Scholar] [CrossRef]
- Rivard, E. Tellurophenes and their emergence as building blocks for polymeric and light-emitting materials. Chem. Lett. 2015, 44, 730–736. [Google Scholar] [CrossRef]
- Parke, S.M.; Narreto, M.A.B.; Hupf, E.; McDonald, R.; Ferguson, M.J.; Hegmann, F.A.; Rivard, E. Understanding the origin of phosphorescence in bismoles: A synthetic and computational study. Inorg. Chem. 2018, 57, 7536–7549. [Google Scholar] [CrossRef] [PubMed]
- Parke, S.M.; Hupf, E.; Matharu, G.K.; de Aguiar, I.; Xu, L.; Yu, H.; Boone, M.P.; de Souza, G.L.C.; McDonald, R.; Ferguson, M.J.; et al. Aerobic solid state red phosphorescence from benzobismole monomers and patternable self-assembled block copolymers. Angew. Chem. Int. Ed. 2018, 57, 14841–14846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Guo, J.; Liu, B.; Tan, Q.; Xu, B. Synthesis of tellurium-containing π-extended aromatics with room-temperature phosphorescence. Org. Lett. 2019, 21, 8328–8333. [Google Scholar] [CrossRef] [PubMed]
- Hupf, E.; Tsuchiya, Y.; Moffat, W.; Xu, L.; Hirai, M.; Zhou, Y.; Ferguson, M.J.; McDonald, R.; Murai, T.; He, G.; et al. A modular approach to phosphorescent π-extended heteroacenes. Inorg. Chem. 2019, 58, 13323–13336. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, B.L.; Berman, J.D. Recommendations for treating Leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am. J. Trop. Med. Hyg. 1992, 46, 296–306. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mitani, M.; Yasuike, S.; Kurita, J.; Kaji, T. An organobismuth compound that exhibits selective cytotoxicity to vascular endothelial cells in vitro. J. Health Sci. 2005, 51, 333–340. [Google Scholar] [CrossRef]
- Kohri, K.; Yoshida, E.; Yasuike, S.; Fujie, T.; Yamamoto, C.; Kaji, T. The cytotoxicity of organobismuth compounds with certain molecular structures can be diminished by replacing the bismuth atom with an antimony atom in the molecules. J. Toxicol. Sci. 2015, 40, 321–327. [Google Scholar] [CrossRef]
- Hara, T.; Nakano, S.; Kitamura, Y.; Yamamoto, C.; Yasuike, S.; Kaji, T. Intracellular accumulation-independent cytotoxicity of pentavalent organoantimony compounds in cultured vascular endothelial cells. J. Toxicol. Sci. 2019, 44, 845–848. [Google Scholar] [CrossRef]
- Sum, H. (Ed.) Biological Chemistry of Aresenic, Antimony and Bismuth; Jhon Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Ashe, A.J.; Drone, F.J. Synthesis of 1-phenylarsole and 1-phenylstibole. Organometallics 1985, 4, 1478–1480. [Google Scholar] [CrossRef]
- Kurita, J.; Ishii, M.; Yasuike, S.; Tsuchiya, T. A versatile synthetic route to 1-benzometalloles involving the first examples of several C-unsubstituted benzometalloles. J. Chem. Soc. Chem. Commun. 1993, 1309–1310. [Google Scholar] [CrossRef]
- Kurita, J.; Ishii, M.; Yasuike, S.; Tsuchiya, T. Synthesis of 1-benzometalloles containing group 14, 15, and 16 heavier elements via a common dilithiostyrene intermediate. Chem. Pharm. Bull 1994, 42, 1437–1441. [Google Scholar] [CrossRef][Green Version]
- Heinekey, D.M.; Millar, I.T. The synthesis of 9-alkyl- or 9-aryl-9-arsafluorenes or -9-stibiafluorenes. J. Chem. Soc. 1959, 3101–3102. [Google Scholar]
- Kurita, J.; Usuda, F.; Yasuike, S.; Tsuchiya, T.; Tsuda, Y.; Kiuchi, F.; Hosoi, S. Resolution of racemic Sb-chiral stibindoles using an optically active ortho-palladated benzylamine derivative, via their diastereomeric complexes. Chem. Commun. 2000, 191–192. [Google Scholar] [CrossRef]
- Christianson, A.M.; Rivard, E.; Gabbaï, F.P. 1λ5-stibaindoles as lewis acidic, π-conjugated, fluoride anion responsive platforms. Organometallics 2017, 36, 2670–2676. [Google Scholar] [CrossRef]
- Christianson, A.M.; Gabbaï, F.P. A lewis acidic, π-conjugated stibaindole with a colorimetric response to anion binding at Sb(III). Organometallics 2017, 36, 3013–3015. [Google Scholar] [CrossRef]
- Ohshita, J.; Fujita, R.; Tanaka, D.; Ooyama, Y.; Kobayashi, N.; Higashimura, H.; Yamamoto, Y. Synthesis and optical properties of dithienostiboles. Chem. Lett. 2012, 41, 1002–1003. [Google Scholar] [CrossRef]
- Ohshita, J.; Yamaji, K.; Ooyama, Y.; Adachi, Y.; Nakamura, M.; Watase, S. Synthesis, properties, and complex formation of antimony- and bismuth-bridged bipyridyls. Organometallics 2019, 38, 1516–1523. [Google Scholar] [CrossRef]
- Yasuike, S.; Iida, T.; Yamaguchi, K.; Seki, H.; Kurita, J. Synthesis, molecular structure and fluxional behavior of (R)-7-p-tolyldinaphtho[2,1-b;1′,2′-d]stibole: The first isolated example of optically active group 15 dinaphthoheteroles. Tetrahedron Lett. 2001, 42, 441–444. [Google Scholar] [CrossRef]
- Matsumura, M.; Kawahata, M.; Muranaka, A.; Hiraiwa, M.; Yamaguchi, K.; Uchiyama, M.; Yasuike, S. Efficient synthesis, structural Characterization, and optical properties of 6H-dibenzo[b,h]carbazole and its derivatives. Eur. J. Org. Chem. 2019, 3788–3793. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates and Resolutions; Krieger Publishing Company: Malabar, FL, USA, 1991. [Google Scholar]
- Wang, Y.; Chen, A.M. Enantioenrichment by crystallization. Org. Process Res. Dev. 2008, 12, 282–290. [Google Scholar] [CrossRef]
- Buchwald, S.L.; Fisher, R.A.; Foxman, B.M. The synthesis and structure of stibaindoles. Angew. Chem. Int. Ed. 1990, 29, 771–772. [Google Scholar] [CrossRef]
- Chen, C.-F.; Shen, Y. Helicene Chemistry: From Synthesis to Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. 2015, C71, 3–8. [Google Scholar]
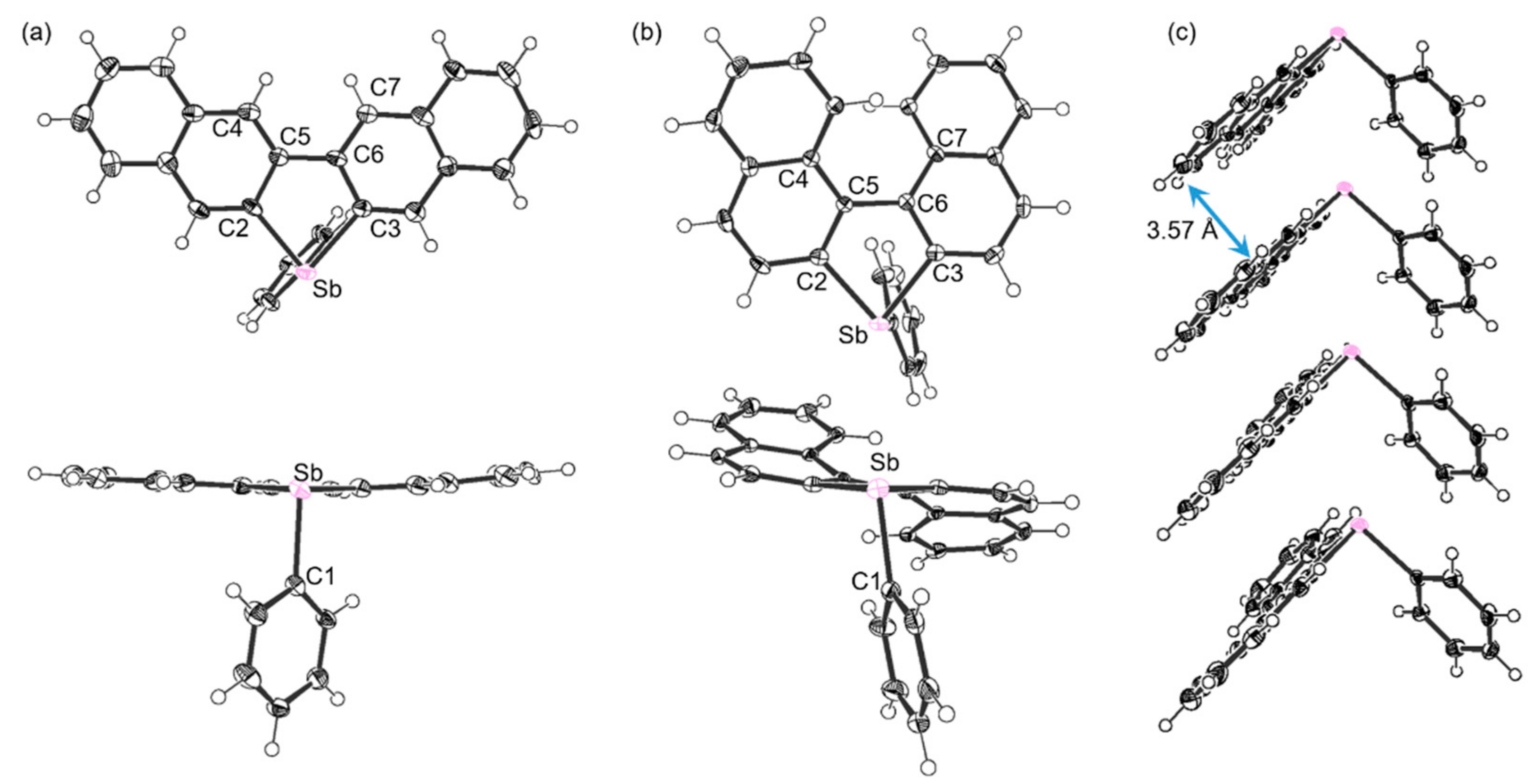

| Compound 3 | Compound 5 | ||
|---|---|---|---|
| Bond length (Å) | C(1)-Sb(1) | 2.182(9) | 2.158(2) |
| C(2)-Sb(1) | 2.155(10) | 2.138(2) | |
| C(3)-Sb(1) | 2.171(8) | 2.146(2) | |
| Bond angle (°) | C(2)-Sb(1)-C(3) | 81.0(3) | 80.03(8) |
| C(1)-Sb(1)-C(2) | 92.7(3) | 100.33(8) | |
| C(1)-Sb(1)-C(3) | 94.6(3) | 89.47(8) | |
| Torsion angle (°) | C(4)-C(5)-C(6)-C(7) | 0.0(13) | 36.3(3) |
| Compd. | UV λabs (ε) (nm) | λflb | Φflb,c | λphosd | τphosd |
|---|---|---|---|---|---|
| (nm) | (%) | (nm) | (ms) | ||
| 2 | 268 (29500), 323 (11300) | 356, 373 | 1.0 | 517, 529 | 40 |
| 3 | 288 (54100), 340 (20700), 360 (3700) | 365, 385 | 0.5 | 551, 588 | 45 |
| 4 | 266 (84800), 341 (15300), 358 (6100) | 362 | 3.0 | 537, 583 | 205 |
| 5 | 363 (12200) | 406.5 | 0.03 | n.d. e | n.d. e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, M.; Matsuhashi, Y.; Kawakubo, M.; Hyodo, T.; Murata, Y.; Kawahata, M.; Yamaguchi, K.; Yasuike, S. Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles. Molecules 2021, 26, 222. https://doi.org/10.3390/molecules26010222
Matsumura M, Matsuhashi Y, Kawakubo M, Hyodo T, Murata Y, Kawahata M, Yamaguchi K, Yasuike S. Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles. Molecules. 2021; 26(1):222. https://doi.org/10.3390/molecules26010222
Chicago/Turabian StyleMatsumura, Mio, Yuki Matsuhashi, Masato Kawakubo, Tadashi Hyodo, Yuki Murata, Masatoshi Kawahata, Kentaro Yamaguchi, and Shuji Yasuike. 2021. "Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles" Molecules 26, no. 1: 222. https://doi.org/10.3390/molecules26010222
APA StyleMatsumura, M., Matsuhashi, Y., Kawakubo, M., Hyodo, T., Murata, Y., Kawahata, M., Yamaguchi, K., & Yasuike, S. (2021). Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles. Molecules, 26(1), 222. https://doi.org/10.3390/molecules26010222