Surface Modification of Magnetic Nanoparticles by Carbon-Coating Can Increase Its Biosafety: Evidences from Biochemical and Neurobehavioral Tests in Zebrafish
Abstract
1. Introduction
2. Material and Methods
2.1. C-MNPs Synthesis and Characterization
2.2. XRD, Raman and FTIR Spectrum
2.3. Zebrafish Ethics and Husbandry
2.4. Zebrafish Exposure to Magnetic Nanoparticles
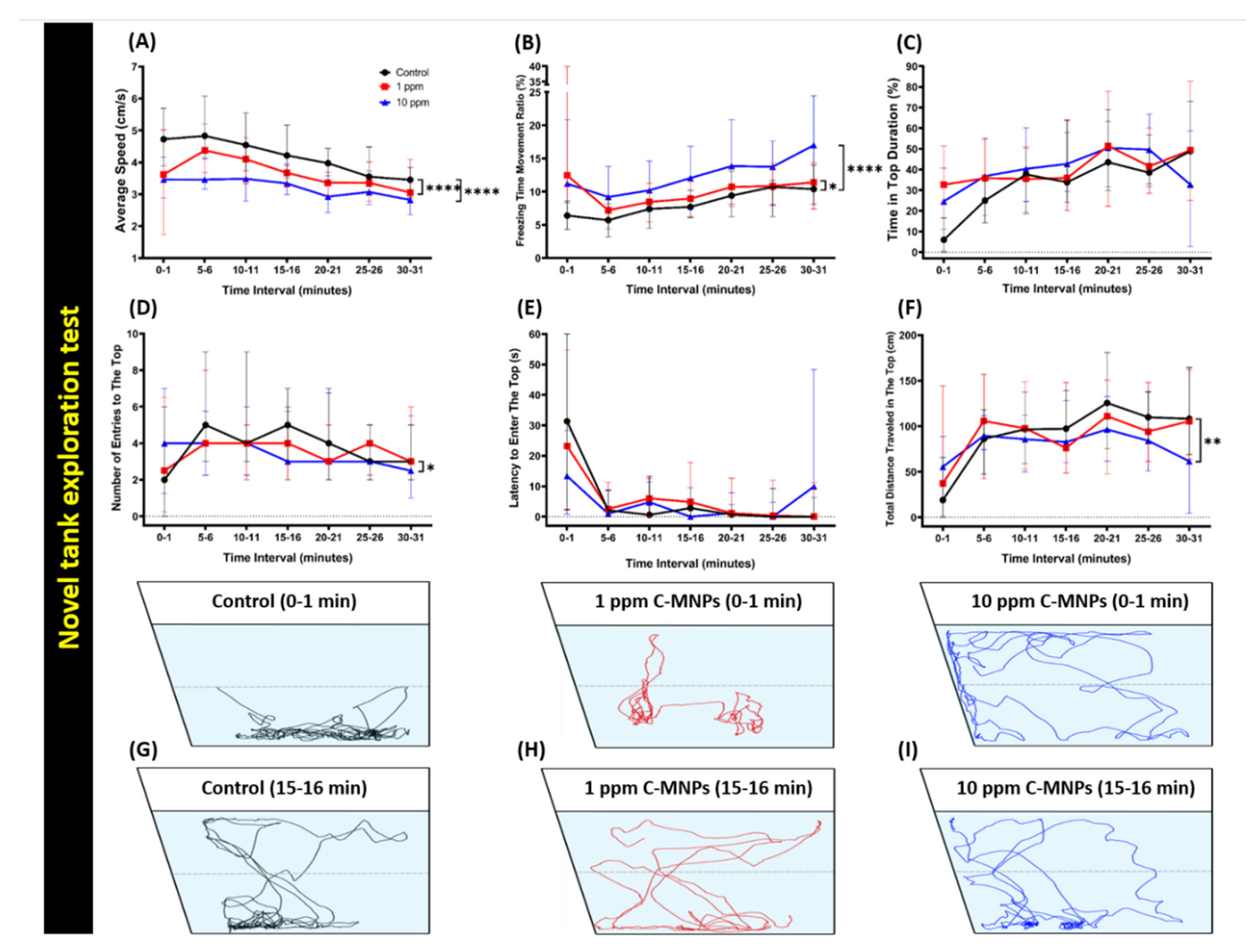
2.5. Novel Tank Test
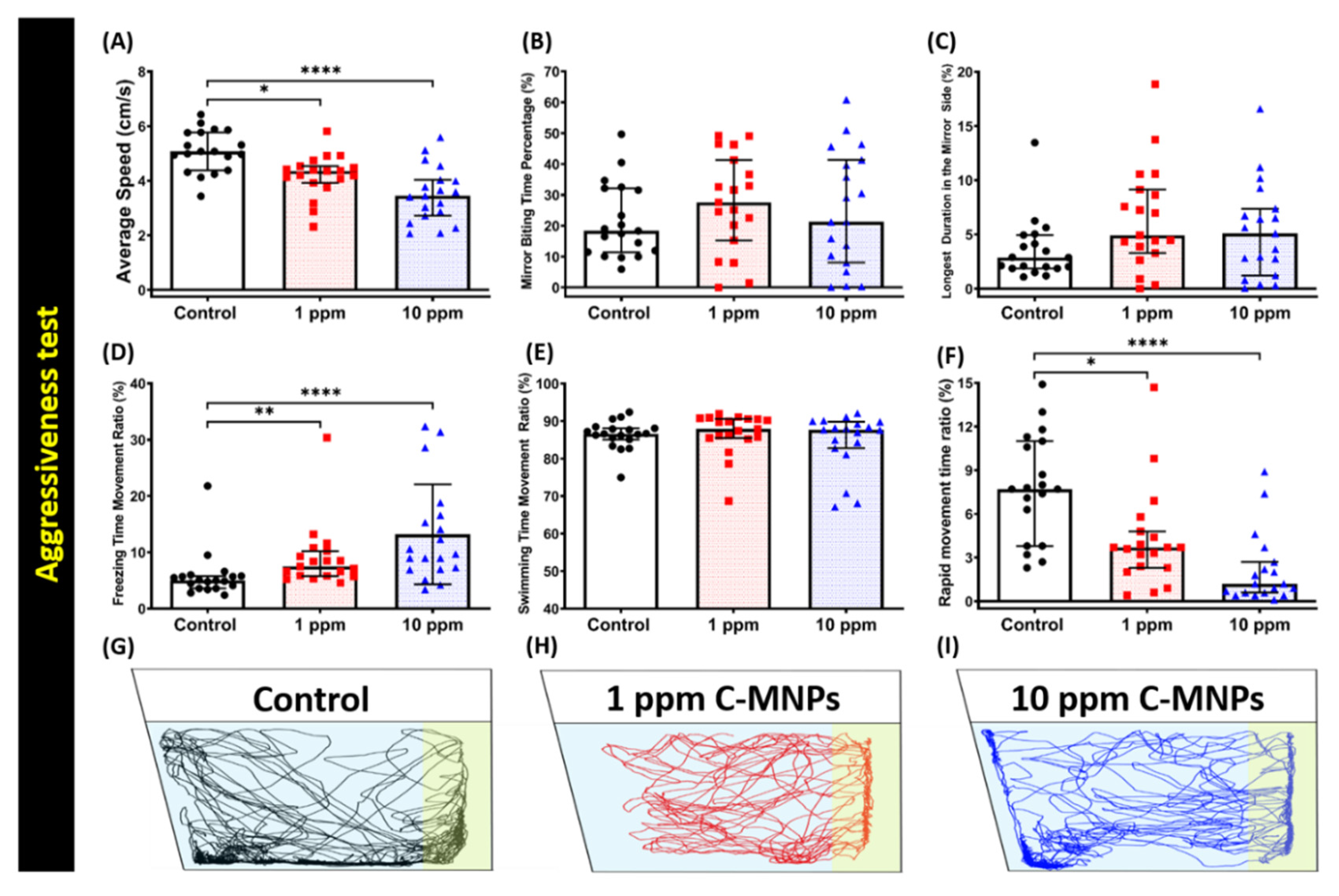
2.6. Aggressiveness Test
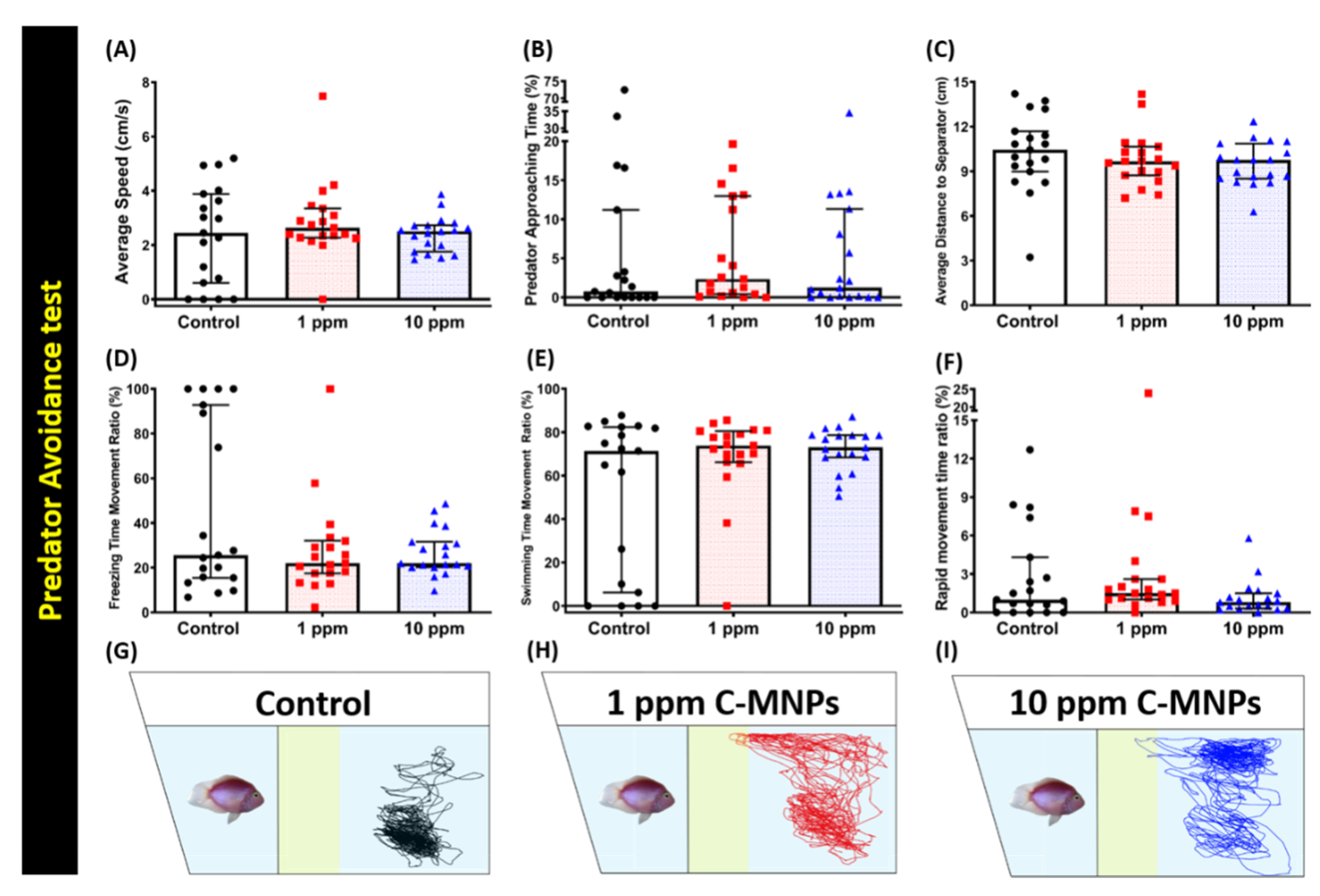
2.7. Predator Avoidance Test
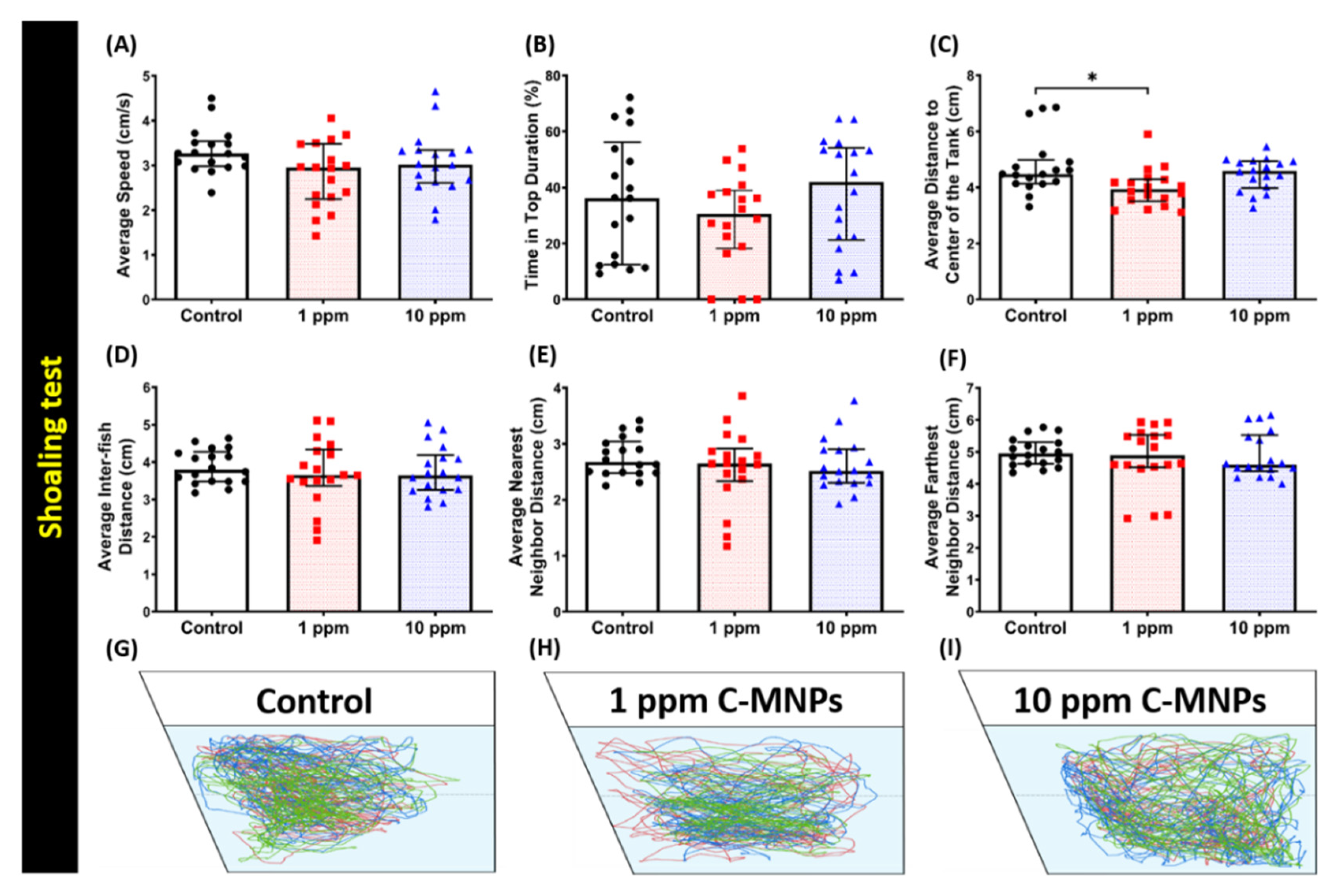
2.8. Shoaling Test
2.9. Social Interaction Test
2.10. Tissue Preparation and Total Protein Determination
2.11. Quantification of Ferric (metal) Content, The Stress Hormone, and Oxidative Stress Markers Were Analyzed in Brain Tissues
2.12. Neurotransmitters Determination in Zebrafish Brain Tissues
2.13. PCA, Heatmap, and Clustering Analysis
2.14. Statistical Analysis
3. Results
3.1. Carbon Magnetic Nanoparticles (C-MNPs) Characterization
3.2. Reduction in Locomotor Activity on Exposure to the Low and High Concentrations of C-MNPs
3.3. C-MNPs Exposure Did Not Change Aggressive Behavior in Zebrafish
3.4. C-MNPs Exposure Did Not Change the Predator Avoidance Behavior in Zebrafish
3.5. C-MNPs Exposure Did Not Alter Shoaling Behavior in the Exposed Zebrafish
3.6. C-MNPs Exposure Did Not Alter Conspecific Social Interaction Interest in Zebrafish
3.7. Effect of C-MNPs Exposure on Ferric (metal) Content, A Stress Hormone and Oxidative Stress Markers in Brain Tissues
3.8. Expression of Neurotransmitters in C-MNPs Exposed Zebrafish Brain
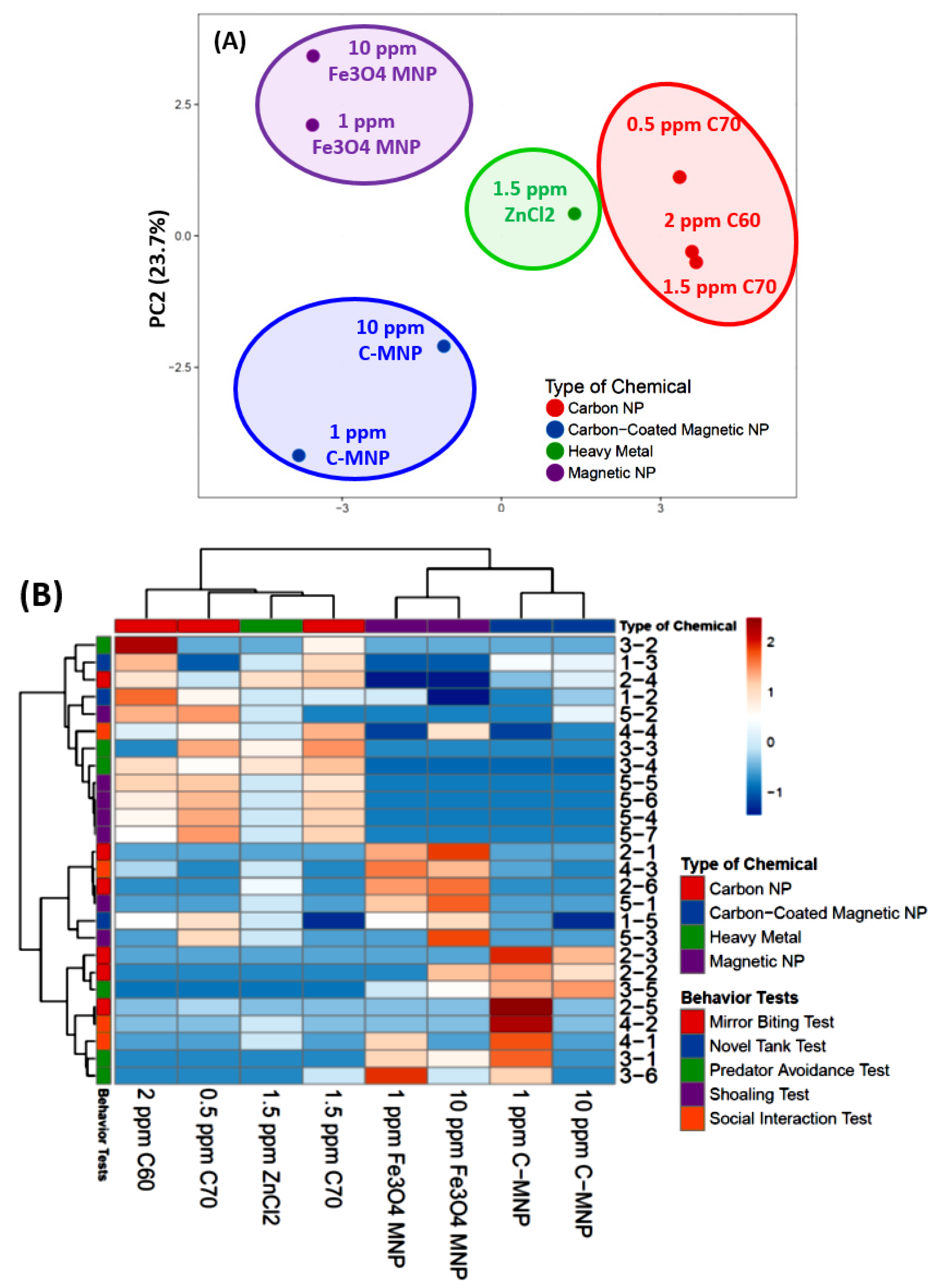
3.9. PCA Analysis and Hierarchical Clustering Analysis of Zebrafish Behavioral Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Zebrafish Behavior Endpoints Legends | ||
|---|---|---|
| 1 | Novel Tank Exploration Test | |
| 1-1 | Average Speed | |
| 1-2 | Freezing Time Movement Ratio | |
| 1-3 | Time in Top Duration | |
| 1-4 | Number of Entries to The Top | |
| 1-5 | Latency to Enter The Top | |
| 1-6 | Total Distance Traveled in The Top | |
| 2 | Mirror Biting Test | |
| 2-1 | Average Speed | |
| 2-2 | Mirror Biting Time Percentage | |
| 2-3 | Longest Duration in The Mirror Side | |
| 2-4 | Freezing Time Movement Ratio | |
| 2-5 | Swimming Time Movement Ratio | |
| 2-6 | Rapid Movement Time Ratio | |
| 3 | Predator Avoidance Test | |
| 3-1 | Average Speed | |
| 3-2 | Predator Approaching Time | |
| 3-3 | Average Distance to Separator | |
| 3-4 | Freezing Time Movement Ratio | |
| 3-5 | Swimming Time Movement Ratio | |
| 3-6 | Rapid Movement Time Ratio | |
| 4 | Social Interaction Test | |
| 4-1 | Interaction Time Percentage | |
| 4-2 | Longest Duration in Separator Side | |
| 4-3 | Average Speed | |
| 4-4 | Average Distance to Separator | |
| 5 | Shoaling Test | |
| 5-1 | Average Speed | |
| 5-2 | Time in Top Duration | |
| 5-3 | Average Distance to Center of the Tank | |
| 5-4 | Average Inter-fish Distance | |
| 5-5 | Average Shoal Area | |
| 5-6 | Average Nearest Neighbor Distance | |
| 5-7 | Average Farthest Neighbor Distance | |
References
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, S.; Simchi, A.A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic Resonance Imaging Tracking of Stem Cells in Vivo Using Iron Oxide Nanoparticles as a Tool for the Advancement of Clinical Regenerative Medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Long, N.J.; Aboagye, E.O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013, 42, 7816. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, D.; Cai, F.-H.; Xi, W.; Peng, L.; Zhu, Z.-F.; He, H.; Hu, M.-L.; He, S. Observation of Multiphoton-Induced Fluorescence from Graphene Oxide Nanoparticles and Applications in In Vivo Functional Bioimaging. Angew. Chem. Int. Ed. 2012, 51, 10570–10575. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Khanna, L.; Verma, N.; Tripathi, S. Burgeoning tool of biomedical applications—Superparamagnetic nanoparticles. J. Alloys Compd. 2018, 752, 332–353. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2017, 7, 1700845. [Google Scholar] [CrossRef]
- Ansari, S.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef]
- Shah, B.; Yin, P.T.; Ghoshal, S.; Lee, K.B. Multimodal magnetic core–shell nanoparticles for effective stem-cell differentiation and imaging. Angew. Chem. Int. Ed. 2013, 52, 6190–6195. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P.; Patrick, C. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Lu, A.-H.; Zhang, X.-Q.; Sun, Q.; Zhang, Y.; Song, Q.; Schüth, F.; Chen, C.; Cheng, F. Precise synthesis of discrete and dispersible carbon-protected magnetic nanoparticles for efficient magnetic resonance imaging and photothermal therapy. Nano Res. 2016, 9, 1460–1469. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Ahmed, W. Nanoparticulate Drug Delivery Systems for Oral Cancer Treatment. In Emerging Nanotechnologies in Dentistry; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 333–345. [Google Scholar]
- Sun, S.-N.; Wei, C.; Zhu, Z.-Z.; Hou, Y.; Venkatraman, S.; Xu, Z.J.; Venkatraman, S. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite–Maghemite Nanoparticles in the 5–15 nm Range: Correlating the Core–Shell Composition and the Surface Structure to the Magnetic Properties. A Total Scattering Study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Xia, T.; Wang, J.; Wu, C.; Meng, F.; Shi, Z.; Lian, J.; Feng, J.; Meng, J. Novel complex-coprecipitation route to form high quality triethanolamine-coated Fe3O4 nanocrystals: Their high saturation magnetizations and excellent water treatment properties. CrystEngComm 2012, 14, 5741–5744. [Google Scholar] [CrossRef]
- Yang, J.; Fan, L.; Xu, Y.; Xia, J. Iron oxide nanoparticles with different polymer coatings for photothermal therapy. J. Nanoparticle Res. 2017, 19, 333. [Google Scholar] [CrossRef]
- Xia, A.; Hu, J.; Wang, C.; Jiang, D. Synthesis of Magnetic Microspheres with Controllable Structure via Polymerization-Triggered Self-Positioning of Nanocrystals. Small 2007, 3, 1811–1817. [Google Scholar] [CrossRef]
- Zeng, J.; Jing, L.; Hou, Y.; Jiao, M.; Qiao, R.; Jia, Q.; Liu, C.; Fang, F.; Lei, H.; Gao, M.; et al. Anchoring Group Effects of Surface Ligands on Magnetic Properties of Fe3O4Nanoparticles: Towards High Performance MRI Contrast Agents. Adv. Mater. 2014, 26, 2694–2698. [Google Scholar] [CrossRef]
- Yue, Q.; Li, J.-L.; Luo, W.; Zhang, Y.; Elzatahry, A.; Wang, X.; Wang, C.; Li, W.; Cheng, X.; Alghamdi, A.; et al. An Interface Coassembly in Biliquid Phase: Toward Core–Shell Magnetic Mesoporous Silica Microspheres with Tunable Pore Size. J. Am. Chem. Soc. 2015, 137, 13282–13289. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Tong, H.-W.; Liu, S.-M.; Yong, G.-P.; Guan, Y.-F. An improved Stöber method towards uniform and monodisperse Fe3O4@C nanospheres. J. Mater. Chem. A 2013, 1, 7488. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell Thickness-Dependent Microwave Absorption of Core–Shell Fe3O4@C Composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Koch, B.; Bachmatiuk, A.; El-Gendy, A.A.; Krupskaya, Y.; Springer, A.; Klingeler, R.; Schmidt, O.G.; Buchner, B.; Sanchez, S.; et al. Synthesis and toxicity characterization of carbon coated iron oxide nanoparticles with highly defined size distributions. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Song, Y.-J.; Hsu, H.-S.; Lin, C.-R.; Huang, J.-Y.; Chen, J. Magnetic enhancement of carbon-encapsulated magnetite nanoparticles. J. Alloys Compd. 2019, 790, 716–722. [Google Scholar] [CrossRef]
- Lu, A.-H.; Hao, G.-P.; Sun, Q.; Zhang, X.-Q.; Li, W.-C. Chemical Synthesis of Carbon Materials With Intriguing Nanostructure and Morphology. Macromol. Chem. Phys. 2012, 213, 1107–1131. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, D.; Zou, Y.; Wu, Z.; Li, F.; Che, R.; Deng, Y.; Tu, B.; Zhao, D. A Low-Concentration Hydrothermal Synthesis of Biocompatible Ordered Mesoporous Carbon Nanospheres with Tunable and Uniform Size. Angew. Chem. Int. Ed. 2010, 49, 7987–7991. [Google Scholar] [CrossRef]
- Kumar, V.; Toffoli, G.; Rizzolio, F. Fluorescent Carbon Nanoparticles in Medicine for Cancer Therapy. ACS Med. Chem. Lett. 2013, 4, 1012–1013. [Google Scholar] [CrossRef]
- Ershova, E.; Sergeeva, V.; Chausheva, A.; Zheglo, D.; Nikitina, V.; Smirnova, T.; Kameneva, L.; Porokhovnik, L.; Kutsev, S.; Troshin, P.; et al. Toxic and DNA damaging effects of a functionalized fullerene in human embryonic lung fibroblasts. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 805, 46–57. [Google Scholar] [CrossRef]
- Kyzyma, E.A.; Tomchuk, A.A.; Bulavin, L.; Petrenko, V.; Almasy, L.; Korobov, M.V.; Volkov, D.; Mikheev, I.; Koshlan, I.V.; Koshlan, N.A.; et al. Structure and toxicity of aqueous fullerene C60 solutions. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 1–5. [Google Scholar] [CrossRef]
- Kuznetsova, G.P.; Larina, O.V.; Petushkova, N.A.; Kisrieva, Y.S.; Samenkova, N.F.; Trifonova, O.P.; Karuzina, I.I.; Ipatova, O.M.; Zolotaryov, K.V.; Romashova, Y.A.; et al. Effects of Fullerene C60 on Proteomic Profile of Danio Rerio Fish Embryos. Bull. Exp. Biol. Med. 2014, 156, 694–698. [Google Scholar] [CrossRef]
- D’Amora, M.; Rodio, M.; Bartelmess, J.; Sancataldo, G.; Brescia, R.; Zanacchi, F.C.; Diaspro, A.; Giordani, S. Biocompatibility and biodistribution of functionalized carbon nano-onions (f-CNOs) in a vertebrate model. Sci. Rep. 2016, 6, 33923. [Google Scholar] [CrossRef]
- AlizadehZeinabad, H.; Zarrabian, A.; Saboury, A.A.; Alizadeh, A.M.; Falahati, M. Interaction of single and multi wall carbon nanotubes with the biological systems: Tau protein and PC12 cells as targets. Sci. Rep. 2016, 6, 26508. [Google Scholar] [CrossRef]
- Zhou, L.; Forman, H.J.; Ge, Y.; Lunec, J. Multi-walled carbon nanotubes: A cytotoxicity study in relation to functionalization, dose and dispersion. Toxicol. In Vitro 2017, 42, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ying, G.-G.; Jones, K.C.; Martin, F.L. Real-world carbon nanoparticle exposures induce brain and gonadal alterations in zebrafish (Danio rerio) as determined by biospectroscopy techniques. Analyst 2015, 140, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.D.S.; Matsubara, E.Y.; Franchi, L.; Martins, I.P.; Rivera, L.M.R.; Rosolen, J.; Grisolia, C.K. Evaluation of carbon nanotubes network toxicity in zebrafish (danio rerio) model. Environ. Res. 2014, 134, 9–16. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tománková, K.B.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zbořil, R. Toxicity of carbon dots – Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Emam, A.N.; Loutfy, S.A.; A Mostafa, A.; Awad, H.M.; Mohamed, M.B. Cyto-toxicity, biocompatibility and cellular response of carbon dots–plasmonic based nano-hybrids for bioimaging. RSC Adv. 2017, 7, 23502–23514. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Kumar, V.B.; Karasik, D.; Gedanken, A. Fluorescent Nanoparticles with Tissue-Dependent Affinity for Live Zebrafish Imaging. ACS Appl. Mater. Interfaces 2017, 9, 18557–18565. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alfawaz, M.A.; Alshatwi, A.A. Carbon nanoparticle induced cytotoxicity in human mesenchymal stem cells through upregulation of TNF3, NFKBIA and BCL2L1 genes. Chemosphere 2016, 144, 275–284. [Google Scholar] [CrossRef]
- Esquivel-Gaón, M.; Nguyen, N.H.; Sgroi, M.F.; Pullini, D.; Gili, F.; Mangherini, D.; Pruna, A.I.; Rosická, P.; Wang, G.; Castagnola, V. In vitro and environmental toxicity of reduced graphene oxide as an additive in automotive lubricants. Nanoscale 2018, 10, 6539–6548. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, J.-R.; Sarasamma, S.; Audira, G.; Siregar, P.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Ger, T.-R.; Hsiao, C.-D. Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing. Nanomaterials 2019, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lu, J.; Zhao, D. Effects of starch-coating of magnetite nanoparticles on cellular uptake, toxicity and gene expression profiles in adult zebrafish. Sci. Total Environ. 2018, 622, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, S.; Cai, Z. Toxicity Assessment of Iron Oxide Nanoparticles in Zebrafish (Danio rerio) Early Life Stages. PLoS ONE 2012, 7, e46286. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Chung, T.-H.; Wu, S.-H.; Yao, M.; Lu, C.-W.; Lin, Y.-S.; Hung, Y.; Mou, C.-Y.; Chen, Y.-C.; Huang, D.-M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef]
- Weil, M.; Meißner, T.; Busch, W.; Springer, A.; Kühnel, D.; Schulz, R.; Duis, K. The oxidized state of the nanocomposite Carbo-Iron® causes no adverse effects on growth, survival and differential gene expression in zebrafish. Sci. Total. Environ. 2015, 530, 198–208. [Google Scholar] [CrossRef]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behav. Ecol. 2007, 18, 556–562. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A Versatile Setup for Measuring Multiple Behavior Endpoints in Zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef] [PubMed]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Lai, Y.-H.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity. Int. J. Mol. Sci. 2018, 19, 3853. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.E.; Rosemberg, D.B. Measures of Anxiety in Zebrafish (Danio rerio): Dissociation of Black/White Preference and Novel Tank Test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Collins, C.; Kyzar, E.J.; Pham, M.; Roth, A.; Gaikwad, S.; Cachat, J.; Stewart, A.M.; Landsman, S.; Grieco, F.; et al. Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 2012, 210, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ. Biol. Fishes 2006, 80, 91–101. [Google Scholar] [CrossRef]
- Ahmed, O.; Séguin, D.; Gerlai, R. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2010, 216, 166–171. [Google Scholar] [CrossRef]
- Abaid, N.; Spinello, C.; Laut, J.; Porfiri, M. Zebrafish (Danio rerio) responds to images animated by mathematical models of animal grouping. Behav. Brain Res. 2012, 232, 406–410. [Google Scholar] [CrossRef]
- Pavlov, D.; Kasumyan, A. Patterns and mechanisms of schooling behavior in fish: A review. J. Ichthyol. 2000, 40, S163. [Google Scholar]
- Shaw, E. The Development of Schooling Behavior in Fishes. Physiol. Zoöl. 1960, 33, 79–86. [Google Scholar] [CrossRef]
- Miller, N.Y.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Da Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, K.S. Possible Role of Oxidative Damage in Metal-Induced Carcinogenesis. Cancer Investig. 1995, 13, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Vallyathan, V.; Shi, X.; Castranova, V. Reactive oxygen species: Their relation to pneumoconiosis and carcinogenesis. Environ. Health Perspect. 1998, 106, 1151–1155. [Google Scholar] [PubMed]
- Liu, T.; Wang, R.; Cao, H.; Lin, A. Polyaspartic acid alleviates heavy metal toxicity in zebrafish (Danio rerio). Chem. Ecol. 2017, 33, 684–693. [Google Scholar] [CrossRef]
- Jemec, A.; Drobne, D.; Tisler, T.; Sepčić, K. Biochemical biomarkers in environmental studies—Lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species. Environ. Sci. Pollut. Res. 2009, 17, 571–581. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, L.; Dong, M.; Wang, J.; Wang, J.; Xie, H.; Liu, T.; Guo, Y. Oxidative Stress and Genotoxicity of the Ionic Liquid 1-Octyl-3-Methylimidazolium Bromide in Zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol. 2014, 67, 261–269. [Google Scholar] [CrossRef]
- Schetinger, M.R.C.; Porto, N.M.; Moretto, M.B.; Morsch, V.M.; Rocha, J.B.T.; Vieira, V.; Moro, F.; Neis, R.T.; Bittencourt, S.; Bonacorso, H.G.; et al. New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem. Res. 2000, 25, 949–955. [Google Scholar] [CrossRef]
- Yang, M.; Ji, L.; Zhang, X.; Fan, Y.; Ren, Z. The relationship between behavior responses and brain acetylcholinesterase (ache) activity of zebrafish (danio rerio) in cadmium stress. Int. J. Fish. Sci. Res. 2017, 1, 1002. [Google Scholar]
- Ren, Q.; Zhang, T.; Li, S.; Ren, Z.; Yang, M.; Pan, H.; Xu, S.; Qi, L.; Chon, T.-S. Integrative Characterization of Toxic Response of Zebra Fish (Danio rerio) to Deltamethrin Based on AChE Activity and Behavior Strength. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Li, T.; Li, X.; Feng, D.; Kuang, X.; Xu, J.; Zhao, X.; Sun, M.; Chen, D.; et al. SiO2 nanoparticles cause depression and anxiety-like behavior in adult zebrafish. RSC Adv. 2017, 7, 2953–2963. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, A.M.M.; Miczek, K.A. Aggressive Behavior, Increased Accumbal Dopamine, and Decreased Cortical Serotonin in Rats. J. Neurosci. 2000, 20, 9320–9325. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, A.M.M.; Miczek, K.A. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology 2006, 191, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Uetake, K.; Okumoto, A.; Tani, N.; Goto, A.; Tanaka, T. Calming effect of orally administered ?-aminobutyric acid in Shih Tzu dogs. Anim. Sci. J. 2012, 83, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Herculano, A.M.; Maximino, C. Serotonergic modulation of zebrafish behavior: Towards a paradox. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Experientia Supplementum (2012); Springer Science and Business Media LLC: London, UK; New York, NY, USA, 2012; Volume 101, pp. 133–164. [Google Scholar]
- Karen, E.; Thomas, M. Reproductive toxicology and teratology. In Principles of Toxicology; Sl. Ewis Puplishers: New York, NY, USA, 1996. [Google Scholar]
- Sarasamma, S.; Audira, G.; Samikannu, P.; Juniardi, S.; Siregar, P.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Behavioral Impairments and Oxidative Stress in the Brain, Muscle, and Gill Caused by Chronic Exposure of C70 Nanoparticles on Adult Zebrafish. Int. J. Mol. Sci. 2019, 20, 5795. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sanetuntikul, J.; Shanmugam, S.; Kim, E. Necrotic cell death caused by exposure to graphitic carbon-coated magnetic nanoparticles. J. Biomed. Mater. Res. Part A 2015, 103, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Grudziński, I.; Bystrzejewski, M.; Cywinska, M.A.; Kosmider, A.; Poplawska, M.; Cieszanowski, A.; Fijalek, Z.; Ostrowska, A. Comparative cytotoxicity studies of carbon-encapsulated iron nanoparticles in murine glioma cells. Colloids Surf. B Biointerfaces 2014, 117, 135–143. [Google Scholar] [CrossRef]
- Goya, G.F.; Marcos-Campos, I.; Fernández-Pacheco, R.; Sáez, B.; Godino, J.; Asin, L.; Lambea, J.; Tabuenca, P.; Mayordomo, J.; Larrad, L. Dendritic cell uptake of iron-based magnetic nanoparticles. Cell Biol. Int. 2008, 32, 1001–1005. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Schimmer, B.B.; Schumacher, C.M.; Gschwind, S.; Kaech, A.; Ziegler, U.; Clavien, P.-A.; Günther, D.; Stark, W.J.; Graf, R.; et al. In vivo risk evaluation of carbon-coated iron carbide nanoparticles based on short- and long-term exposure scenarios. Nanomedicine 2016, 11, 783–796. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the carbon-coated Fe3O4 magnetic nanoparticles are available from the authors. |


| Biomarker | WT | C-MNP | C-MNP | Unit | Significance | ANOVA | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| (1 ppm) | (10 ppm) | F Value | |||||||
| Ferric ion | 1.070 ± 0.4689 | 1.821 ± 0.652 | ns | 0.9054 ± 1.025 | ns | pg/ug of total protein | NO | F (2,6) = 1.089 | p = 0.3949 |
| Metallothionein | 34.41 ± 9.015 | 40.69 ± 14.05 | ns | 27.81 ± 3.921 | ns | pg/ug of total protein | NO | F (2,6) = 0.423 | p = 0.6728 |
| ROS | 317.5 ± 70.68 | 428.8 ± 98.14 | ns | 223.6 ± 9.179 | ns | U/ug of total protein | NO | F (2,6) = 2.152 | p = 0.1975 |
| CAT | 29.05 ± 7.592 | 42.22 ± 10.61 | ns | 23.05 ± 1.903 | ns | U/ug of total protein | NO | F (2,6) = 1.658 | p = 0.2672 |
| TBARS | 183.4 ± 52.11 | 183.9 ± 37.91 | ns | 97.49 ± 6.354 | ns | ng/ug of total protein | NO | F (2,6) = 1.770 | p = 0.2488 |
| Cortisol | 290.6 ± 80.02 | 260.7 ± 85.90 | ns | 142.3 ± 10.09 | ns | pg/ug of total protein | NO | F (2,6) = 1.330 | p = 0.3326 |
| Hif-1α | 147.7 ± 40.59 | 200.5 ± 60.90 | ns | 109.4 ± 4.572 | ns | pg/ug of total protein | NO | F (2,6) =1.167 | p = 0.3732 |
| ATP | 3074 ± 933.2 | 3896 ± 1235 | ns | 1999 ± 152.5 | ns | pg/ug of total protein | NO | F (2,6) = 1.123 | p = 0.3852 |
| Hif-1α | 147.7 ± 40.59 | 200.5 ± 60.90 | ns | 109.4 ± 4.572 | ns | pg/ug of total protein | NO | F (2,6) =1.167 | p = 0.3732 |
| LDH | 24.93 ± 6.257 | 35.61 ± 9.463 | ns | 18.76 ± 1.228 | ns | ng/ug of total protein | NO | F (2,6) = 1.673 | p = 0.2646 |
| Acetylcholine esterase | 50.59 ± 15.76 | 86.45 ± 29.01 | ns | 55.37 ± 2.924 | ns | U/ug of total protein | NO | F (2,6) = 1.035 | p = 0.4109 |
| Acetylcholine | 242.9 ± 58.99 | 414.5 ± 131.4 | ns | 221.6 ± 12.99 | ns | U/ug of total protein | NO | F (2,6) = 1.605 | p = 0.2764 |
| 5-HT | 6.242 ± 1.861 | 8.573 ± 2.885 | ns | 4.663 ± 0.1496 | ns | ng/ug of total protein | NO | F (2,6) = 0.983 | p = 0.4273 |
| Dopamine | 377.3 ± 88.96 | 521.1 ± 186.3 | ns | 297.7 ± 5.453 | ns | pg/ug of total protein | NO | F (2,6) = 0.901 | p = 0.4545 |
| GABA | 1.747 ± 0.4157 | 2.688 ± 0.7480 | ns | 1.491 ± 0.0231 | ns | U/ug of total protein | NO | F (2,6) = 1.402 | p = 0.3165 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, N.; Audira, G.; Chen, J.-R.; Siregar, P.; Hsu, H.-S.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Surface Modification of Magnetic Nanoparticles by Carbon-Coating Can Increase Its Biosafety: Evidences from Biochemical and Neurobehavioral Tests in Zebrafish. Molecules 2020, 25, 2256. https://doi.org/10.3390/molecules25092256
Malhotra N, Audira G, Chen J-R, Siregar P, Hsu H-S, Lee J-S, Ger T-R, Hsiao C-D. Surface Modification of Magnetic Nanoparticles by Carbon-Coating Can Increase Its Biosafety: Evidences from Biochemical and Neurobehavioral Tests in Zebrafish. Molecules. 2020; 25(9):2256. https://doi.org/10.3390/molecules25092256
Chicago/Turabian StyleMalhotra, Nemi, Gilbert Audira, Jung-Ren Chen, Petrus Siregar, Hua-Shu Hsu, Jiann-Shing Lee, Tzong-Rong Ger, and Chung-Der Hsiao. 2020. "Surface Modification of Magnetic Nanoparticles by Carbon-Coating Can Increase Its Biosafety: Evidences from Biochemical and Neurobehavioral Tests in Zebrafish" Molecules 25, no. 9: 2256. https://doi.org/10.3390/molecules25092256
APA StyleMalhotra, N., Audira, G., Chen, J.-R., Siregar, P., Hsu, H.-S., Lee, J.-S., Ger, T.-R., & Hsiao, C.-D. (2020). Surface Modification of Magnetic Nanoparticles by Carbon-Coating Can Increase Its Biosafety: Evidences from Biochemical and Neurobehavioral Tests in Zebrafish. Molecules, 25(9), 2256. https://doi.org/10.3390/molecules25092256









