Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents
Abstract
1. Introduction
2. Results and Discussion
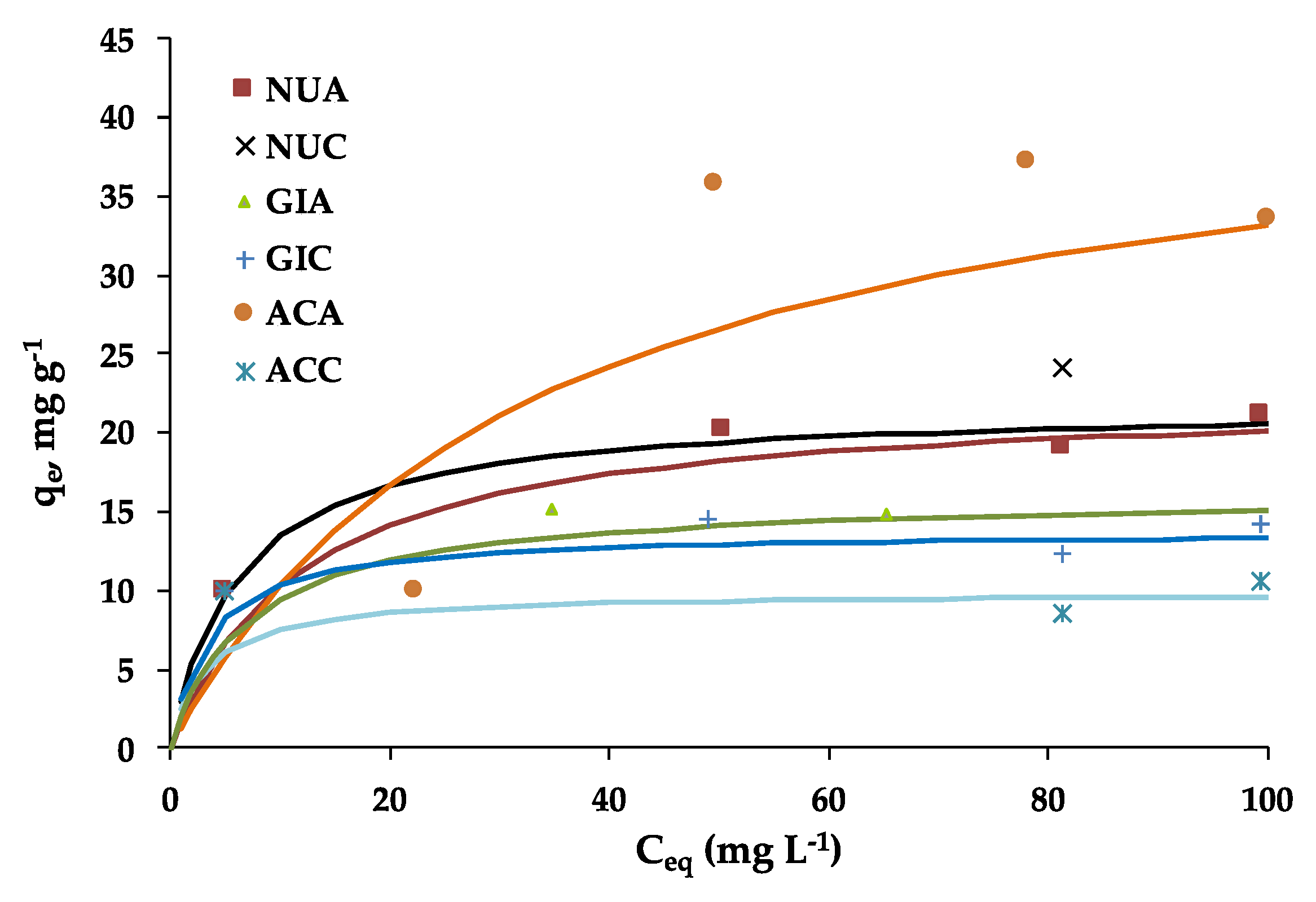
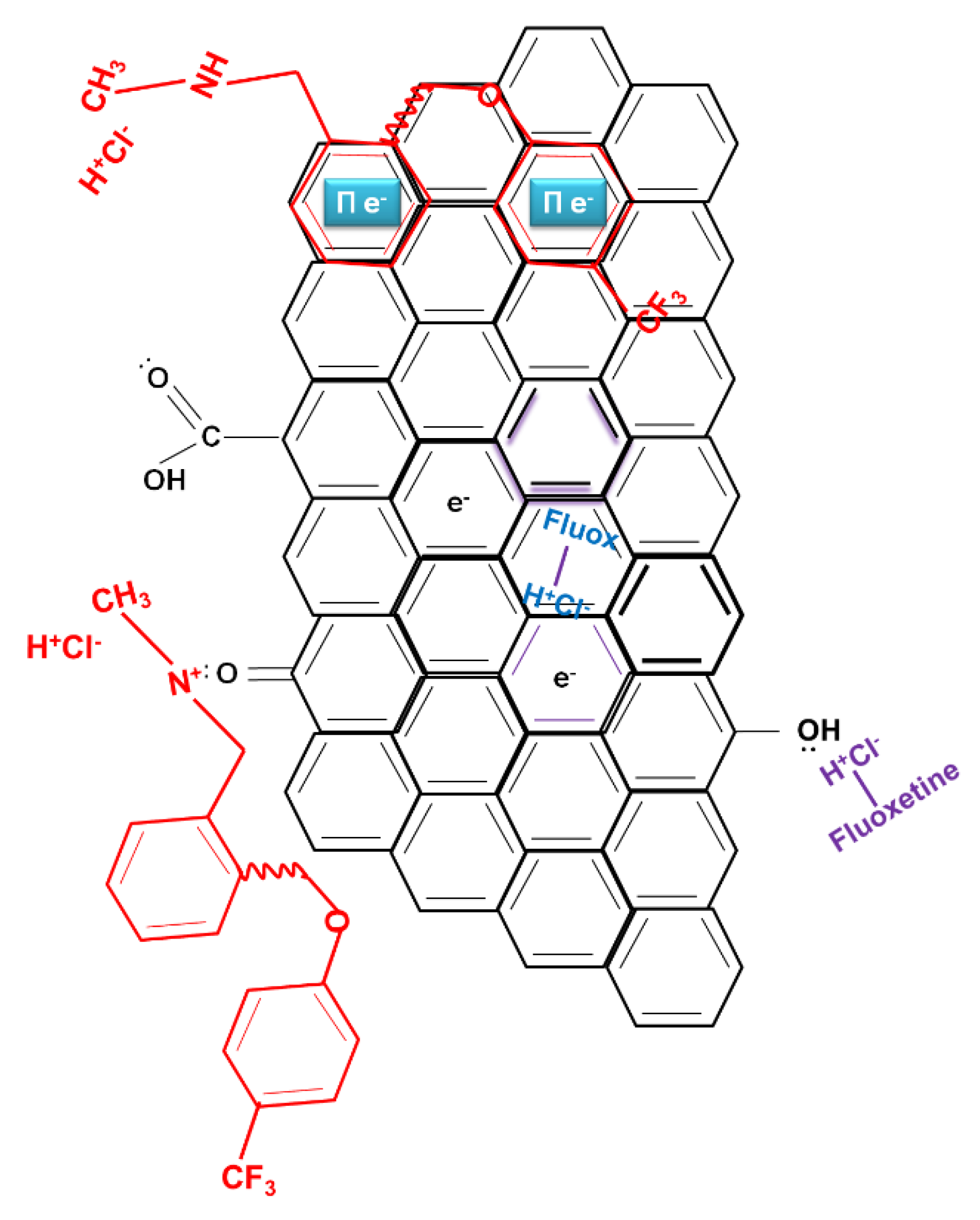
2.1. Fluoxetine Adsorption
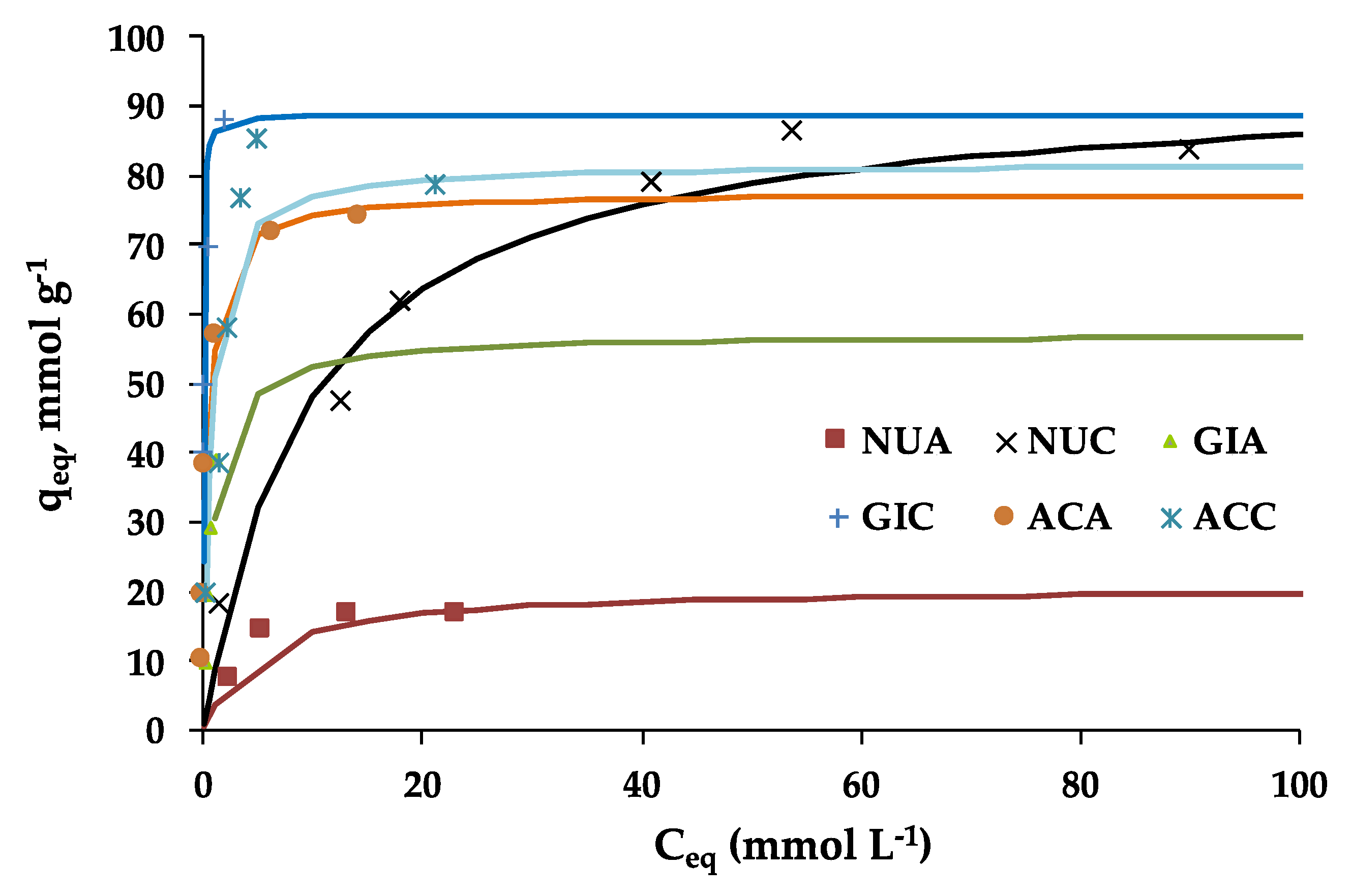
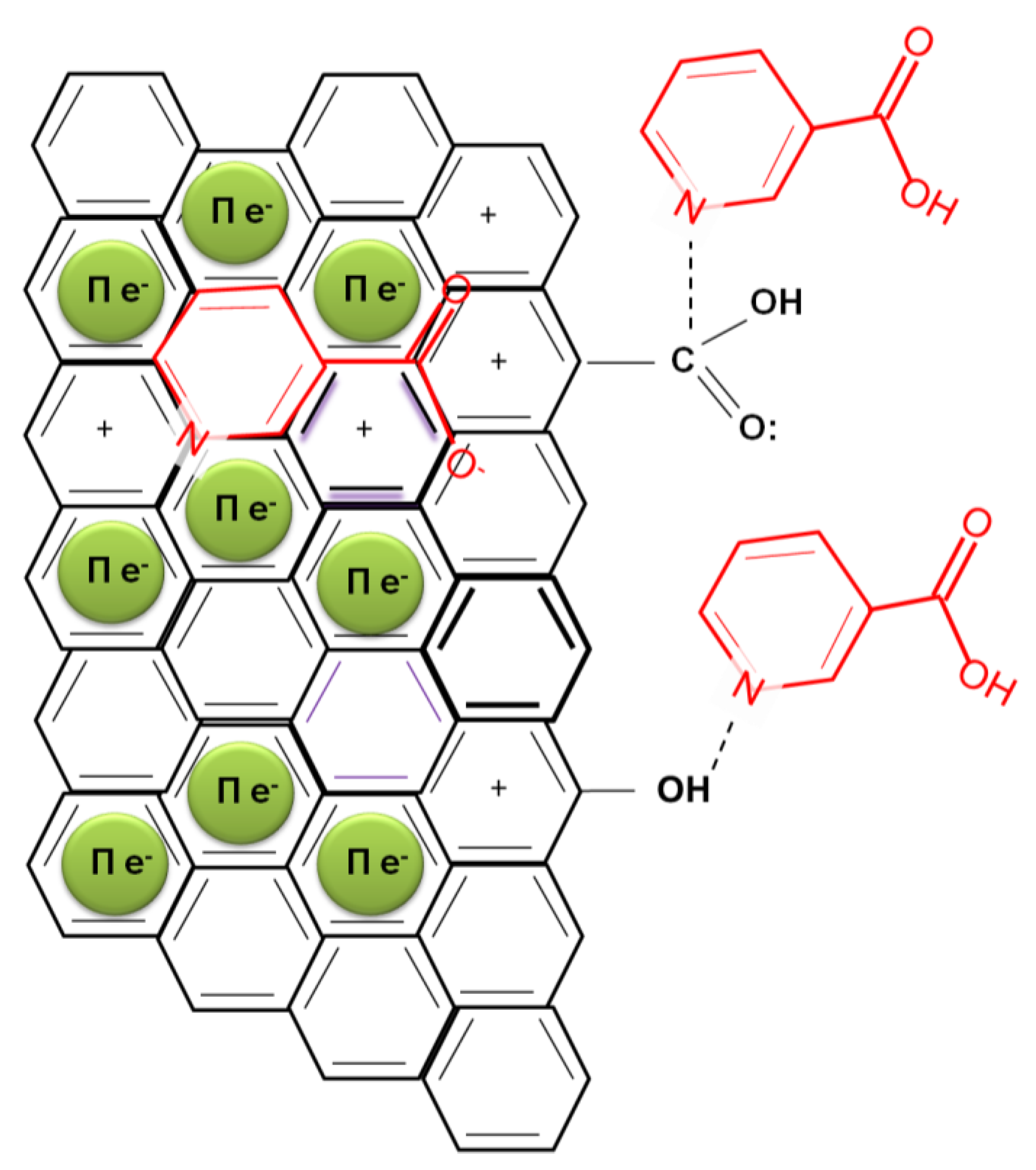
2.2. Nicotinic Acid Adsorption
3. Materials and Methods
3.1. Materials
3.2. Adsorption Isotherms
3.3. Adsorption Models
- (a)
- The Langmuir model:where qe (mg g−1) is the measured adsorption at a drug equilibrium concentration of Ce. Q0 (mg g−1) is the maximum adsorption capacity of the monolayer and KL (L mg−1) is the Langmuir constant, related to the free energy of adsorption.qe = (Q0CeKL)/(1 + CeKL)
- (b)
- The Freundlich model:where KF (mg g−1)(L mg−1)1/n is the Freundlich constant representing the adsorption capacity and n (dimensionless) is the constant depicting the adsorption intensity.qe = KFCe1/n
- (c)
- The Redlich–Peterson model [33] is an empirical isotherm incorporating three parameters (Equation (3)). It combines elements from the Langmuir and Freundlich equation, and the mechanism of adsorption does not follow ideal monolayer adsorption.where KR is an isotherm constant (L g−1), aR is the Redlich–Peterson isotherm parameter (L mg−1), and g is the Redlich–Peterson isotherm exponent.qe = (KRCe)/(1 + aRCeg)
- (d)
- The SIPs model [34] uses an equation similar to the Freundlich equation, but it has a finite limit when the concentration is sufficiently high; in this way, this model avoids the continuous increase in the adsorbed amount with an increasing concentration.where αS is the Sips maximum adsorption capacity (mg g−1), KS the Sips equilibrium constant (L mg−1), and βS the Sips model exponent.qe = (KSCeβS)/(1 + αSCeβS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel. Bioprod. Bior. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Falco, C.; Marco-Lozar, J.P.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M.; Lozano-Castello, D. Tailoring the porosity of chemically activated hydrothermal carbons: Influence of the precursor and hydrothermal carbonization temperature. Carbon 2013, 62, 346–355. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; Álvarez, A.; Coronella, C.; Qaramaleki, S.V. Suitability of hydrothermal carbonization to convert water hyacinth to added-value products. Renew. Energy 2020, 146, 1649–1658. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications: A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Y.Q.; Zhang, Z.B.; Cao, X.H.; Nie, W.B.; Li, Q.; Hua, R. Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl. Surf. Sci. 2013, 273, 68–74. [Google Scholar] [CrossRef]
- Kyzas, G.; Deliyanni, E.A. Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem. Eng. Res. Des. 2015, 97, 135–144. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Biores. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Biores. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, L.; Li, S.; Geng, J.; Song, Q.; Liu, J.; Wang, C.; Wang, H.; Li, J.; Qin, Z.; et al. Simple approach to carboxyl-rich materials through low-temperature heat treatment of hydrothermal carbon in air. Appl. Surf. Sci. 2011, 257, 8686–8691. [Google Scholar] [CrossRef]
- Román, S.; Valente Nabais, J.M.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Micropor. Mesopor. Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Mourão, P.A.M.; Laginhas, C.; Custódio, F.; Nabais, J.M.V.; Carrott, P.J.M.; Carrott, M.M.L. Influence of oxidation process on the adsorption capacity of activated carbons from lignocellulosic precursors. Fuel Process. Technol. 2011, 92, 241–246. [Google Scholar] [CrossRef]
- Farooq, A.; Reinert, L.; Levêque, J.M.; Papaiconomou, N.; Irfan, N.; Duclaux, L. Adsorption of ionic liquids onto activated carbons: Effect of pH and temperature. Micropor. Mesopor. Mater. 2012, 158, 55–63. [Google Scholar] [CrossRef]
- Nunell, G.V.; Fernández, M.E.; Bonelli, P.R.; Cukierman, A.L. Conversion of biomass from an invasive species into activated carbons for removal of nitrate from wastewater. Biomass Bioenerg 2012, 44, 87–95. [Google Scholar] [CrossRef]
- OECD/European Union (2018). Health at a Glance: Europe 2018: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Li, Y.; Zhang, S.; Zhang, W.; Xiong, W.; Ye, Q.; Hou, X.; Wang, C.; Wang, P. Life cycle assessment of advanced wastewater treatment processes: Involving 126 pharmaceuticals and personal care products in life cycle inventory. J. Env. Manag. 2019, 238, 442–450. [Google Scholar] [CrossRef]
- Kato, M.; Fukuda, T.; Serretti, A.; Wakeno, M.; Okugawa, G.; Ikenaga, Y.; Hosoi, Y.; Takekita, Y.; Mandelli, L.; Azuma, J.; et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog. Neuro. Psychoph. 2008, 32, 398–404. [Google Scholar] [CrossRef]
- Sousa-Moura, D.; Matsubara, E.Y.; Ferraz, I.B.M.; de Oliveira, R.; Szlachetka, I.O.; da Silva, S.W.; Camargo, N.S.; Rosolen, J.M.; Grisolia, C.K.; da Rocha, M.C.O. CNTs coated charcoal as a hybrid composite material: Adsorption of fluoxetine probeby zebrafish embryos and its potential for environmental remediation. Chemosphere 2019, 230, 369–376. [Google Scholar] [CrossRef]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lagoa, A.; Nevesa, I.C.; Tavares, T. Waste-based biosorbents as cost-effective alternatives to commercial adsorbents for the retention of fluoxetine from water. Sep. Purif. 2020, 235, 116–139. [Google Scholar] [CrossRef]
- Bruckert, E.; Labreuche, J.; Amarenco, P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis 2010, 210, 353–361. [Google Scholar] [CrossRef]
- Datta, D.; Sah, S.; Rawat, N.; Kumar, R. Application of Magnetically Activated Carbon for the Separation of Nicotinic Acid from Aqueous Solution. J. Chem. Eng. Data 2017, 62, 712–719. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I, Theoretical. J. Colloid Interf. Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteve, I. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J. Colloid Interf. Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Nabais, J.M.V.; González, J.F.; González-García, C.M.; Ortiz, A.L. Study of the Contributions of Non-Specific and Specific Interactions during Fluoxetine Adsorption onto Activated Carbons. Clean-Soil Air Water 2012, 40, 698–705. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Mouquinho, A.; Galacho, C.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. In Vitro Adsorption Study of Fluoxetine in Activated Carbons and Activated Carbon Fibres. Fuel Process. Technol. 2008, 89, 549–555. [Google Scholar] [CrossRef]
- Apple, C.A.; Wittenberg, B.A.; Wittenberg, B.J. Nicotinic Acid as a Ligand Affecting Leghemoglobin Structure and Oxygen Reactivity. PNAS 1973, 70, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, E.; Duman, O. Adsorption of aromatic organic acids onto high area activated carbon cloth in relation to wastewater purification. J. Hazard. Mater. 2006, 36, 542–552. [Google Scholar] [CrossRef]
- Qureshi, M.; Varshney, K.G.; Zuber, K.; Ahmad, A. Adsorption of Tertiary Nitrogen-Containing Compounds on Activated Carbon. I. Equilibrium Studies of Nicotinic Acid in Aqueous Systems. Colloid Surf. 1990, 50, 7–16. [Google Scholar] [CrossRef]
- Román, S.; González, J.F.; Encinar, J.M. Olive stone: A source of energy generation and a suitable precursor for activated carbon production. In Proceedings of the International Conference on Renewable Energies and Power Quality, Santander, Spain, 13–15 March 2008. [Google Scholar]
- Roman, S.; González, J.F.; González-García, C.M.; Martínez, G.; Encinar, J.M. The adsorptive properties of air activated carbons from walnut-shells. In Proceedings of the 11th Mediterranean Congress of Chemical Engineering, Barcelona, Spain, 21–24 October 2008. [Google Scholar]
- Domínguez, F. Empleo de hydrochars biomásicos como precursores de carbones activos ácidos y básicos para la adsorción de compuestos iónicos. Ph.D. Thesis, University of Extremadura, Badajoz, Spain, 2013. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1026. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Fluoxetine | Parameters | SFA | SFC | OSA | OSC | WSA | WSC |
| Langmuir | q0 (mg g−1) | 16.05 | 13.77 | 44.07 | 9.87 | 22.43 | 26.26 |
| KL (L mg−1) | 0.14 | 0.77 | 0.03 | 0.44 | 0.07 | 0.40 | |
| R2 | 1.00 | 0.98 | 0.95 | 0.99 | 0.99 | 0.98 | |
| Freundlich | KF (mg g−1)(L mg−1)1/n | 6.10 | 8.59 | 2.28 | 5.06 | 0.26 | 9.70 |
| n | 3.02 | 9.35 | 1.62 | 15.40 | 4.26 | 4.72 | |
| R2 | 0.97 | 0.71 | 0.95 | 0.85 | 0.97 | 0.75 | |
| Redlich-Peterson | KR (L g−1) | 23.00 | 32.00 | 72.40 | 25.70 | 26.02 | 20.10 |
| aR (L mg−1) | 1.45 | 2.10 | 1.24 | 3.12 | 2.41 | 0.82 | |
| g | 0.91 | 1.00 | 0.98 | 0.98 | 0.91 | 1.03 | |
| R2 | 0.88 | 0.75 | 0.93 | 0.83 | 0.78 | 0.78 | |
| Sips | KS (L mg−1) | 0.66 | 0.77 | 0.85 | 0.94 | 0.76 | 0.88 |
| αS (mg g−1) | 16.20 | 14.20 | 34.50 | 9.55 | 21.20 | 24.50 | |
| βS | 0.85 | 1.35 | 0.46 | 0.68 | 0.59 | 0.71 | |
| R2 | 0.96 | 0.96 | 0.93 | 0.81 | 0.96 | 0.96 | |
| Nicotinic Acid | Parameters | SFA | SFC | OSA | OSC | WSA | WSC |
| Langmuir | q0 (mg g−1) | 57.21 | 89.59 | 77.44 | 81.93 | 16.18 | 91.91 |
| KL (L mg−1) | 1.14 | 4.41 | 2.42 | 1.64 | 0.86 | 0.12 | |
| R2 | 1.000 | 0.999 | 1.000 | 0.814 | 0.999 | 0.992 | |
| Freundlich | KF (mg g−1)(L mg−1)1/n | 20.42 | 66.31 | 21.68 | 41.53 | 5.77 | 17.43 |
| n | 4.49 | 2.90 | 7.25 | 4.09 | 3.82 | 2.58 | |
| R2 | 0.76 | 0.98 | 0.98 | 0.84 | 0.89 | 0.96 | |
| Redlich-Peterson | KR (L g−1) | 28.90 | 286.55 | 212.00 | 224.50 | 26.02 | 30.61 |
| aR (L mg−1) | 0.49 | 3.12 | 2.33 | 3.60 | 2.13 | 1.14 | |
| g | 1.00 | 0.99 | 0.65 | 0.85 | 0.99 | 0.69 | |
| R2 | 0.81 | 0.96 | 0.92 | 0.74 | 0.94 | 0.99 | |
| Sips | KS (L mg−1) | 4.20 | 4.35 | 3.90 | 4.20 | 4.70 | 4.15 |
| αS (mg g−1) | 52.00 | 89.20 | 75.50 | 78.60 | 13.70 | 88.50 | |
| βS | 1.60 | 1.75 | 1.03 | 1.98 | 0.36 | 0.18 | |
| R2 | 0.99 | 0.99 | 0.89 | 0.82 | 0.92 | 0.94 |
| Samples | SBET m2 g−1 | Vmi cm3 g−1 | Vme cm3 g−1 | Vma cm3 g−1 | PZC |
|---|---|---|---|---|---|
| WSA | 213 | 0.105 | 0.052 | 2.361 | 4.43 |
| WSC | 379 | 0.196 | 0.017 | 2.253 | 8.53 |
| SFA | 434 | 0.228 | 0.031 | 6.292 | 4.25 |
| SFC | 438 | 0.230 | 0.047 | 5.211 | 8.12 |
| OSA | 204 | 0.115 | 0.002 | 2.094 | 4.05 |
| OSC | 438 | 0.231 | 0.006 | 3.558 | 9.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, S.; Valente Nabais, J.M.; Ledesma, B.; Laginhas, C.; Titirici, M.-M. Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents. Molecules 2020, 25, 2264. https://doi.org/10.3390/molecules25092264
Román S, Valente Nabais JM, Ledesma B, Laginhas C, Titirici M-M. Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents. Molecules. 2020; 25(9):2264. https://doi.org/10.3390/molecules25092264
Chicago/Turabian StyleRomán, Silvia, Joâo Manuel Valente Nabais, Beatriz Ledesma, Carlos Laginhas, and Maria-Magdalena Titirici. 2020. "Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents" Molecules 25, no. 9: 2264. https://doi.org/10.3390/molecules25092264
APA StyleRomán, S., Valente Nabais, J. M., Ledesma, B., Laginhas, C., & Titirici, M.-M. (2020). Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents. Molecules, 25(9), 2264. https://doi.org/10.3390/molecules25092264