Therapeutic Nanoparticles and Their Targeted Delivery Applications
Abstract
1. Introduction
2. Designing Nanoparticles for Therapeutics
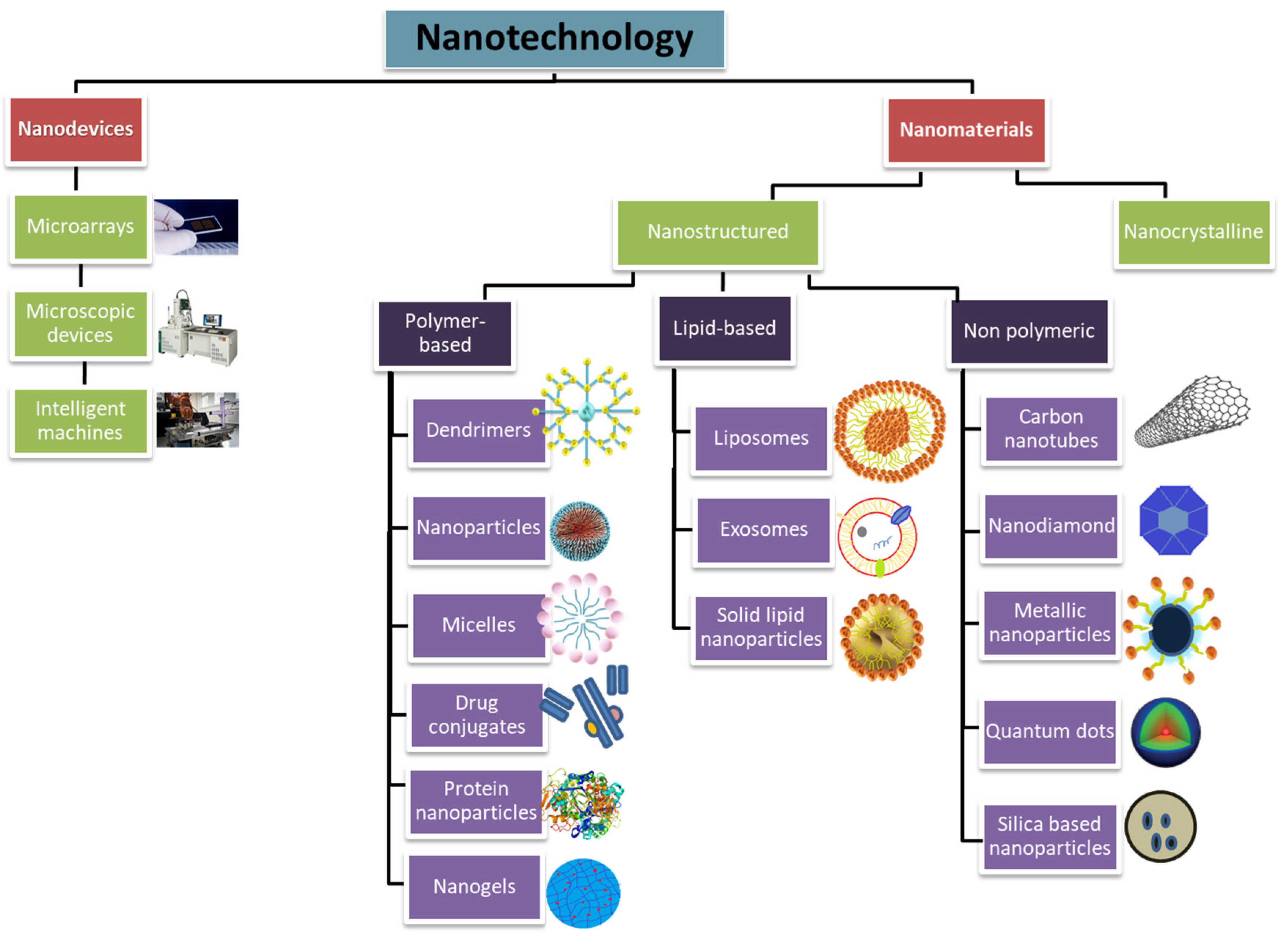
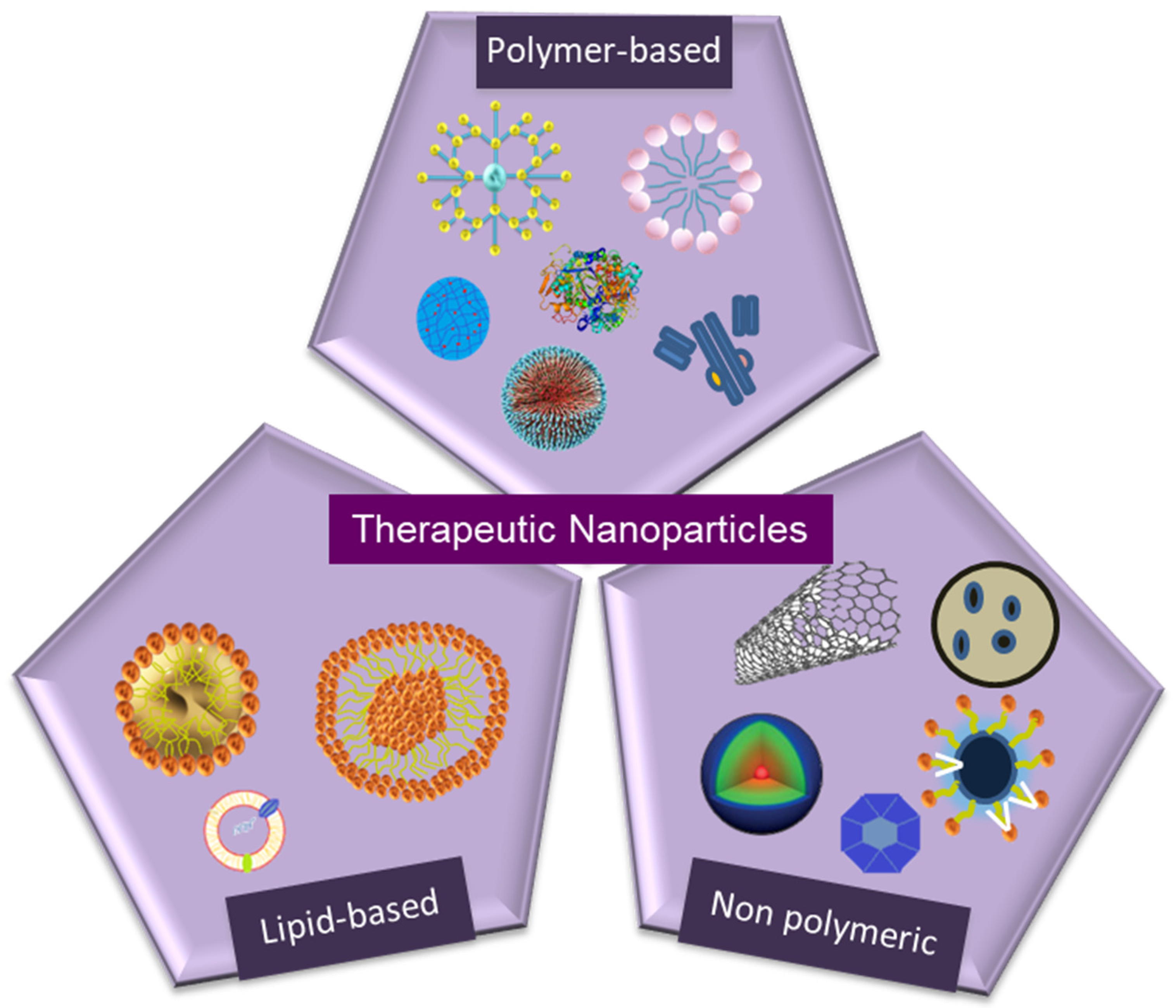
3. Types of Therapeutic Nanoparticles
3.1. Nano-Structured Particles
3.1.1. Polymer-Based Particles
Dendrimers
Nanoparticles
Micelles
Drug Conjugates
Protein Nanoparticles
Nanogels
3.1.2. Non-Polymeric Particles
Carbon Nanotubes
Nanodiamonds (NDs)
Metallic Nanoparticles
Quantum Dots
Silica-Based Nanoparticles
3.1.3. Lipid-Based Nanoparticles
Liposomes
Exosomes
Solid Lipid Nanoparticles (SLN)
3.2. Nanocrystalline Particles
4. Targeted Delivery Applications of Therapeutic Nanoparticles
4.1. Cancer
4.2. Infectious Diseases
4.3. Autoimmune Diseases
4.4. Cardiovascular Diseases
4.5. Neurodegenerative Diseases
4.6. Ocular Diseases
4.7. Pulmonary Diseases
4.8. Regenerative Therapy
5. Limitations and Disadvantages of Therapeutic Nanoparticles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chandrasekhar, S.; Iyer, L.K.; Panchal, J.P.; Topp, E.M.; Cannon, J.B.; Ranade, V.V. Microarrays and microneedle arrays for delivery of peptides, proteins, vaccines and other applications. Expert Opin. Drug Deliv. 2013, 10, 1155–1170. [Google Scholar] [CrossRef]
- Rabl, P.; Kolkowitz, S.J.; Koppens, F.H.L.; Harris, J.G.E.; Zoller, P.; Lukin, M.D. A quantum spin transducer based on nanoelectromechanical resonator arrays. Nat. Phys. 2010, 6, 602–608. [Google Scholar] [CrossRef]
- Shabnashmi, P.S.; Naga Kani, S.; Vithya, V.; Vijaya Lakshmi, B.; Jasmine, R. Therapeutic applications of Nanorobots-Respirocytes and Microbivores. J. Chem. Pharm. Res. 2016, 8, 605–609. [Google Scholar]
- Kadam, R.S.; Bourne, D.W.; Kompella, U.B. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: Contribution of reduced clearance. Drug Metab. Dispos 2012, 40, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, J.W.; Pullan, J.E.; Dailey, K.M.; Mallik, S.; Brooks, A.E. Overcoming Hurdles in Nanoparticle Clinical Translation: The Influence of Experimental Design and Surface Modification. Int. J. Mol. Sci. 2019, 20, 6056. [Google Scholar] [CrossRef] [PubMed]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.B.; Li, J.S.; Durkan, C.; Wang, N.; Wang, G.X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038–3058. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Bhatia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-Dependent Endocytosis of Nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, T.; Li, Y.; Huang, G.; White, M.A.; Gao, J. Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes. Adv. Drug Deliv. Rev. 2017, 113, 87–96. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Letchford, K.; Liggins, R.; Wasan, K.M.; Burt, H. In vitro human plasma distribution of nanoparticulate paclitaxel is dependent on the physicochemical properties of poly(ethylene glycol)-block-poly(caprolactone) nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 196–206. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Markovsky, E.; Baabur-Cohen, H.; Satchi-Fainaro, R. Anticancer polymeric nanomedicine bearing synergistic drug combination is superior to a mixture of individually-conjugated drugs. J. Control. Release 2014, 187, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Mondal, G.; Wen, D.; Mahato, R.I. Combination therapy of paclitaxel and cyclopamine polymer-drug conjugates to treat advanced prostate cancer. Nanomed. -Uk 2017, 13, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Jiang, Y.; Xiao, Q.; Leung, A.W.; Hua, H.; Xu, C. pH-responsive polymer-drug conjugates: Design and progress. J. Control. Release 2016, 222, 116–129. [Google Scholar] [CrossRef]
- Lv, S.; Tang, Z.; Zhang, D.; Song, W.; Li, M.; Lin, J.; Liu, H.; Chen, X. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J. Control. Release 2014, 194, 220–227. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Cheetham, A.G.; Moon, J.H.; Moxley, J.W., Jr.; Lin, Y.A.; Cui, H. Controlled release of free doxorubicin from peptide-drug conjugates by drug loading. J. Control. Release 2014, 191, 123–130. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J. Control. Release 2015, 212, 94–102. [Google Scholar] [CrossRef]
- Hill, B.D.; Zak, A.; Khera, E.; Wen, F. Engineering Virus-like Particles for Antigen and Drug Delivery. Curr. Protein Pept. Sci. 2018, 19, 112–127. [Google Scholar] [CrossRef]
- Lopez-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.-W. Protein-based nanoparticles in cancer vaccine development. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 164–174. [Google Scholar] [CrossRef]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed. Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef]
- Herrera Estrada, L.P.; Champion, J.A. Protein nanoparticles for therapeutic protein delivery. Biomater. Sci. 2015, 3, 787–799. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Garg, T.; Aman, A.; Panchal, K.; Sharma, R.; Kumar, S.; Markandeywar, T. Nanogel—An advanced drug delivery tool: Current and future. Artif. Cells Nanomed. Biotechnol. 2016, 44, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Reilly, R.M. Carbon nanotubes: Potential benefits and risks of nanotechnology in nuclear medicine. J. Nucl. Med. 2007, 48, 1039–1042. [Google Scholar] [CrossRef]
- Mroz, P.; Pawlak, A.; Satti, M.; Lee, H.; Wharton, T.; Gali, H.; Sarna, T.; Hamblin, M.R. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic Biol. Med. 2007, 43, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Demidova, T.N.; Arcila-lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic Fullerenes Are Effective and Selective Antimicrobial Photosensitizers. NIH Public Access. 2011, 12, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Bosi, S.; Da Ros, T.; Castellano, S.; Banfi, E.; Prato, M. Antimycobacterial activity of ionic fullerene derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1043–1045. [Google Scholar] [CrossRef]
- Ji, H.; Yang, Z.; Jiang, W.; Geng, C.; Gong, M.; Xiao, H.; Wang, Z.; Cheng, L. Antiviral activity of nano carbon fullerene lipidosome against influenza virus in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 243–246. [Google Scholar] [CrossRef]
- Cai, X.; Jia, H.; Liu, Z.; Hou, B.; Luo, C.; Feng, Z.; Li, W.; Liu, J. Polyhydroxylated fullerene derivative C(60)(OH)(24) prevents mitochondrial dysfunction and oxidative damage in an MPP(+) -induced cellular model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 3622–3634. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Torres Sangiao, E.; Holban, A.M.; Gestal, M.C. Applications of Nanodiamonds in the Detection and Therapy of Infectious Diseases. Mater. (Basel) 2019, 12, 1639. [Google Scholar] [CrossRef]
- Man, H.B.; Ho, D. Nanodiamonds as platforms for biology and medicine. J. Lab. Autom 2013, 18, 12–18. [Google Scholar] [CrossRef]
- Ho, D.; Wang, C.H.; Chow, E.K. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef]
- van der Laan, K.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds for In Vivo Applications. Small 2018, 14, 1703838. [Google Scholar] [CrossRef]
- Tinwala, H.; Wairkar, S. Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics. Mater. Sci. Eng. C 2019, 97, 913–931. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Chakrabarti, R.; Chattopadhyay, K.K.; Chaudhuri, S.; Pal, A.K. Nano-diamond films produced from CVD of camphor. Diam. Relat. Mater. 1998, 7, 845–852. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Jiang, H.; Hochwald, S.N.; Delano, M.; Cance, W.G.; Grobmyer, S.R. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 2006, 107, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Gao, W.; Chen, Z.; Fan, H.; Li, M.; Deng, F.; Chen, Z. Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int J. Pharm. 2011, 404, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Andocs, G.; Renner, H.; Balogh, L.; Fonyad, L.; Jakab, C.; Szasz, A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol 2009, 185, 120–126. [Google Scholar] [CrossRef]
- Pandey, B.; Shetake, N.; Balla, M.S.; Kumar, A. Magnetic hyperthermia therapy: An emerging modality of cancer treatment in combination with radiotherapy. J. Radiat. Cancer Res. 2016, 7, 13. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Tian, L.; Lu, L.; Qiao, Y.; Ravi, S.; Salatan, F.; Melancon, M.P. Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy. J. Funct. Biomater. 2016, 7, 19. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W.; et al. Multifunctional Photonic Nanomaterials for Diagnostic, Therapeutic, and Theranostic Applications. Adv. Mater. 2018, 30, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Iga, A.M.; Robertson, J.H.; Winslet, M.C.; Seifalian, A.M. Clinical potential of quantum dots. J. Biomed. Biotechnol. 2007, 2007, 76087. [Google Scholar] [CrossRef] [PubMed]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.E.; Smith, A.M.; Nie, S. Quantum dots in biology and medicine. Phys. E Low-Dimens. Syst. Nanostructures 2004, 25, 1–12. [Google Scholar] [CrossRef]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Bharali, D.J.; Klejbor, I.; Stachowiak, E.K.; Dutta, P.; Roy, I.; Kaur, N.; Bergey, E.J.; Prasad, P.N.; Stachowiak, M.K. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11539–11544. [Google Scholar] [CrossRef]
- Bagheri, E.; Ansari, L.; Abnous, K.; Taghdisi, S.M.; Charbgoo, F.; Ramezani, M.; Alibolandi, M. Silica based hybrid materials for drug delivery and bioimaging. J. Control. Release 2018, 277, 57–76. [Google Scholar] [CrossRef]
- Leucuta, S.E. Nanotechnology for delivery of drugs and biomedical applications. Curr. Clin. Pharm. 2010, 5, 257–280. [Google Scholar] [CrossRef]
- Buse, J.; El-Aneed, A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: Current research and advances. Nanomedicine (Lond) 2010, 5, 1237–1260. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Oberholzer, T.; Luisi, P.L. The use of liposomes for constructing cell models. J. Biol. Phys. 2002, 28, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharm. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Shimasaki, T.; Yamamoto, S.; Arisawa, T. Exosome Research and Co-culture Study. Biol. Pharm. Bull. 2018, 41, 1311–1321. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol. Pharm Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Ferrara, G.; Lanni, M.; Emiliani, C. Exosome-based strategies for Diagnosis and Therapy. Recent Pat. Cns Drug Discov. 2015, 10, 10–27. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.B.; Zuhorn, I.S. Solid lipid nanoparticles as nucleic acid delivery system: Properties and molecular mechanisms. J. Control. Release 2015, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers - a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Ezzati Nazhad Dolatabadi, J.; Valizadeh, H.; Hamishehkar, H. Solid Lipid Nanoparticles as Efficient Drug and Gene Delivery Systems: Recent Breakthroughs. Adv. Pharm. Bull. 2015, 5, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shi, S.; Zhang, Z.; Gong, T.; Sun, X. Coating Solid Lipid Nanoparticles with Hyaluronic Acid Enhances Antitumor Activity against Melanoma Stem-like Cells. Theranostics 2015, 5, 755–771. [Google Scholar] [CrossRef]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharm. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Liu, Y.; Noe, D.; Mertz, J.; Bargfrede, M.; Marbury, T.; Farbakhsh, K.; Oliva, C.; Milton, A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment. Br. J. Clin. Pharm. 2014, 77, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. Onco Targets 2016, 9, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Komlosh, A.; Weinstein, V.; Loupe, P.; Hasson, T.; Timan, B.; Konya, A.; Alexander, J.; Melamed-Gal, S.; Nock, S. Physicochemical and Biological Examination of Two Glatiramer Acetate Products. Biomedicines 2019, 7, 49. [Google Scholar] [CrossRef]
- Kim, M.T.; Chen, Y.; Marhoul, J.; Jacobson, F. Statistical modeling of the drug load distribution on trastuzumab emtansine (Kadcyla), a lysine-linked antibody drug conjugate. Bioconjug Chem. 2014, 25, 1223–1232. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Chelle, P.; Yeung, C.H.T.; Croteau, S.E.; Lissick, J.; Balasa, V.; Ashburner, C.; Park, Y.S.; Bonanad, S.; Megías-Vericat, J.E.; Nagao, A.; et al. Development and Validation of a Population-Pharmacokinetic Model for Rurioctacog Alfa Pegol (Adynovate®): A Report on Behalf of the WAPPS-Hemo Investigators Ad Hoc Subgroup. Clin. Pharmacokinet. 2020, 59, 245–256. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine (Lond) 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Bullivant, J.P.; Zhao, S.; Willenberg, B.J.; Kozissnik, B.; Batich, C.D.; Dobson, J. Materials characterization of Feraheme/ferumoxytol and preliminary evaluation of its potential for magnetic fluid hyperthermia. Int. J. Mol. Sci. 2013, 14, 17501–17510. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A.; Keating, G.M. Ferric Carboxymaltose. Drugs 2009, 69, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, H.K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Kaduk, J.A.; Dmitrienko, A.O.; Gindhart, A.M.; Blanton, T.N. Crystal structure of paliperidone palmitate (INVEGA SUSTENNA®), C39H57FN4O4. Powder Diffr. 2017, 32, 222–227. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef]
- Stavrovskaya, A.A. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000, 65, 95–106. [Google Scholar]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. -UK 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J. Am. Chem Soc. 2012, 134, 8848–8855. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Schiller, G.; Lister, J.; Damon, L.; Goldberg, S.; Aulitzky, W.; Ben-Yehuda, D.; Stock, W.; Coutre, S.; Douer, D.; et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J. Clin. Oncol. 2013, 31, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Cohen, S.M.; Rockefeller, N.; Mukerji, R.; Durham, D.; Forrest, M.L.; Cai, S.; Cohen, M.S.; Shnayder, Y. Efficacy and toxicity of peritumoral delivery of nanoconjugated cisplatin in an in vivo murine model of head and neck squamous cell carcinoma. JAMA Otolaryngol. Head Neck Surg 2013, 139, 382–387. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Boulikas, T. Lipoplatin formulation review article. J. Drug Deliv. 2012, 2012, 581363. [Google Scholar] [CrossRef]
- Boulikas, T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol. Rep. 2004, 12, 3–12. [Google Scholar] [CrossRef]
- Boulikas, T. Clinical overview on Lipoplatin: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef]
- Farhat, F.; Kattan, J.; Ibrahim, K.; Bitar, N.; Haddad, N.; Tamraz, S.; Hatoum, H.; Shamseddine, A. 457 Preliminary results of a phase II study of lipoplatin (liposomal cisplatin)–vinorelbine combination as first line treatment in HER2/neu negative metastatic breast cancer (MBC). Eur. J. Cancer Suppl. 2010, 8, 192. [Google Scholar] [CrossRef]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. MAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, D.; Jiang, Z.; Tong, R.; Jiang, P.; Bai, L.; Chen, L.; Zhu, Y.; Guo, C.; Shi, J.; et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur. J. Med. Chem. 2019, 183, 111682. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Shion, H.; Yu, C.; Yu, Y.Q.; Zhu, L.; Li, M.; Chen, W.; Gao, K. In-depth structural characterization of Kadcyla(R) (ado-trastuzumab emtansine) and its biosimilar candidate. MAbs 2016, 8, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharm. 2006, 7, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Zhang, Q.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. (Camb) 2016, 52, 978–981. [Google Scholar] [CrossRef]
- A Clinical Study to Measure the Effect of OP-101 after Being Administered Subcutaneous in Healthy Volunteers. Available online: https://ClinicalTrials.gov/show/NCT04321980:2018 (accessed on 13 April 2020).
- Dai, L.; Yu, Y.; Luo, Z.; Li, M.; Chen, W.; Shen, X.; Chen, F.; Sun, Q.; Zhang, Q.; Gu, H.; et al. Photosensitizer enhanced disassembly of amphiphilic micelle for ROS-response targeted tumor therapy in vivo. Biomaterials 2016, 104, 1–17. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef]
- Bhirde, A.A.; Patel, S.; Sousa, A.A.; Patel, V.; Molinolo, A.A.; Ji, Y.; Leapman, R.D.; Gutkind, J.S.; Rusling, J.F. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomed. (Lond. Engl.) 2010, 5, 1535–1546. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, W.; Hao, Y.; Wang, Y.; Zhang, H.; Hou, L.; Zhang, Z. Programmed near-infrared light-responsive drug delivery system for combined magnetic tumor-targeting magnetic resonance imaging and chemo-phototherapy. Acta Biomater 2017, 49, 402–413. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, W.; Shan, X.; Yuan, Y.; Zhang, H.; Hou, L.; Zhang, Z. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater 2016, 38, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, L.; Liu, F.; Qi, X.; Ge, Y.; Shen, S. Azo-functionalized Fe3O4 nanoparticles: A near-infrared light triggered drug delivery system for combined therapy of cancer with low toxicity. J. Mater. Chem. B 2016, 4, 3660–3669. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Zhao, X.; Wu, Q.; Zhu, H.; Mao, Z.; Gao, C. Doxorubicin-conjugated pH-responsive gold nanorods for combined photothermal therapy and chemotherapy of cancer. Bioact Mater. 2018, 3, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A.N.; Feinstein, J.A.; Cramer, J.A.; Boothroyd, J.A.; Shishkina, E.V.; Shur, V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: Long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017, 13, 345–363. [Google Scholar] [CrossRef]
- Choi, J.; Park, Y.; Choi, E.B.; Kim, H.O.; Kim, D.J.; Hong, Y.; Ryu, S.H.; Lee, J.H.; Suh, J.S.; Yang, J.; et al. Aptamer-conjugated gold nanorod for photothermal ablation of epidermal growth factor receptor-overexpressed epithelial cancer. J. Biomed. Opt. 2014, 19, 051203. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Li, J.; Luo, Z.; Hu, Y.; Liu, J.; Dai, L.; Zhou, J.; Hou, C.; Cai, K. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology 2015, 26, 145102. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Lim, W.Q.; Luo, Z.; Phua, S.Z.F.; Huo, R.; Li, L.; Li, K.; Dai, L.; Liu, J.; et al. A Transferrin-Conjugated Hollow Nanoplatform for Redox-Controlled and Targeted Chemotherapy of Tumor with Reduced Inflammatory Reactions. Theranostics 2018, 8, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Li, P.-Z.; Nguyen, K.T.; Wang, X.-J.; Luo, Z.; Zhang, H.; Tan, N.S.; Zhao, Y. Biocompatible, Uniform, and Redispersible Mesoporous Silica Nanoparticles for Cancer-Targeted Drug Delivery In Vivo. Adv. Funct. Mater. 2014, 24, 2450–2461. [Google Scholar] [CrossRef]
- Pei, Y.; Li, M.; Hou, Y.; Hu, Y.; Chu, G.; Dai, L.; Li, K.; Xing, Y.; Tao, B.; Yu, Y.; et al. An autonomous tumor-targeted nanoprodrug for reactive oxygen species-activatable dual-cytochrome c/doxorubicin antitumor therapy. Nanoscale 2018, 10, 11418–11429. [Google Scholar] [CrossRef]
- Canullo, L.; Dellavia, C.; Heinemann, F. Maxillary sinus floor augmentation using a nano-crystalline hydroxyapatite silica gel: Case series and 3-month preliminary histological results. Ann. Anat. - Anat. Anz. 2012, 194, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Sharma, A.; Toma, M.; Samaniego, S.; Gallud, A.; Wang, E.; Pal, K.; Mukhopadhyay, D.; Fadeel, B. Tumor selective uptake of drug-nanodiamond complexes improves therapeutic outcome in pancreatic cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Pal, K.; Keshavan, S.; Caulfield, T.R.; Dutta, S.K.; Wang, E.; Fadeel, B.; Mukhopadhyay, D. Development of multi-drug loaded PEGylated nanodiamonds to inhibit tumor growth and metastasis in genetically engineered mouse models of pancreatic cancer. Nanoscale 2019, 11, 22006–22018. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Proctor, R.A. Staphylococcus aureus as an intracellular pathogen: The role of small colony variants. Trends Microbiol. 2009, 17, 54–58. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Corporation, A. ARD-3150 Pulmaquin® and ARD-3100 Lipoquin®. Available online: www.aradigm.com/products_3100.html (accessed on 22 March 2020).
- Walsh, T.J.; Finberg, R.W.; Arndt, C.; Hiemenz, J.; Schwartz, C.; Bodensteiner, D.; Pappas, P.; Seibel, N.; Greenberg, R.N.; Dummer, S.; et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N. Engl. J. Med. 1999, 340, 764–771. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Bresnik, M.; Ebrahimi, R.; Ullmann, A.J.; Bouza, E.; Heussel, C.P.; Lortholary, O.; Rieger, C.; Boehme, A.; et al. Liposomal amphotericin B as initial therapy for invasive mold infection: A randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 2007, 44, 1289–1297. [Google Scholar] [CrossRef]
- Ghaffar, K.A.; Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Y.; Zhang, Y.; Deng, J.; Lin, C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015, 10, 7241–7252. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Beal, S.G.; Ciurca, J.; Smith, G.; John, J.; Lee, F.; Doern, C.D.; Gander, R.M. Evaluation of the nanosphere verigene gram-positive blood culture assay with the VersaTREK blood culture system and assessment of possible impact on selected patients. J. Clin. Microbiol. 2013, 51, 3988–3992. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Santos, J.P.; Morey, A.T.; Marcato, P.D.; Duran, N.; Pinge-Filho, P.; Nakazato, G.; Yamada-Ogatta, S.F.; Yamauchi, L.M. Combination of fluconazole with silver nanoparticles produced by Fusarium oxysporum improves antifungal effect against planktonic cells and biofilm of drug-resistant Candida albicans. Med. Mycol. 2016, 54, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef]
- Chakraborti, S.; Mandal, A.K.; Sarwar, S.; Singh, P.; Chakraborty, R.; Chakrabarti, P. Bactericidal effect of polyethyleneimine capped ZnO nanoparticles on multiple antibiotic resistant bacteria harboring genes of high-pathogenicity island. Colloids Surf. B Biointerfaces 2014, 121, 44–53. [Google Scholar] [CrossRef]
- Shimizu, N.; Otsuka, K.; Sawada, H.; Maejima, T.; Shirotake, S. Bacteriolysis by vancomycin-conjugated acryl nanoparticles and morphological component analysis. Drug Dev. Ind. Pharm. 2014, 40, 813–818. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tsang, K.W.; Wang, L.; Xu, B. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J. Am. Chem. Soc. 2003, 125, 15702–15703. [Google Scholar] [CrossRef]
- Shaik, N.; Pan, G.; Elmquist, W.F. Interactions of pluronic block copolymers on P-gp efflux activity: Experience with HIV-1 protease inhibitors. J. Pharm. Sci. 2008, 97, 5421–5433. [Google Scholar] [CrossRef]
- Alipour, M.; Halwani, M.; Omri, A.; Suntres, Z.E. Antimicrobial effectiveness of liposomal polymyxin B against resistant Gram-negative bacterial strains. Int. J. Pharm. 2008, 355, 293–298. [Google Scholar] [CrossRef]
- Aditya, N.P.; Vathsala, P.G.; Vieira, V.; Murthy, R.S.; Souto, E.B. Advances in nanomedicines for malaria treatment. Adv. Colloid Interface Sci. 2013, 201–202, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.P.; Sahu, S.K.; Pramanik, P.; Roy, S. In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int. J. Pharm. 2012, 436, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; Perepletchikova, F.; et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 417–424.e5. [Google Scholar] [CrossRef] [PubMed]
- Turos, E.; Reddy, G.S.; Greenhalgh, K.; Ramaraju, P.; Abeylath, S.C.; Jang, S.; Dickey, S.; Lim, D.V. Penicillin-bound polyacrylate nanoparticles: Restoring the activity of beta-lactam antibiotics against MRSA. Bioorg. Med. Chem. Lett. 2007, 17, 3468–3472. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.P.; Miller, K.P.; Cash, B.M.; Jones, S.; Glenn, S.; Benicewicz, B.C.; Decho, A.W. Functionalised nanoparticles complexed with antibiotic efficiently kill MRSA and other bacteria. Chem. Commun. (Camb) 2014, 50, 12030–12033. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Ismail, M.; Webster, T.J. Synthesis, characterization, controlled release, and antibacterial studies of a novel streptomycin chitosan magnetic nanoantibiotic. Int. J. Nanomed. 2014, 9, 549–557. [Google Scholar] [CrossRef]
- Abeylath, S.C.; Turos, E.; Dickey, S.; Lim, D.V. Glyconanobiotics: Novel carbohydrated nanoparticle antibiotics for MRSA and Bacillus anthracis. Bioorg. Med. Chem. 2008, 16, 2412–2418. [Google Scholar] [CrossRef]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.H.; Lee, H.T.; Lee, J.U.; Son, J.Y.; Shin, W.; Heo, Y.S. Structural Biology of the TNFalpha Antagonists Used in the Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Horton, S.; Walsh, C.; Emery, P. Certolizumab pegol for the treatment of rheumatoid arthritis. Expert Opin. Biol. 2012, 12, 235–249. [Google Scholar] [CrossRef]
- Yudoh, K.; Karasawa, R.; Masuko, K.; Kato, T. Water-soluble fullerene (C60) inhibits the development of arthritis in the rat model of arthritis. Int. J. Nanomed. 2009, 4, 217–225. [Google Scholar] [CrossRef] [PubMed]
- de Castro, S.; Camarasa, M.J. Polypharmacology in HIV inhibition: Can a drug with simultaneous action against two relevant targets be an alternative to combination therapy? Eur. J. Med. Chem. 2018, 150, 206–227. [Google Scholar] [CrossRef] [PubMed]
- Herskovitz, J.; Gendelman, H.E. HIV and the Macrophage: From Cell Reservoirs to Drug Delivery to Viral Eradication. J. Neuroimmune Pharm. 2019, 14, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Garg, M.; Jain, N.K. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur. J. Pharm. Sci. 2008, 34, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Dembri, A.; Montisci, M.J.; Gantier, J.C.; Chacun, H.; Ponchel, G. Targeting of 3’-azido 3’-deoxythymidine (AZT)-loaded poly(isohexylcyanoacrylate) nanospheres to the gastrointestinal mucosa and associated lymphoid tissues. Pharm. Res. 2001, 18, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lobenberg, R.; Maas, J.; Kreuter, J. Improved body distribution of 14C-labelled AZT bound to nanoparticles in rats determined by radioluminography. J. Drug Target. 1998, 5, 171–179. [Google Scholar] [CrossRef]
- Dutta, T.; Agashe, H.B.; Garg, M.; Balakrishnan, P.; Kabra, M.; Jain, N.K. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J. Drug Target. 2007, 15, 89–98. [Google Scholar] [CrossRef]
- Pham, K.; Li, D.; Guo, S.; Penzak, S.; Dong, X. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Control. Release 2016, 226, 88–97. [Google Scholar] [CrossRef]
- Liptrott, N.J.; Giardiello, M.; McDonald, T.O.; Rannard, S.P.; Owen, A. Assessment of interactions of efavirenz solid drug nanoparticles with human immunological and haematological systems. J. Nanobiotechnol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Govender, T.; Ojewole, E.; Naidoo, P.; Mackraj, I. Polymeric nanoparticles for enhancing antiretroviral drug therapy. Drug Deliv. 2008, 15, 493–501. [Google Scholar] [CrossRef]
- Shah, L.K.; Amiji, M.M. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm. Res. 2006, 23, 2638–2645. [Google Scholar] [CrossRef]
- Garg, M.; Asthana, A.; Agashe, H.B.; Agrawal, G.P.; Jain, N.K. Stavudine-loaded mannosylated liposomes: In-vitro anti-HIV-I activity, tissue distribution and pharmacokinetics. J. Pharm. Pharm. 2006, 58, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Poirier, P.; Despres, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Haeri, A.; Sadeghian, S.; Rabbani, S.; Anvari, M.S.; Ghassemi, S.; Radfar, F.; Dadashzadeh, S. Effective attenuation of vascular restenosis following local delivery of chitosan decorated sirolimus liposomes. Carbohydr. Polym. 2017, 157, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Arzani, G.; Haeri, A.; Daeihamed, M.; Bakhtiari-Kaboutaraki, H.; Dadashzadeh, S. Niosomal carriers enhance oral bioavailability of carvedilol: Effects of bile salt-enriched vesicles and carrier surface charge. Int. J. Nanomed. 2015, 10, 4797–4813. [Google Scholar] [CrossRef]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- Formiga, F.R.; Pelacho, B.; Garbayo, E.; Abizanda, G.; Gavira, J.J.; Simon-Yarza, T.; Mazo, M.; Tamayo, E.; Jauquicoa, C.; Ortiz-de-Solorzano, C.; et al. Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia-reperfusion model. J. Control. Release 2010, 147, 30–37. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Formiga, F.R.; Tamayo, E.; Pelacho, B.; Prosper, F.; Blanco-Prieto, M.J. PEGylated-PLGA microparticles containing VEGF for long term drug delivery. Int. J. Pharm. 2013, 440, 13–18. [Google Scholar] [CrossRef]
- Phillips, M.A.; Gran, M.L.; Peppas, N.A. Targeted Nanodelivery of Drugs and Diagnostics. Nano Today 2010, 5, 143–159. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Fan, C.H.; Ting, C.Y.; Yeh, C.K. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Klafki, H.W.; Staufenbiel, M.; Kornhuber, J.; Wiltfang, J. Therapeutic approaches to Alzheimer’s disease. Brain 2006, 129, 2840–2855. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.; Paramakrishnan, N.; Suresh, B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Patel, P.M. Dendrimer applications - A review. Int. J. Pharma Bio Sci. 2013, 4, 454–463. [Google Scholar]
- Laserra, S.; Basit, A.; Sozio, P.; Marinelli, L.; Fornasari, E.; Cacciatore, I.; Ciulla, M.; Turkez, H.; Geyikoglu, F.; Di Stefano, A. Solid lipid nanoparticles loaded with lipoyl-memantine codrug: Preparation and characterization. Int. J. Pharm. 2015, 485, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Qiao, Y.; Meng, F.; Liu, X.; Ding, C. Enhanced apatite-forming ability and cytocompatibility of porous and nanostructured TiO2/CaSiO3 coating on titanium. Colloids Surf. B Biointerfaces 2013, 101, 83–90. [Google Scholar] [CrossRef]
- Joshi, S.A.; Chavhan, S.S.; Sawant, K.K. Rivastigmine-loaded PLGA and PBCA nanoparticles: Preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur. J. Pharm. Biopharm. 2010, 76, 189–199. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Silva Adaya, D.; Aguirre-Cruz, L.; Guevara, J.; Ortiz-Islas, E. Nanobiomaterials’ applications in neurodegenerative diseases. J. Biomater Appl. 2017, 31, 953–984. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.H. Updates in the medical management of Parkinson disease. Cleve Clin. J. Med. 2012, 79, 28–35. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.A.; MacDonald, A.A.; Seergobin, K.N.; Tamjeedi, R.; Ganjavi, H.; Provost, J.S.; Monchi, O. The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson’s disease: Support from functional MRI. Brain 2011, 134, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: A mini review. J. Drug Target. 2015, 23, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Talegaonkar, S.; Negi, L.M.; Ahmad, F.J.; Khar, R.K.; Iqbal, Z. Oil based nanocarrier system for transdermal delivery of ropinirole: A mechanistic, pharmacokinetic and biochemical investigation. Int. J. Pharm. 2012, 422, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef]
- Gendelman, H.E.; Anantharam, V.; Bronich, T.; Ghaisas, S.; Jin, H.; Kanthasamy, A.G.; Liu, X.; McMillan, J.; Mosley, R.L.; Narasimhan, B.; et al. Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases. Nanomed. -Uk 2015, 11, 751–767. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Reynolds, A.D.; Mosley, R.L.; Tatiana, K.; Kabanov, A.V.; Gendelman, H.E. A Macrophage−Nanozyme Delivery System for Parkinson‘s Disease. NIH Public Access. 2009, 18, 1498–1506. [Google Scholar] [CrossRef]
- Crotty, S.; Fitzgerald, P.; Tuohy, E.; Harris, D.M.; Fisher, A.; Mandel, A.; Bolton, A.E.; Sullivan, A.M.; Nolan, Y. Neuroprotective effects of novel phosphatidylglycerol-based phospholipids in the 6-hydroxydopamine model of Parkinson’s disease. Eur. J. Neurosci. 2008, 27, 294–300. [Google Scholar] [CrossRef]
- Xia, C.F.; Boado, R.J.; Zhang, Y.; Chu, C.; Pardridge, W.M. Intravenous glial-derived neurotrophic factor gene therapy of experimental Parkinson‘s disease with Trojan horse liposomes and a tyrosine hydroxylase promoter. J. Gene Med. 2008, 10, 306–315. [Google Scholar] [CrossRef]
- Konishi, M.; Kawamoto, K.; Izumikawa, M.; Kuriyama, H.; Yamashita, T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008, 10, 610–618. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Bjorklund, A. Cell therapeutics in Parkinson‘s disease. Neurotherapeutics 2011, 8, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S. Biomedical applications of nanotechnology—Implications for drug targeting and gene therapy. Trends Biotechnol. 1997, 15, 217–224. [Google Scholar] [CrossRef]
- Yurek, D.M.; Flectcher, A.M.; Kowalczyk, T.H.; Padegimas, L.; Cooper, J. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. NIH Public Access. 2009, 18, 1183–1196. [Google Scholar] [CrossRef]
- Bondi, M.L.; Craparo, E.F.; Giammona, G.; Drago, F. Brain-targeted solid lipid nanoparticles containing riluzole: Preparation, characterization and biodistribution. Nanomedicine (Lond) 2010, 5, 25–32. [Google Scholar] [CrossRef]
- Mazibuko, Z.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Pillay, V. A review of the potential role of nano-enabled drug delivery technologies in amyotrophic lateral sclerosis: Lessons learned from other neurodegenerative disorders. J. Pharm. Sci. 2015, 104, 1213–1229. [Google Scholar] [CrossRef]
- Basso, A.S.; Frenkel, D.; Quintana, F.J.; Costa-Pinto, F.A.; Petrovic-Stojkovic, S.; Puckett, L.; Monsonego, A.; Bar-Shir, A.; Engel, Y.; Gozin, M.; et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Investig. 2008, 118, 1532–1543. [Google Scholar] [CrossRef]
- Bell, C.; Anderson, J.; Ganguly, T.; Prescott, J.; Capila, I.; Lansing, J.C.; Sachleben, R.; Iyer, M.; Fier, I.; Roach, J.; et al. Development of Glatopa(R) (Glatiramer Acetate): The First FDA-Approved Generic Disease-Modifying Therapy for Relapsing Forms of Multiple Sclerosis. J. Pharm. Pr. 2018, 31, 481–488. [Google Scholar] [CrossRef]
- Sharaf, M.G.; Cetinel, S.; Heckler, L.; Damji, K.; Unsworth, L.; Montemagno, C. Nanotechnology-Based Approaches for Ophthalmology Applications: Therapeutic and Diagnostic Strategies. Asia Pac. J. Ophthalmol (Phila) 2014, 3, 172–180. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Offerta, A.; Carbone, C.; Bonina, F.; Pignatello, R.; Puglisi, G. Lipid nanocarriers (LNC) and their applications in ocular drug delivery. Curr. Med. Chem. 2015, 22, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. Aaps Pharmscitech 2015, 16, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Chetoni, P.; Zambito, Y. Mucoadhesive nano-sized supramolecular assemblies for improved pre-corneal drug residence time. Drug Dev. Ind. Pharm. 2015, 41, 2069–2076. [Google Scholar] [CrossRef]
- El-Salamounia, N.S.; Farida, R.M. Recent Drug Delivery Systems for Treatment of Glaucoma. Glaucoma 2016, 1–13. [Google Scholar]
- Ibrahim, M.M.; Abd-Elgawad, A.H.; Soliman, O.A.; Jablonski, M.M. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Transl. Vis. Sci. Technol. 2015, 4, 12. [Google Scholar] [CrossRef]
- Cetinel, S.; Montemagno, C. Nanotechnology Applications for Glaucoma. Asia Pac. J. Ophthalmol. (Phila) 2016, 5, 70–78. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, Q.; Boylan, N.J.; Lamb, N.W.; Emmert, D.G.; Yang, J.C.; Tang, L.; Heflin, T.; Alwadani, S.; Eberhart, C.G.; et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J. Control. Release 2015, 201, 32–40. [Google Scholar] [CrossRef]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine (Lond) 2017, 12, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Nikaido, H. Properties of AdeABC and AdeIJK efflux systems of Acinetobacter baumannii compared with those of the AcrAB-TolC system of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 7250–7257. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Im, J.; Nho, R.S. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J. Clin. Med. 2016, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Tiemann, K.M.; Hunstad, D.A.; Elsabahy, M.; Wooley, K.L. Polymeric nanoparticles in development for treatment of pulmonary infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 842–871. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Najlah, M.; D’Emanuele, A.; Elhissi, A. PAMAM dendrimers as aerosol drug nanocarriers for pulmonary delivery via nebulization. Int. J. Pharm. 2014, 461, 242–250. [Google Scholar] [CrossRef]
- Mohamud, R.; Xiang, S.D.; Selomulya, C.; Rolland, J.M.; O’Hehir, R.E.; Hardy, C.L.; Plebanski, M. The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metab. Rev. 2014, 46, 176–190. [Google Scholar] [CrossRef]
- Gera, S.; Sampathi, S.; Dodoala, S. Role of Nanoparticles in Drug Delivery and Regenerative Therapy for Bone Diseases. Curr. Drug Deliv. 2017, 14, 904–916. [Google Scholar] [CrossRef]
- Alves Cardoso, D.; Jansen, J.A.; Leeuwenburgh, S.C. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2316–2326. [Google Scholar] [CrossRef]
- Ding, X.; Wang, Y. Weak Bond-Based Injectable and Stimuli Responsive Hydrogels for Biomedical Applications. J. Mater. Chem. B 2017, 5, 887–906. [Google Scholar] [CrossRef]
- Unal, S.; Ekren, N.; Sengil, A.Z.; Oktar, F.N.; Irmak, S.; Oral, O.; Sahin, Y.M.; Kilic, O.; Agathopoulos, S.; Gunduz, O. Synthesis, characterization, and biological properties of composites of hydroxyapatite and hexagonal boron nitride. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2384–2392. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Jodar-Reyes, A.B.; Peula-Garcia, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. Biomed. Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yi, S.W.; Kim, H.J.; Kim, S.M.; Park, K.H. Regulation of Cell Signaling Factors Using PLGA Nanoparticles Coated/Loaded with Genes and Proteins for Osteogenesis of Human Mesenchymal Stem Cells. Acs Appl. Mater. Interfaces 2016, 8, 30387–30397. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Lin, J.; Yu, M.; Yu, L.; Li, J.; Weng, W.; Cheng, K.; Wang, H. Enhanced loading and controlled release of rhBMP-2 in thin mineralized collagen coatings with the aid of chitosan nanospheres and its biological evaluations. J. Mater. Chem. B 2014, 2, 4572–4582. [Google Scholar] [CrossRef]
- Zhang, S.; Kucharski, C.; Doschak, M.R.; Sebald, W.; Uludag, H. Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials 2010, 31, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Giger, E.V.; Castagner, B.; Leroux, J.C. Biomedical applications of bisphosphonates. J. Control. Release 2013, 167, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Lee, T.C.; Dou, Q.; Deen, G.R. Utilising inorganic nanocarriers for gene delivery. Biomater. Sci. 2016, 4, 70–86. [Google Scholar] [CrossRef]
- FDA. EquivaBone Osteoinductive Bone Graft Substitute. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf9/K090855.pdf (accessed on 11 March 2020).
- Warabi, S.; Tachibana, Y.; Kumegawa, M.; Hakeda, Y. Dexamethasone inhibits bone resorption by indirectly inducing apoptosis of the bone-resorbing osteoclasts via the action of osteoblastic cells. Cytotechnology 2001, 35, 25–34. [Google Scholar] [CrossRef]
- Goncalves, R.M.; Pereira, A.C.; Pereira, I.O.; Oliveira, M.J.; Barbosa, M.A. Macrophage response to chitosan/poly-(gamma-glutamic acid) nanoparticles carrying an anti-inflammatory drug. J. Mater. Sci Mater. Med. 2015, 26, 167. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Su, C.K.; Sun, Y.C. In vivo monitoring of distributional transport kinetics and extravasation of quantum dots in living rat liver. Nanotechnology 2013, 24, 165101. [Google Scholar] [CrossRef]
- Chang, S.; Chen, D.; Kang, B.; Dai, Y. UV-enhanced cytotoxicity of CdTe quantum dots in PANC-1 cells depend on their size distribution and surface modification. J. Nanosci. Nanotechnol. 2013, 13, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Angra, P.K.; Rizvi, S.A.A.; Oettinger, C.W.; D’Souza, M.J. Novel approach for preparing nontoxic stealth microspheres for drug delivery. Eur. J. Chem. 2011, 2, 125–129. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Shape and aggregation control of nanoparticles: Not shaken, not stirred. J. Am. Chem. Soc. 2006, 128, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Sadauskas, E.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Wallin, H. Protracted elimination of gold nanoparticles from mouse liver. Nanomed. -Uk 2009, 5, 162–169. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Stealth Nanoparticles: High Density but Sheddable PEG is a Key for Tumor Targeting. J. Control. Release 2010, 145, 178–181. [Google Scholar] [CrossRef]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef]
- Fan, J.; Wang, S.; Zhang, X.; Chen, W.; Li, Y.; Yang, P.; Cao, Z.; Wang, Y.; Lu, W.; Ju, D. Quantum Dots Elicit Hepatotoxicity through Lysosome-Dependent Autophagy Activation and Reactive Oxygen Species Production. Acs Biomater. Sci. Eng. 2018, 4, 1418–1427. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, S.; Chen, H.; He, M.; Deng, Y.; Cao, Z.; Pi, H.; Chen, C.; Li, M.; Ma, Q.; et al. CdSe/ZnS quantum dots induce hepatocyte pyroptosis and liver inflammation via NLRP3 inflammasome activation. Biomaterials 2016, 90, 27–39. [Google Scholar] [CrossRef]
- Grabowska-Jadach, I.; Zuchowska, A.; Olesik, M.; Drozd, M.; Pietrzak, M.; Malinowska, E.; Brzozka, Z. Cytotoxicity studies of selected cadmium-based quantum dots on 2D vs. 3D cell cultures. New J. Chem. 2018, 42, 12787–12795. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Guan, F.; Pan, Y.; Xiao, X.; Zhang, M.; Zhang, H.; Chen, L. Selective inhibition of liver cancer growth realized by the intrinsic toxicity of a quantum dot-lipid complex. Int. J. Nanomed. 2014, 9, 5753–5769. [Google Scholar] [CrossRef] [PubMed]
| Nanostructure | Product | Nanoparticle Formulation | Drug | Indication(s) | Approval | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | Marqibo® | Sphingomyelin and cholesterol | Vincristine sulfate | Acute lymphoid leukemia | FDA 2012 | [92] |
| Mepact® | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and 1,2-Dioleoyl-sn-glycero-3-phospho-l-serine liposomes | Mifamurtide | Non-metastasizing osteosarcoma | Europe 2009 | [93] | |
| Onivyde® | Nanoliposomes | Irinotecan | Pancreatic cancer, Colorectal cancer | FDA 2015 Europe 2016 | [94] | |
| Vyxeos® | Distearoylphosphatidylcholine, Distearoylphosphatidylglycerol, Cholesterol | Daunorubicin Cytarabine | Acute myeloid leukemia | FDA 2017 | [95] | |
| Lipid-based (Non-liposoma) | Onpattro® | Lipid nanoparticles | Transthyretin targeted siRNA | Transthyretin-mediated amyloidosis | FDA 2018 | [96] |
| Polymer-based | Glatopa® | l-glutamic acid polymer with l-alanine, l-lysine, and l-tyrosine (Glatiramer) | - | Multiple sclerosis | FDA 2015 | [97] |
| Protein-drug conjugates | Kadcyla® | Maytansine derivative, DM1 | Trastuzumab | HER2+ breast cancer | FDA 2013 | [98] |
| Abraxane® | Albumin | Paclitaxel | Non-small lung cancer, Pancreatic cancer | FDA 2012 Europe 2005, FDA 2013 Europe 2008 | [99] | |
| Krystexxa® | PEGylated uricase | Pegloticase | Gout disease | FDA 2010 Europe 2013 | [100] | |
| Plegridy® | PEGylated interferon β-1a | Interferon β-1a | Multiple sclerosis | FDA 2014 Europe 2014 | [100] | |
| Adynovate® | PEGylated factor VIII | Factor VIII | Hemophilia | FDA 2015 | [101] | |
| Rebinyn® | Glycopegylated coagulation factor IX | Factor IX | Hemophilia | FDA 2017 | [102] | |
| Metallic nanoparticles | Feraheme® | Superparamagnetic iron oxide nanoparticle (SPION) covered with dextran | - | Anemia in chronic kidney disease | FDA 2009 Europe 2012 | [103] |
| Ferinject® | Nanoparticles of ferric oxide core-carboxymaltose shell | - | Iron deficiency anemia in chronic kidney disease | FDA 2013 | [104] | |
| NanoTherm® | Nanoparticles of superparamagnetic iron oxide coated with amino silane | - | Glioblastoma, prostate, pancreatic cancer | Europe 2009 | [105] | |
| Nanocrystals | EquivaBone® | Hydroxyapatite | - | Osteoinductive bone graft substitute | FDA 2009 | [106] |
| Invega® Sustenna® | Paliperidone palmitate | Paliperidone | Schizophrenia | FDA 2009/2015 Europe 2011 | [107] | |
| Ryanodex® | Dantrolene sodium | Dantrolene | Malignant hyperthermia | FDA 2014 | [100] |
| Nanostructure | Nanoparticle | Conjugated Drug | Evaluation | Ref |
|---|---|---|---|---|
| Dendrimer | Polyethylene glycol (PEG)-platinum | α-cyclodextrin | pre-clinical | [127] |
| Polyamidoamine dendrimer | N-acetyl-cysteine | clinical/phase I | [128] | |
| Micelle | Polypropylene sulfide-PEG- serine-folic acid zinc phtalocyanine | doxorubicin | pre-clinical | [129] |
| PEG-polyaspartate polymeric micelle | paclitaxel | clinical/phase III | [130] | |
| Carbon nanotube | PEGylated single walled CNT | epidermal growth factor (EGF), cisplatin | pre-clinical | [131] |
| Metallic nanoparticles | Hollow mesoporous copper sulfide nanoparticle with iron oxides | doxorubicin | pre-clinical | [132] |
| Hollow mesoporous copper sulfide nanoparticle with hyaluronic acid | doxorubicin | pre-clinical | [133] | |
| PEGylated MoS nanosheets | pre-clinical | [134] | ||
| Azo-functionalized magnetite nanoparticles | doxorubicin | pre-clinical | [135] | |
| PEGylated gold nanorods (AuNR) | doxorubicin | pre-clinical | [136] | |
| Iron oxide magnetic silica-gold nanoparticles | clinical/phase I | [137] | ||
| PEGylated gold nanorod | aptamer | pre-clinical | [138] | |
| Silica based nanoparticles | Peptide-functionalized mesoporous silica | lactobionic acid, doxorubicin | pre-clinical | [139] |
| Transferrin mesoporous silica | doxorubicin | pre-clinical | [140] | |
| PEGylated mesoporous silica | amino-β-cyclodextrin, doxorubicin | pre-clinical | [141] | |
| mesoporous silica | cytochrome C conjugated lactobionic acid-doxorubicin | pre-clinical | [142] | |
| Hydroxyapatite nano-crystalline nano-structured silica gel | clinical/phase 0 | [143] | ||
| Nanodiamonds | PEGylated nanodiamonds | doxorubicin | pre-clinical | [144] |
| PEGylated nanodiamonds | irinotecan, curcumin | pre-clinical | [145] |
| Pathogen | Nanoparticle | Conjugated Drug | Evaluation | Ref |
|---|---|---|---|---|
| C. Albicans | Metallic nanoparticle (AgNP) | Fluconazole | In vitro | [157] |
| E. Coli | Metallic nanoparticle (AuNP and AgNP) | Ampicillin | In vitro | [158] |
| E. Coli | Metallic nanoparticle (ZnO-PEI) | Tetracycline | In vitro | [159] |
| Enterococci | Metallic nanoparticle (AuNP) | Vancomycin | In vitro | [160] |
| Liposome | In vitro | [161] | ||
| HIV-infected cells | Polymeric nanoparticle (Micelle) | Nelfinavir, saquinavir | In vitro | [162] |
| P. Aeruginosa | Liposome | Polymyxin B | In vitro | [163] |
| P. Aeruginosa | Metallic nanoparticle (AuNP) | Ampicillin | In vitro | [158] |
| Plasmodium sp. | Liposome | Chloroquine | In vitro | [164] |
| S. Aureus | Chitosan NP | Vancomycin | In vitro | [165] |
| Metallic nanoparticle (AuNP) | In vitro | [166] | ||
| Polymeric nanoparticle (PLA NP) | Penicillin | In vitro | [167] | |
| Silica nanoparticle | In vitro | [168] | ||
| Chitosan NP | Streptomycin | In vitro | [169] | |
| Liposome | β-Lactam, penicillin | In vitro | [170] | |
| Metallic nanoparticle (AuNP and AgNP) | Ampicillin | In vitro | [158,166] |
| Nanostructure | Nanoparticle | Conjugated Drug | Evaluation | Ref |
|---|---|---|---|---|
| Polymeric nanoparticle | Poly(hexylcyanoacrylate) nanoparticles | Zidovudine | Pre-clinical | [178] |
| Poly(isohexyl cyanate) nanoparticles | Zidovudine | Pre-clinical | [179] | |
| Poly(propyleneimine) dendrimers | Efavirenz | In vitro | [177] | |
| PPI dendrimer | Efavirenz | In vitro | [180] | |
| PLGA nanoparticles | Ritonavir, Lopinavir, Efavirenz | Pre-clinical | [181,182] | |
| PBCA and MMA-SPM nanoparticles | Stavudine, Zidovudine, Lamivudine | In vitro | [183] | |
| Poly(epsilon-caprolactone) | Saquinavir | In vitro | [184] | |
| Liposome | Mannosylated and galactosylated liposomes | Stavudine | In vitro | [185] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. https://doi.org/10.3390/molecules25092193
Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules. 2020; 25(9):2193. https://doi.org/10.3390/molecules25092193
Chicago/Turabian StyleYetisgin, Abuzer Alp, Sibel Cetinel, Merve Zuvin, Ali Kosar, and Ozlem Kutlu. 2020. "Therapeutic Nanoparticles and Their Targeted Delivery Applications" Molecules 25, no. 9: 2193. https://doi.org/10.3390/molecules25092193
APA StyleYetisgin, A. A., Cetinel, S., Zuvin, M., Kosar, A., & Kutlu, O. (2020). Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules, 25(9), 2193. https://doi.org/10.3390/molecules25092193







