Chemical Separation of Uranium and Precise Measurement of 234U/238U and 235U/238U Ratios in Soil Samples Using Multi Collector Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Analytical Validation of U Measurement Using ICP-MS
2.2. Measurement of U and Some Selected Elements in Soil Samples Using ICP-MS and X-ray Fluorescence Spectrometer
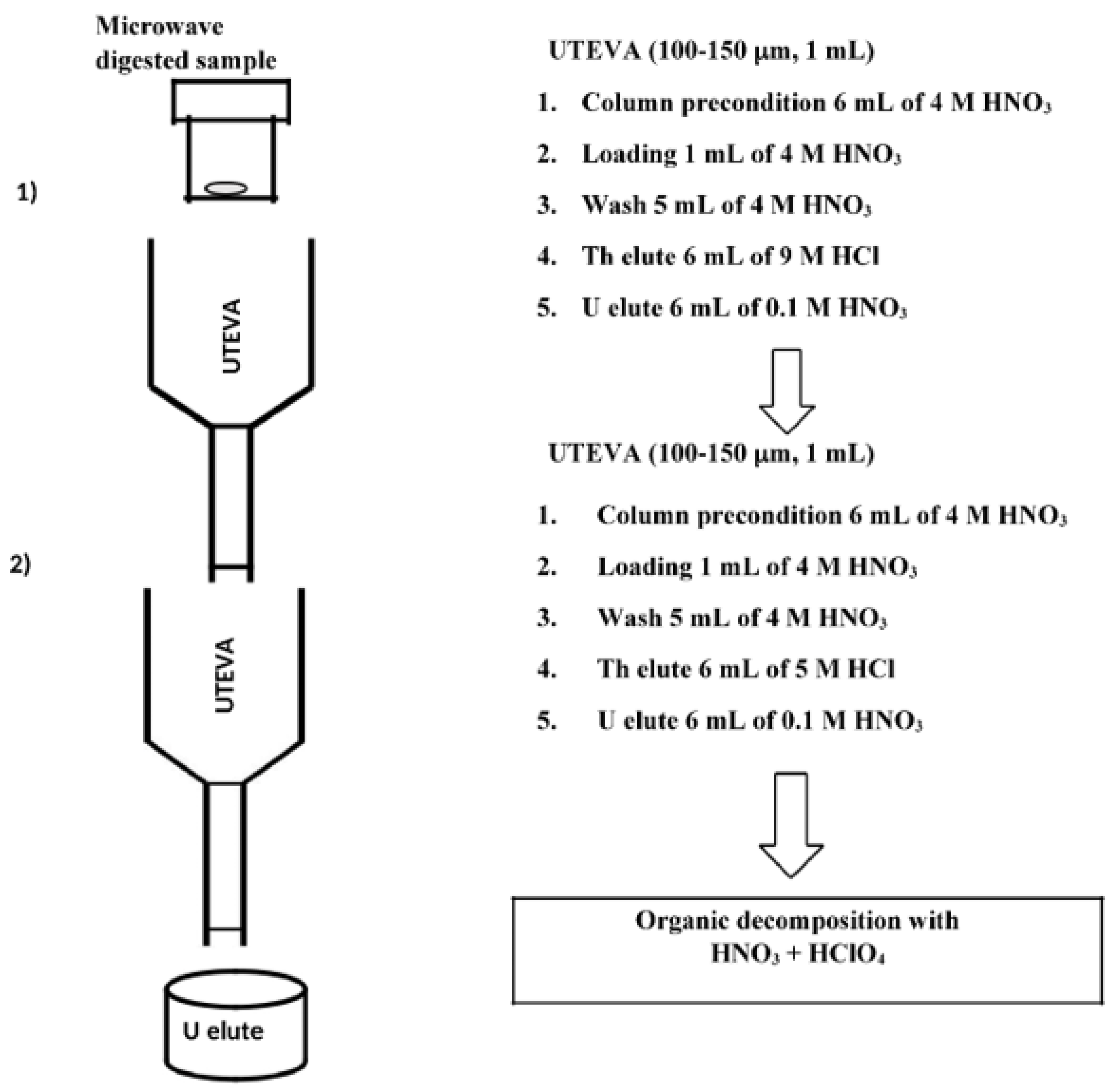
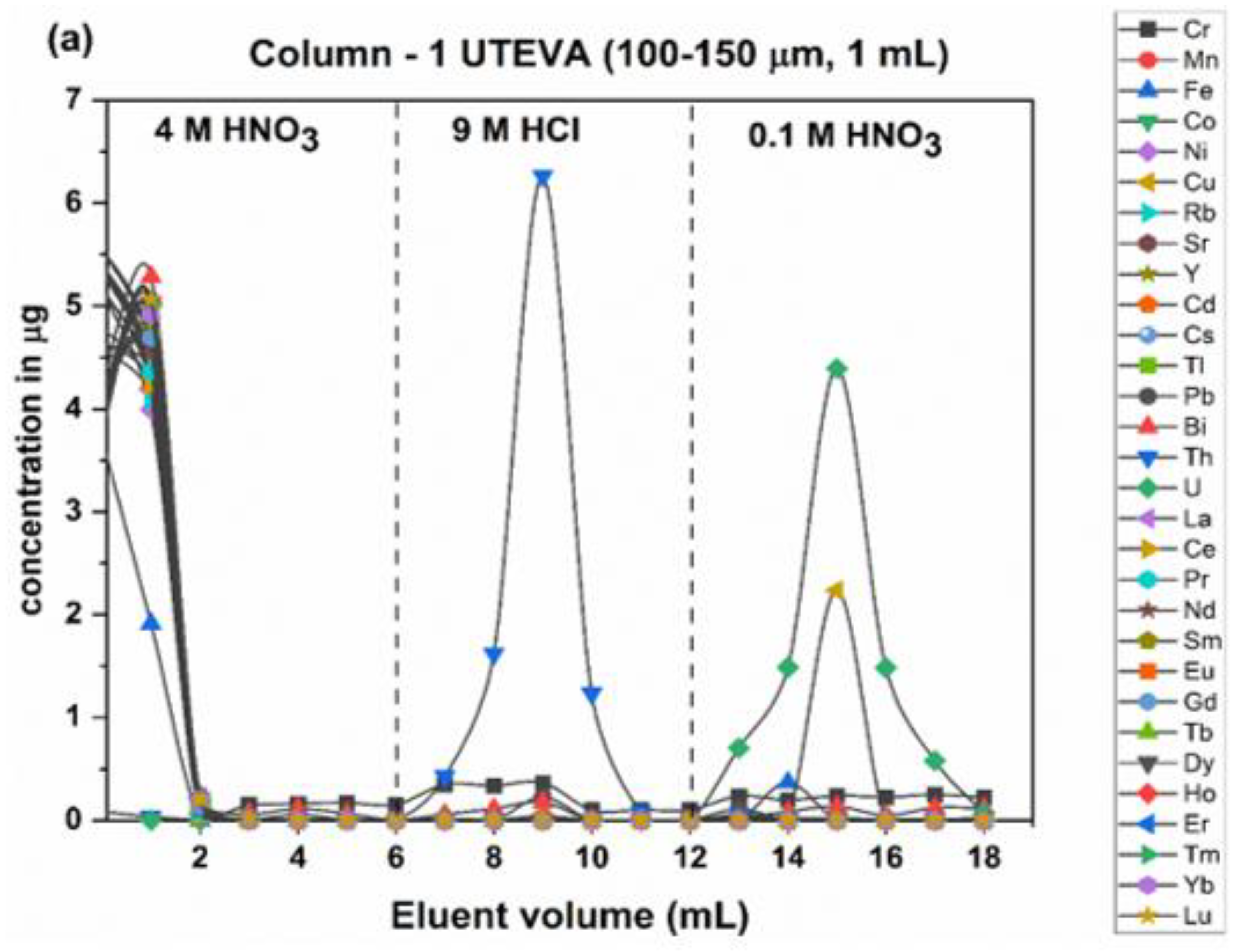
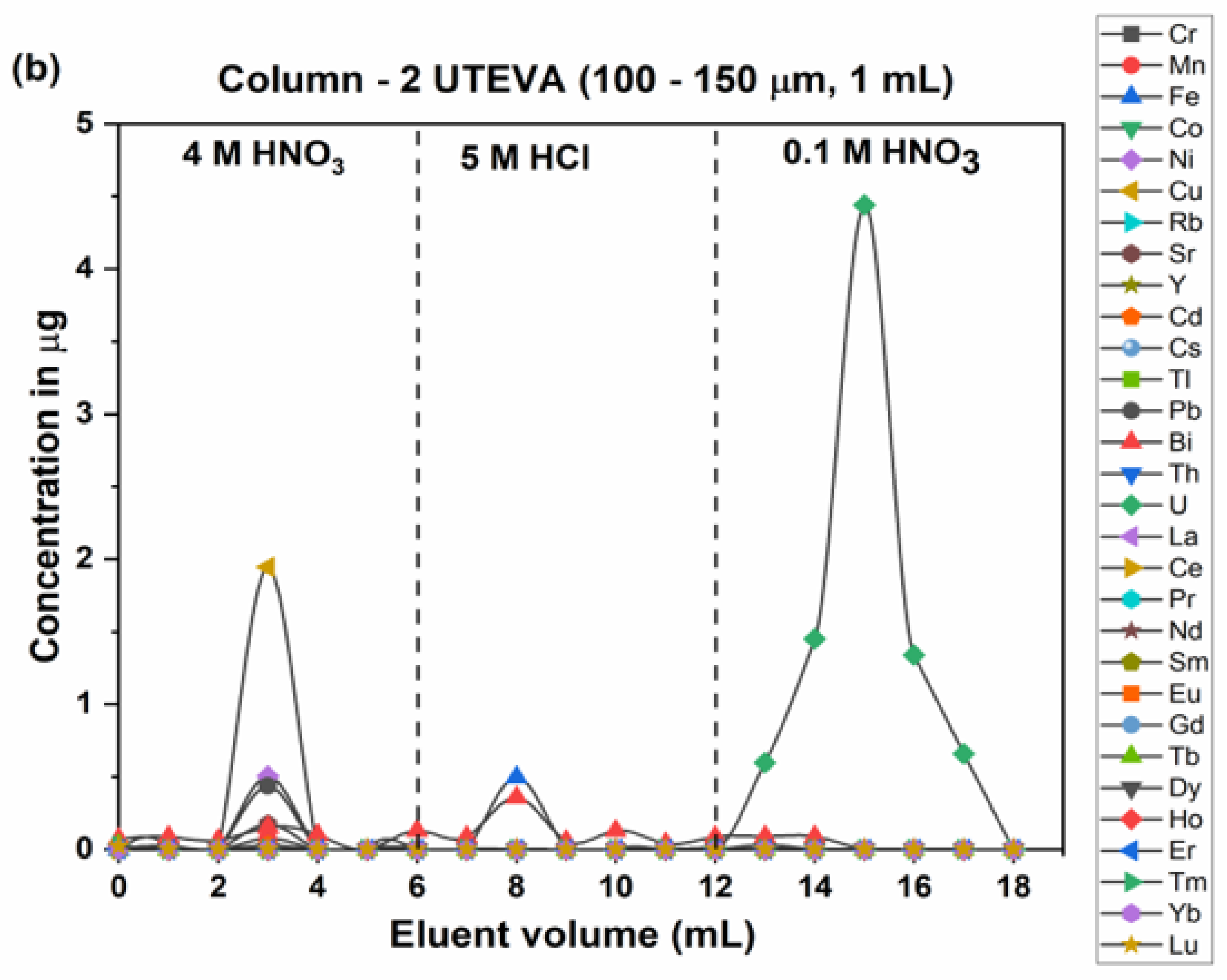
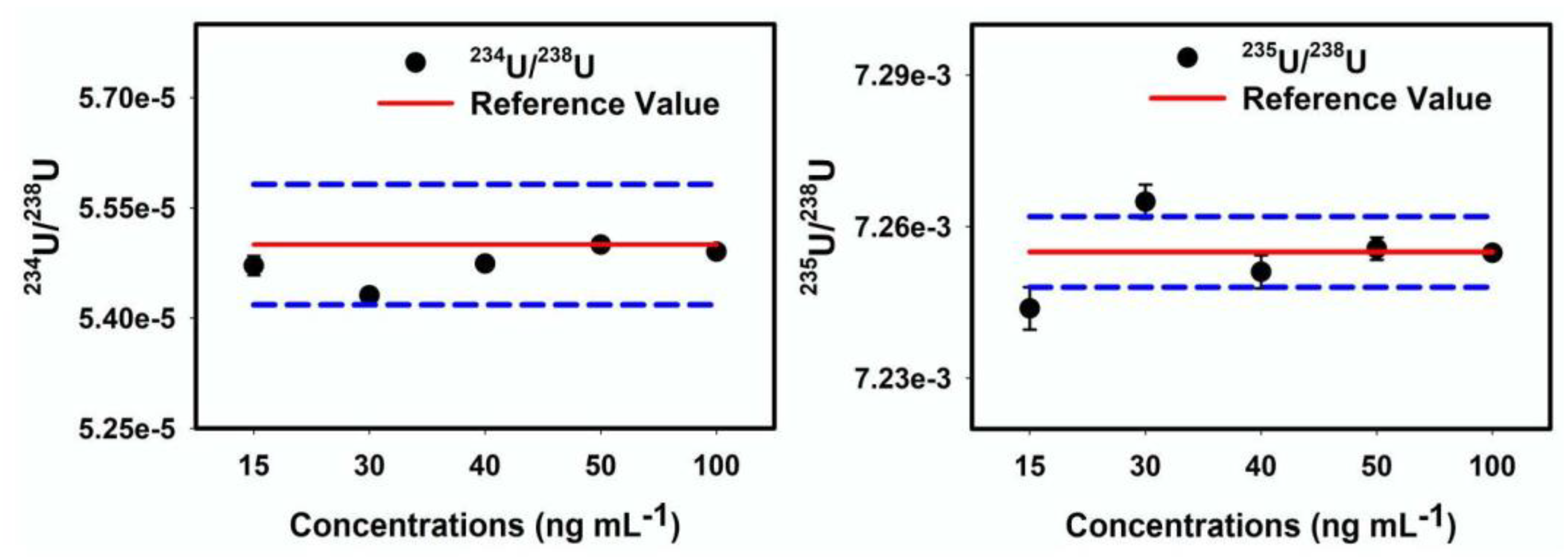
2.3. Analytical Chemical Separation of Uranium from a Synthetic Mixture and Samples
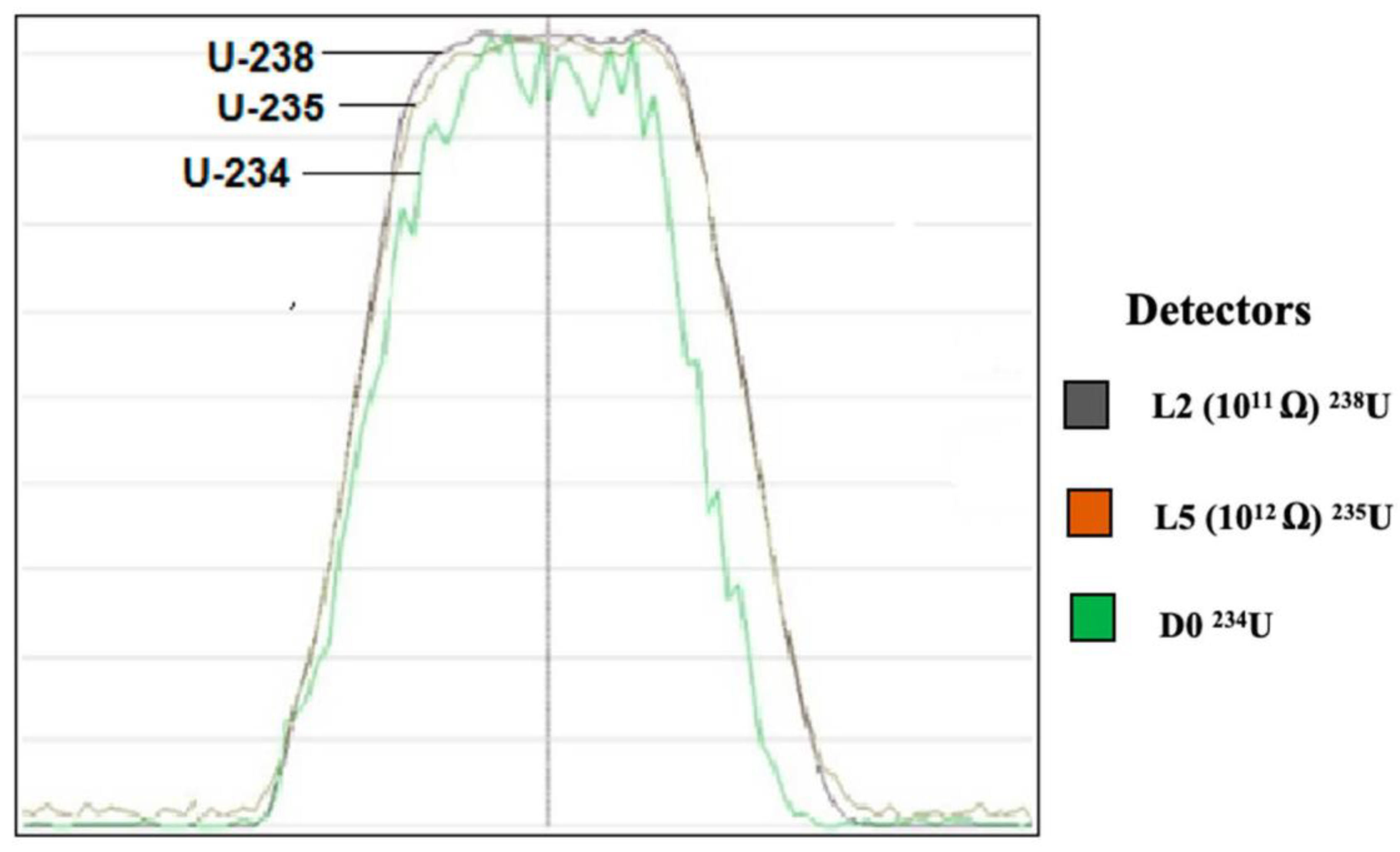
2.4. Optimization of MC-ICP-MS for U Isotope Ratios Measurement
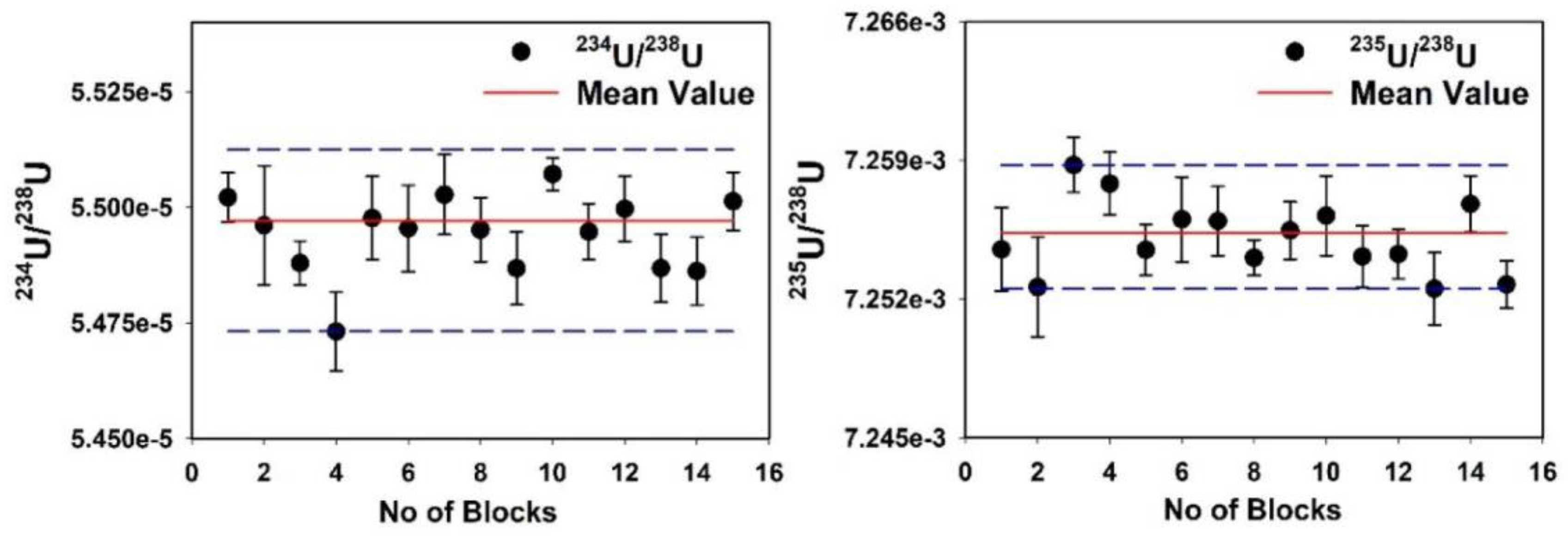
2.5. Measurement of U Isotope Ratios for in-House Standard Using MC-ICP-MS
2.6. Validation of U Isotope Ratio Measurement Using MC-ICP-MS
2.6.1. Accuracy and Precision for In-House Standard (JB-1) Using MC-ICP-MS
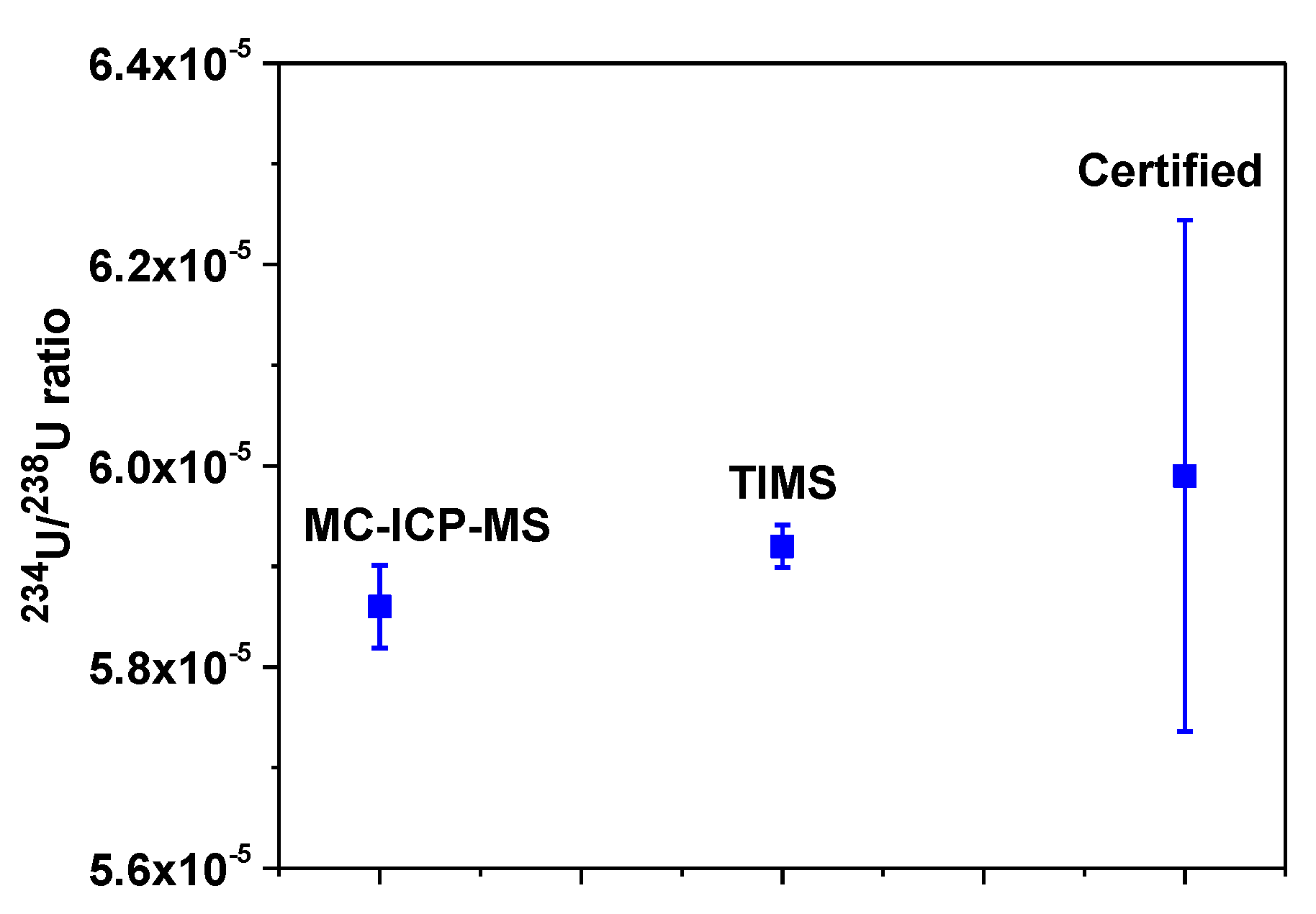
2.6.2. Validation of 234U/238U Ratio for NIST SRM 4350B
2.7. Measurement of 234U/238U and 235U/238U Isotope Ratios in Reference Materials and Environmental Samples
3. Materials and Methods
3.1. Reagents
3.2. Samples
3.3. Sample Preparation
3.4. Extraction Chromatography Resin (UTEVA)
3.5. Instrumentation
3.5.1. XRF
3.5.2. ICP-MS
3.5.3. MC-ICP-MS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ivanovich, M.; Harmon, R.S. Uranium Series Disequilibrium: Applications to Earth, Marine and Environmental Sciences, 2nd ed.; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Murphy, M.J.; Froehlich, M.B.; Fifield, L.K.; Turner, S.P.; Schaefer, B.F. In situ production of natural 236U in groundwaters and ores in high-grade uranium deposits. Chem. Geol. 2015, 410, 213–222. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Nakamura, Y.; Siraishi, K.; Masuda, A. Accurate measurement of uranium isotope ratios in soil samples using thermal ionization mass spectrometry equipped with a warp energy filter. Int. J. Environ. Anal. Chem. 2004, 84, 919–926. [Google Scholar] [CrossRef]
- Rovan, L.; Strok, M. Optimization of the sample preparation and measurement protocol for the analysis of uranium isotopes by MC-ICP-MS without spike addition. J. Anal. At. Spectrom. 2019, 34, 1882–1891. [Google Scholar] [CrossRef]
- Andersen, M.B.; Stirling, C.H.; Weyer, S. Uranium isotope fractionation. Rev. Mineral. Geochem. 2017, 83, 799–850. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.K.; Chaudhury, P.; Pradeepkumar, K.S. Measurement and validation of uranium isotope ratio in uranium ore for isotopic fingerprinting. Radiat. Prot. Environ. 2017, 40, 3–8. [Google Scholar] [CrossRef]
- Uvarova, Y.A.; Kyser, T.K.; Geagea, M.L.; Chipley, D. Variations in the uranium isotopic compositions of uranium ores from different types of uranium deposits. Geochim. Cosmochim. Acta 2014, 146, 1–17. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Matusevich, J.L.; Mironov, V.P.; Kudrjashov, V.P.; Halicz, L.; Segal, I.; McLean, J.A.; Monaser, A.; Becker, J.S. Determination of 236U/238U isotope ratio in contaminated environmental samples using different ICP-MS instruments. J. Anal. At. Spectrom. 2002, 17, 958–964. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Kimura, S.; Watanabe, Y.; Shiraishi, K.; Masuda, A. Detection of 236U and variation of uranium isotope composition in the soil samples affected by the JCO criticality accident. Proc. Jpn. Acad. Ser. B 2002, 78, 196–200. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Sabine Becker, J.; Matusevitch, J.L.; Dietze, H.J. Isotope ratio measurements of spent nuclear uranium in environmental samples by using inductively coupled plasma mass spectrometry. Int. J. Mass Spectrom. 2000, 203, 143–154. [Google Scholar] [CrossRef]
- Croudace, I.W.; Russel, B.C.; Warwick, P.W. Plasma source mass spectrometry for radioactive waste characterization in support of nuclear decommissioning; a review. J. Anal. At. Spectrom. 2017, 32, 494–526. [Google Scholar] [CrossRef]
- Baxter, D.C.; Rodushkin, I.; Engstrom, E. Isotope abundance ratio measured by inductively coupled plasma-sector field mass spectrometry. J. Anal. At. Spectrom. 2012, 27, 1355–1381. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Koepf, A.; Kapper, K.S.; Macsik, Z.; Stadelmann, G. Uranium isotope analysis by MC-ICP-MS in sub-ng sized samples. J. Anal. At. Spectrom. 2016, 31, 2272–2284. [Google Scholar] [CrossRef]
- Pollington, A.D.; Kinman, W.S.; Hanson, K.S.; Steiner, R.E. Polyatomic interferences on high precision uranium isotope ratio measurement by MC-ICP-MS: Application to environmental sampling for nuclear safeguards. J. Radioanal. Nucl. Chem. 2016, 307, 2109–2115. [Google Scholar] [CrossRef]
- Varga, Z.; Krachler, M.; Nicholl, A.; Ernstberger, M.; Wiss, T.; Wallenius, M.; Mayer, K. Accurate measurement of uranium isotope ratios in solid samples by laser ablation multi-collector inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2018, 33, 1076–1080. [Google Scholar] [CrossRef]
- Adriaens, A.G.; Fassett, J.D.; Kelly, W.R.; Simons, D.S.; Adams, F.C. Determination of Uranium and Thorium concentrations in soils: Comparison of isotope dilution-secondary ion mass spectrometry and isotope dilution-thermal ionization mass spectrometry. Anal. Chem. 1992, 64, 2945–2950. [Google Scholar] [CrossRef]
- Croudace, I.; Warwick, P.; Taylor, R.; Dee, S. Rapid procedure for plutonium and uranium determination in soils using a borate fusion followed by ion-exchange and extraction chromatography. Anal. Chim. Acta 1998, 371, 217–225. [Google Scholar] [CrossRef]
- Yokoyama, T.; Makishima, A.; Nakamura, E. Separation of thorium and uranium from silicate rock samples using two commercial extraction chromatographic resins. Anal. Chem. 1999, 71, 135–141. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.K.; Arae, H.; Watanabe, Y.; Mietelski, J.W. Activity ratio of caesium, strontium and uranium with site specific distribution coefficients in contaminated soil near vicinity of Fukushima dai-ichi nuclear power plant. J. Chromatogr. Sep. Technol. 2014, 5, 1–6. [Google Scholar]
- Chang, L.L.Y.; Howie, R.A.; Zussman, J. Rock Forming Minerals, Non-Silicates, Sulphides, Carbonates, Phosphates and Halides, 2nd ed.; The Geological Society: London, UK, 1998; pp. 336–349. [Google Scholar]
- Sahoo, S.K.; Kierepko, R.; Sorimachi, A.; Omori, Y.; Ishikawa, T.; Tokonami, S.; Prasad, G.; Gusain, G.S.; Ramola, R.C. Natural radioactivity level and elemental composition of soil samples from a high background radiation area on eastern coast of India (Odisha). Radiat. Prot. Dosim. 2016, 171, 172–178. [Google Scholar] [CrossRef]
- Kasar, S.; Mishra, S.; Omori, Y.; Sahoo, S.K.; Kavasi, N.; Arae, H.; Sorimachi, A. Sorption and desorption studies of Cs and Sr in contaminated soil samples around Fukushima Daiichi Nuclear Power Plant. J. Soils Sediments 2020, 20, 392–403. [Google Scholar] [CrossRef]
- Kritsananuwat, R.; Sahoo, S.K.; Fukushi, M.; Chanyotha, S. Distribution of rare earth elements, thorium and uranium in Gulf of Thailand’s sediments. Environ. Earth Sci. 2015, 73, 3361–3374. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1239. [Google Scholar] [CrossRef]
- Kasar, S.; Sahoo, S.K.; Area, H.; Mishra, S.; Tokonami, S.; Aono, T. Uranium, thorium, and rare earth elements distribution in Fukushima soil samples. Radiat. Prot. Dosim. 2019, 184, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Sengupta, D.; Das, S.K.; Saha, S.K.; Van, K.V. Natural radioactivity and radiation exposure in the high background area at Chhatrapur beach placer deposit of Orissa, India. J. Environ. Radioact. 2004, 75, 15–33. [Google Scholar] [CrossRef]
- Mishra, S.; Kasar, S.; Takamasa, A.; Veerasamy, N.; Sahoo, S.K. Measurement of uranium distribution coefficient and 235U/238U ratio in soils affected by Fukushima dai-ichi nuclear power plant accident. J. Eniviron. Radioact. 2019, 196, 36–42. [Google Scholar] [CrossRef]
- Yang, L.; Tong, S.; Zhou, L.; Hu, Z.; Mester, Z.; Meija, J. A critical review on isotopic fractionation correction methods for accurate isotope amount ratio measurements by MC-ICP-MS. J. Anal. At. Spectrom. 2018, 33, 1849–1861. [Google Scholar] [CrossRef]
- ISO. Guide to the Expression of Uncertainty in Measurement, 1st ed.; International Standards Organisation ISO: Geneva, Switzerland, 2008; ISBN 92-67-10188-9. [Google Scholar]
- Ellison, S.L.R.; Williams, A. Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; EURACHEM: Torino, Italy, 2012; ISBN 978-0-948926-30-3. [Google Scholar]
- NIST 4350B. Source River Sediments. Standard Reference Material Program, Certified Standard Reference Material 4350B; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1981.
- Qiao, J.; Lagerkvist, P.; Rodushkin, I.; Paatero, S.S.; Roos, P.; Lierhagen, S.; Jensen, K.A.; Engstrom, E.; Lahaye, Y.; Skipperud, L. On the application of ICP-MS techniques for measuring uranium and plutonium: A Nordic inter-laboratory comparison exercise. J. Radioanal. Nucl. Chem. 2018, 315, 565–580. [Google Scholar] [CrossRef]
- Makishima, A.; Nakamura, E. High-resolution MC-ICPMS employing amplifiers with a 1012ohm resistor for bulk sulfur determination in biological and geological samples. J. Anal. At. Spectrom. 2012, 27, 891–895. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Sample Name | Certified Value of U (μg g−1) | Measured Value of U (μg g−1) in Soil | RSD (%) |
|---|---|---|---|
| SRM 4350 | 2.51 | 2.50 ± 0.5 | 1.9 |
| JSd-2 | 1.1 | 1.02 ± 0.01 | 3.8 |
| JLk-1 | 3.83 | 3.77 ± 0.14 | 3.7 |
| JB-1 | 1.67 | 1.65 ± 0.03 | 1.7 |
| JB-3 | 0.48 | 0.46 ± 0.01 | 4.5 |
| Sample Name | GPS Co-Ordinates | D | La | Ce | Nd | Th | U | TiO2 | Fe2O3 | P2O5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nGy h−1 | μg g−1 | wt% | |||||||||
| HBRA-1 | N 19.323 | E 84.960 | 572 | 18 ±1.3 | 2454 ± 2 | 826 ± 0.2 | 497 ± 0.4 | 16 ± 0.02 | 9.4 | 10.5 | 0.29 |
| HBRA-2 | N 19.258 | E 84.905 | 1064 | 15.5 ± 2 | 2344 ± 7 | 783 ± 1 | 587 ± 1 | 13 ± 0.01 | 7.7 | 8.2 | 0.24 |
| HBRA-3 | N 19.347 | E 84.946 | 1224 | 1127 ± 0.3 | 2317 ± 5 | 946 ± 0.1 | 634 ±0.1 | 30 ± 0.2 | 8.9 | 12 | 0.18 |
| Fuk-1 | N 37.552 | E 140.918 | 7507 | 23.6 ± 0.3 | 45 ± 0.5 | 19.1 ± 0.3 | 11 ± 0.07 | 2.1± 0.01 | 0.7 | 6.3 | 0.09 |
| Fuk-2 | N 37.498 | E 140.969 | 19562 | 17.6 ± 0.2 | 36 ± 0.6 | 14 ± 0.2 | 9.8 ± 0.1 | 2 ± 0.03 | 0.5 | 4.9 | 0.07 |
| UCC | 32 | 65 | 26 | 10 | 2.5 | 0.5 | 4.9 | 0.1 | |||
| RF Power | 1300 W |
|---|---|
| Acceleration Potential (V) | 6000 |
| Sampler cone | Ni cone |
| Skimmer cone | Ni wide angle cone |
| Resolution | Low |
| Cool gas | 13.4 L min−1 |
| Auxiliary gas | 0.90 L min−1 |
| Wet Plasma | |
| Sample | Conventional Spray chamber |
| Nebulizer | Micromist, 200 µL min−1 |
| Nebulizer gas | 1.14 L min−1 |
| Sweep Ar Gas | - |
| Cycles/Blocks | 10 cycles/15 blocks |
| Sample Concentration | 50 ng mL−1 |
| Typical Sensitivity | 50 V per µg mL−1 |
| Washout time 2% HNO3 | 10–15 min |
| 238U Beam intensity | 2.6–3.6 V |
| Date | 234U/238U | RSE% | 235U/238U | RSE% |
|---|---|---|---|---|
| 2020-Feb-27 | 0.0000553 (01) | 0.253 | 0.0072550 (30) | 0.045 |
| 2020-Feb-28 | 0.0000554 (01) | 0.248 | 0.0072467 (27) | 0.040 |
| 2020-Mar-02 | 0.0000553 (13) | 0.240 | 0.0072557 (29) | 0.040 |
| 2020-Mar-03 | 0.0000554 (02) | 0.246 | 0.0072489 (26) | 0.035 |
| 2020-Mar-09 | 0.0000547 (02) | 0.231 | 0.0072567 (26) | 0.039 |
| 2020-Mar-10 | 0.0000546 (02) | 0.254 | 0.0072506 (28) | 0.039 |
| 2020-Mar-11 | 0.0000546 (06) | 0.249 | 0.0072566 (31) | 0.046 |
| Mean | 0.0000551 (04) | 0.246 | 0.0072529 (28) | 0.045 |
| Sample Name | 234U/238U | 235U/238U |
|---|---|---|
| SRM 4350B | 0.0000586 (01) | 0.0072527 (50) |
| JSd-2 | 0.0000566 (02) | 0.0072597 (57) |
| JLk-1 | 0.0000587 (01) | 0.0072499 (43) |
| JB-1 | 0.0000547 (02) | 0.0072543 (30) |
| JB-3 | 0.0000551 (03) | 0.0072560 (68) |
| HBRA-1 | 0.0000539 (04) | 0.0072594 (05) |
| HBRA-2 | 0.0000546 (03) | 0.0072500 (09) |
| HBRA-3 | 0.0000541 (04) | 0.0072545 (69) |
| Fuk-1 | 0.0000552 (02) | 0.0072523 (20) |
| Fuk-2 | 0.0000564 (04) | 0.0072585 (19) |
| Detectors | L7 | D4 | D3 | D2 | D1 | L6 | D0 | L5 | L4 | L3 | L2 | L1 | Ax | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Configuration | 234U | 235U | 238U | 238UH+ | |||||||||||||||||
| 2nd Configuration | 235U | 238U | 238UH+ | ||||||||||||||||||
| Faraday Cup resistors | 1012Ω | 1012Ω | 1012Ω | 1011Ω | 1011Ω | 1011Ω | 1011Ω | 1011Ω | 1011Ω | 1011Ω | 1011 Ω | 1011Ω | 1011Ω | 1011Ω | 1011Ω | 1011Ω |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veerasamy, N.; Takamasa, A.; Murugan, R.; Kasar, S.; Aono, T.; Inoue, K.; Fukushi, M.; Sahoo, S.K. Chemical Separation of Uranium and Precise Measurement of 234U/238U and 235U/238U Ratios in Soil Samples Using Multi Collector Inductively Coupled Plasma Mass Spectrometry. Molecules 2020, 25, 2138. https://doi.org/10.3390/molecules25092138
Veerasamy N, Takamasa A, Murugan R, Kasar S, Aono T, Inoue K, Fukushi M, Sahoo SK. Chemical Separation of Uranium and Precise Measurement of 234U/238U and 235U/238U Ratios in Soil Samples Using Multi Collector Inductively Coupled Plasma Mass Spectrometry. Molecules. 2020; 25(9):2138. https://doi.org/10.3390/molecules25092138
Chicago/Turabian StyleVeerasamy, Nimelan, Asako Takamasa, Rajamanickam Murugan, Sharayu Kasar, Tatsuo Aono, Kazumasa Inoue, Masahiro Fukushi, and Sarata Kumar Sahoo. 2020. "Chemical Separation of Uranium and Precise Measurement of 234U/238U and 235U/238U Ratios in Soil Samples Using Multi Collector Inductively Coupled Plasma Mass Spectrometry" Molecules 25, no. 9: 2138. https://doi.org/10.3390/molecules25092138
APA StyleVeerasamy, N., Takamasa, A., Murugan, R., Kasar, S., Aono, T., Inoue, K., Fukushi, M., & Sahoo, S. K. (2020). Chemical Separation of Uranium and Precise Measurement of 234U/238U and 235U/238U Ratios in Soil Samples Using Multi Collector Inductively Coupled Plasma Mass Spectrometry. Molecules, 25(9), 2138. https://doi.org/10.3390/molecules25092138






