Microwave-Assisted Heating Reactions of N-Acetylglucosamine (GlcNAc) in Sulfolane as a Method Generating 1,6-Anhydrosugars Consisting of Amino Monosaccharide Backbones
Abstract
1. Introduction
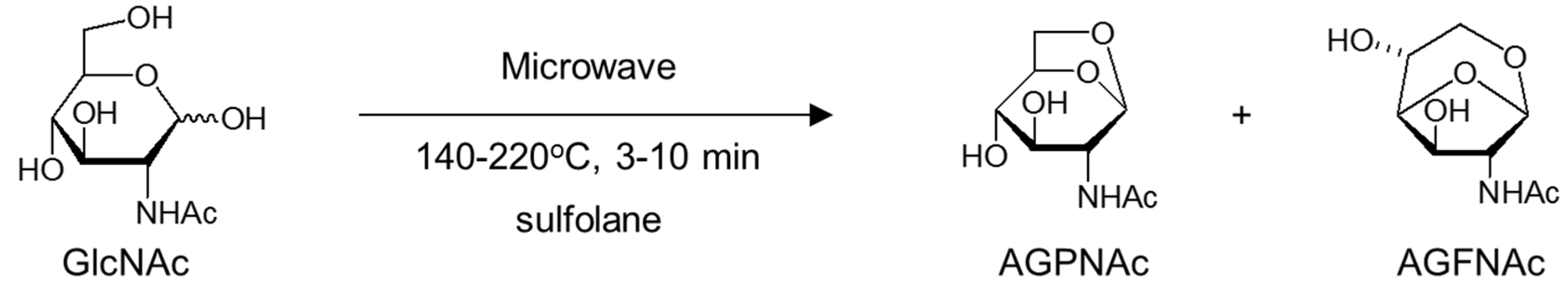
2. Results and Discussion
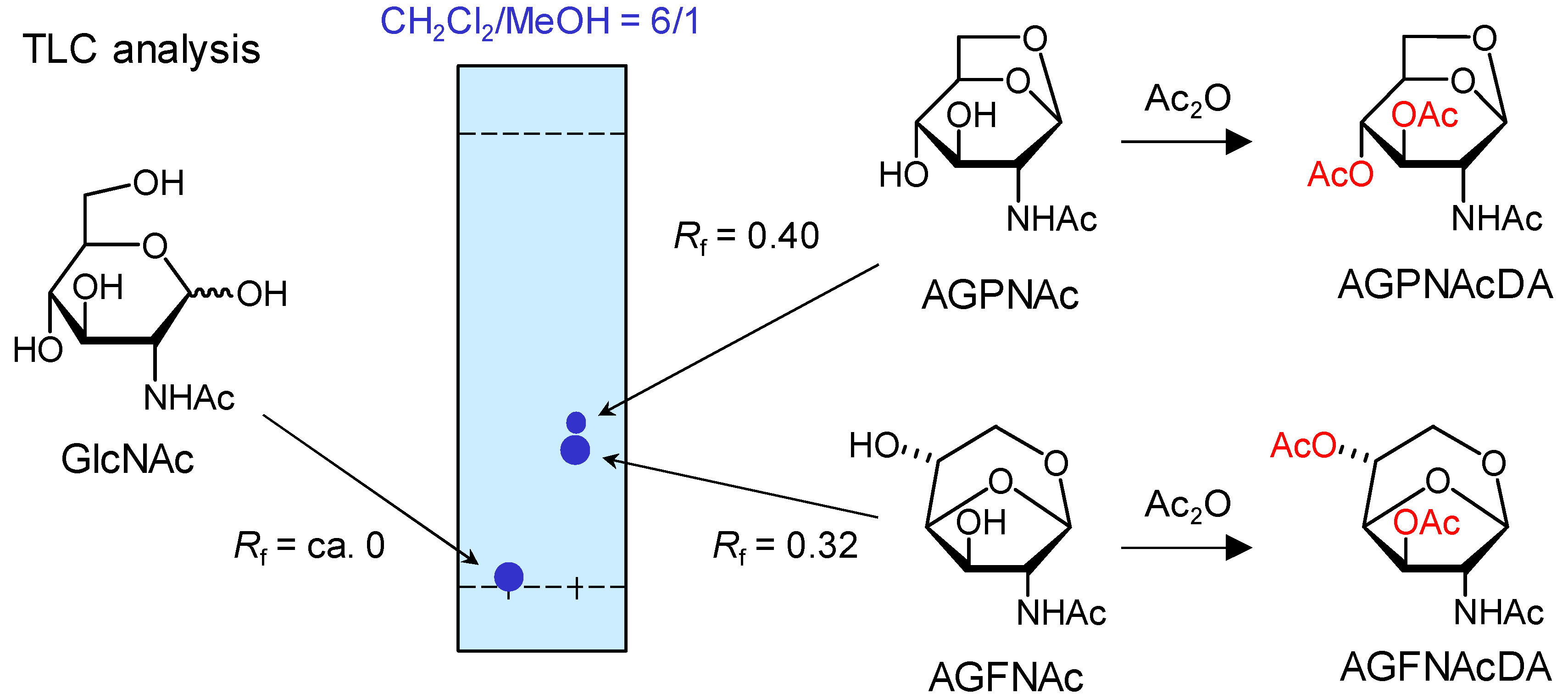
2.1. Products Assignments
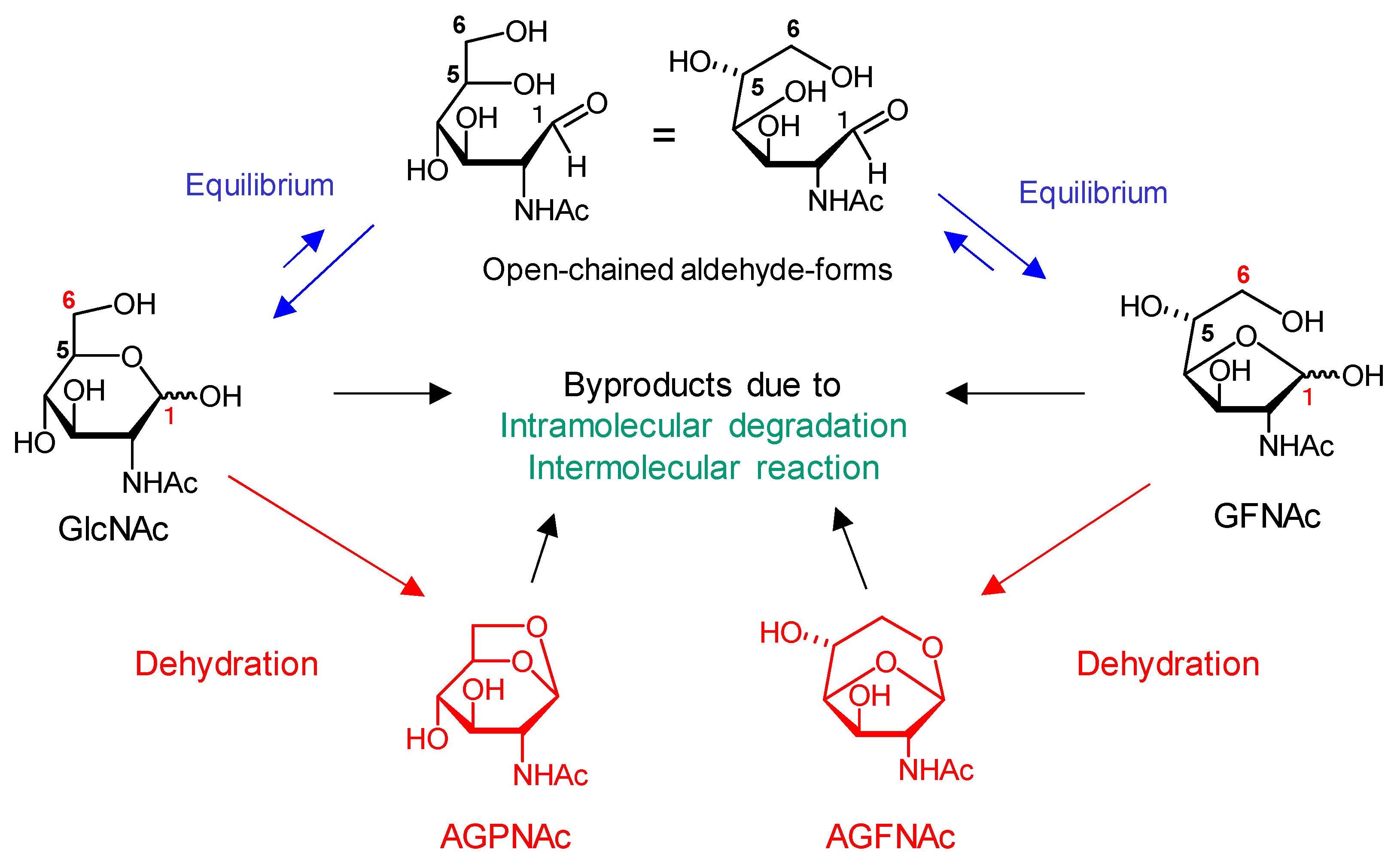
2.2. Reaction System Clarification
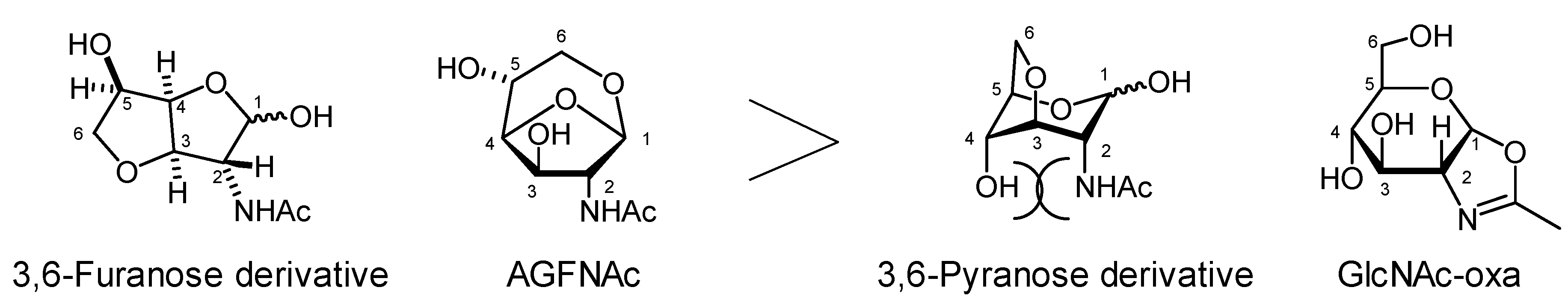
2.3. Selectivity to Form Pyranose Ring Versus Furanose Ring Differed from other Systems
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Microwave-Assisted Heating Reaction and HPLC Analysis
3.4. Isolation of 1,6-Anhydro-2-acetamido-2-deoxy-β-d-glucopyranose (AGPNAc) and 1,6-Anhydro-2-acetamido-2-deoxy-β-d-glucofuranose (AGFNAc)
3.5. Acetylation of AGPNAc
3.6. Acetylation of AGFNAc
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cerny, M. Chemistry of anhydro sugars. Adv. Carbohydr. Chem. Biochem. 2003, 58, 121–198. [Google Scholar] [CrossRef]
- Ruckel, E.; Schuerch, C.J. Preparation of High Polymers from 1,6-Anhydro-2,3,4-tri-O-Substituted β-d-Glucopyranose. J. Org. Chem. 1966, 31, 2233–2239. [Google Scholar] [CrossRef]
- Uryu, T.; Schuerch, C.J. Preparation of High Molecular Weight 2,3,4-Tri-O-benzyl-[1→6]-α-d-gluco- and -galactopyranan and [1→6]-α-d-Glucopyranan. Macromology 1971, 4, 342–345. [Google Scholar] [CrossRef]
- Kakuchi, T.; Kusuno, A.; Miura, M.; Kaga, H. Cationic ring-opening polymerization of 1,6-anhydro-2,3,4-tri-O-allyl-β-d-glucopyranose as a convenient synthesis of dextran. Macromol. Rapid Commun. 2000, 21, 1003–1006. [Google Scholar] [CrossRef]
- Satoh, T.; Imai, T.; Ishihara, H.; Maeda, T.; Kitajyo, Y.; Narumi, A.; Kaga, H.; Kaneko, N.; Kakuchi, T. Synthesis of hyperbranched polysaccharide by thermally induced cationic polymerization of 1,6-anhydro-β-d-mannopyranose. Macromolecules 2003, 36, 6364–6370. [Google Scholar] [CrossRef]
- Hattori, K.; Yoshida, T.; Uryu, T. Ring-opening polymerization of new 1,6-anhydro-β-d-glucosamine derivatives. Carbohyd. Polym. 1998, 36, 129–135. [Google Scholar] [CrossRef]
- Kadokawa, J.; Sato, M.; Karasu, M.; Tagaya, H.; Chiba, K. Synthesis of hyperbranched aminopolysaccharides. Angew. Chem. Int. Ed. 1998, 37, 2373–2376. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Honjo, K.; Kitagawa, S.; Uemura, T. Preparation of Porous Polysaccharides Templated by Coordination Polymer with Three-Dimensional Nanochannels. ACS Appl. Mater. Interfaces 2017, 9, 11373–11379. [Google Scholar] [CrossRef]
- Hoai, N.T.; Sasaki, A.; Sasaki, M.; Kaga, H.; Kakuchi, T.; Satoh, T. Selective synthesis of 1,6-anhydro-β-d-mannopyranose and -mannofuranose using microwave-assisted heating. Carbohydr. Res. 2011, 346, 1747–1751. [Google Scholar] [CrossRef]
- Hoai, N.T.; Sasaki, A.; Sasaki, M.; Kaga, H.; Kakuchi, T.; Satoh, T. Synthesis, Characterization, and Lectin Recognition of Hyperbranched Polysaccharide Obtained from 1,6-Anhydro-d-hexofuranose. Biomacromolecules 2011, 12, 1891–1899. [Google Scholar] [CrossRef]
- McMurry, J.E. Organic Chemistry, 9th ed.; Brooks/Cole Pub Co.: Belmont, CA, USA, 2015; pp. 832–869. [Google Scholar]
- McMurry, J.; Ballantine, D.S.; Hoeger, C.A.; Peterson, V.E. Fundamentals of General, Organic, and Biological Chemistry, 7th ed.; Pearson Education Limited: Essex, UK, 2014; pp. 713–752. [Google Scholar]
- Hesek, D.; Lee, M.; Zhang, W.L.; Noll, B.C.; Mobashery, S. Total Synthesis of N-Acetylglucosamine-1,6-anhydro-N-acetylmuramylpentapeptide and Evaluation of Its Turnover by AmpD from Escherichia coli. J. Am. Chem. Soc. 2009, 131, 5187–5193. [Google Scholar] [CrossRef]
- Akagi, M.; Tejima, S.; Haga, M. Synthesis of Anomers of 6-O-Tosyl-1, 3, 4-tri-O-acetyl-N-acetyl-d-glucosamine and 1, 6-Anhydro-N-acetyl-β-d-glucosamine. Chem. Pharm. Bull. 1962, 10, 1039–1042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabinsohn, Y.; Acher, A.J.; Shapiro, D. Derivatives of 1,6-anhydroglucosamine and their use as aglycons in disaccharide synthesis. J. Org. Chem. 1973, 38, 202–204. [Google Scholar] [CrossRef]
- Lafont, D.; Boullanger, P.; Cadas, O.; Descotes, G. A Mild Procedure for the Preparation of 1,6-Anhydro-β-d-Hexopyranoses and Derivatives. Synthesis 1989, 1989, 191–194. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Oros, D.R.; Elias, V.O. Molecular tracers for smoke from charring/burning of chitin biopolymer. Chemosphere Glob. Chang. Sci. 2000, 2, 101–105. [Google Scholar] [CrossRef]
- Schmitt, F.; Sinaÿ, P. Synthèse de disaccharides à liaison (1→4) posseédant un résidu réducteur 2-acétamido-2-désoxy-d-glucopyranose: Emploi de dérivés du 2-acétamido-1,6-anhydro-2-désoxy-β-d-glucopyranose. Carbohydr. Res. 1973, 29, 99–111. [Google Scholar] [CrossRef]
- Ogata, M.; Hattori, T.; Takeuchi, R.; Usui, T. Novel and facile synthesis of furanodictines A and B based on transformation of 2-acetamido-2-deoxy-d-glucose into 3,6-anhydro hexofuranoses. Carbohydr. Res. 2010, 345, 230–234. [Google Scholar] [CrossRef]
- Sasaki, M.; Takahashi, K.; Haneda, Y.; Satoh, H.; Sasaki, A.; Narumi, A.; Satoh, T.; Kakuchi, T.; Kaga, H. Thermochemical transformation of glucose to 1,6-anhydroglucose in high-temperature steam. Carbohydr. Res. 2008, 343, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Kaga, H.; Kakuchi, T.; Satoh, T.; Takahashi, K. Formation of Anhydroglucose in Ionic Liquids by Microwave Heating-Temperature and Chloride Ion Effects. Chem. Lett. 2009, 38, 1178–1179. [Google Scholar] [CrossRef]
- Köll, P.; Borchers, G.; Metzger, J.O. Thermal-Degradation of Chitin and Cellulose. J. Anal. Appl. Pyrol. 1991, 19, 119–129. [Google Scholar]
- Miura, M.; Kaga, H.; Sakurai, A.; Kakuchi, T.; Takahashi, K. Rapid pyrolysis of wood block by microwave heating. J. Anal. Appl. Pyrol. 2004, 71, 187–199. [Google Scholar] [CrossRef]
- Miura, M.; Kaga, H.; Yoshida, T.; Ando, K. Microwave pyrolysis of cellulosic materials for the production of anhydrosugars. J. Wood Sci. 2001, 47, 502–506. [Google Scholar] [CrossRef]
- Pazur, J.H.; Miskiel, F.J.; Liu, B.L. Identification of Furanose and Pyranose Ring Forms of Carbohydrates by Methylation, Gas-Liquid-Chromatography and Mass-Spectrometry. J. Chromatogr. 1987, 396, 139–147. [Google Scholar] [CrossRef]
- Mackie, W.; Perlin, A.S. Pyranose–furanose and anomeric equilibria: Influence of solvent and of partial methylation. Can. J. Chem. 1966, 44, 2039–2049. [Google Scholar] [CrossRef]
- Franks, F.; Lillford, P.J.; Robinson, G. Isomeric Equilibria of Monosaccharides in Solution-Influence of Solvent and Temperature. J. Chem. Soc. Faraday Trans. I. 1989, 85, 2417–2426. [Google Scholar] [CrossRef]
- Drover, M.W.; Omari, K.W.; Murphy, J.N.; Kerton, F.M. Formation of a renewable amide, 3-acetamido-5-acetylfuran, via direct conversion of N-acetyl-d-glucosamine. RSC Adv. 2012, 2, 4642–4644. [Google Scholar] [CrossRef]
- Wang, J.; Zang, H.J.; Jiao, S.L.; Wang, K.; Shang, Z.; Li, H.X.; Lou, J. Efficient conversion of N-acetyl-d-glucosamine into nitrogen-containing compound 3-acetamido-5-acetylfuran using amino acid ionic liquid as the recyclable catalyst. Sci. Total Env. 2020, 710. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaga, H.; Enomoto, M.; Shimizu, H.; Nagashima, I.; Matsuda, K.; Kawaguchi, S.; Narumi, A. Microwave-Assisted Heating Reactions of N-Acetylglucosamine (GlcNAc) in Sulfolane as a Method Generating 1,6-Anhydrosugars Consisting of Amino Monosaccharide Backbones. Molecules 2020, 25, 1944. https://doi.org/10.3390/molecules25081944
Kaga H, Enomoto M, Shimizu H, Nagashima I, Matsuda K, Kawaguchi S, Narumi A. Microwave-Assisted Heating Reactions of N-Acetylglucosamine (GlcNAc) in Sulfolane as a Method Generating 1,6-Anhydrosugars Consisting of Amino Monosaccharide Backbones. Molecules. 2020; 25(8):1944. https://doi.org/10.3390/molecules25081944
Chicago/Turabian StyleKaga, Harumi, Masaru Enomoto, Hiroki Shimizu, Izuru Nagashima, Keigo Matsuda, Seigou Kawaguchi, and Atsushi Narumi. 2020. "Microwave-Assisted Heating Reactions of N-Acetylglucosamine (GlcNAc) in Sulfolane as a Method Generating 1,6-Anhydrosugars Consisting of Amino Monosaccharide Backbones" Molecules 25, no. 8: 1944. https://doi.org/10.3390/molecules25081944
APA StyleKaga, H., Enomoto, M., Shimizu, H., Nagashima, I., Matsuda, K., Kawaguchi, S., & Narumi, A. (2020). Microwave-Assisted Heating Reactions of N-Acetylglucosamine (GlcNAc) in Sulfolane as a Method Generating 1,6-Anhydrosugars Consisting of Amino Monosaccharide Backbones. Molecules, 25(8), 1944. https://doi.org/10.3390/molecules25081944




