Experimental Activation of Endocannabinoid System Reveals Antilipotoxic Effects on Cardiac Myocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Model
2.2. Lipid Analyses
2.3. Plasma Measurements
2.4. Immunoblotting Analysis
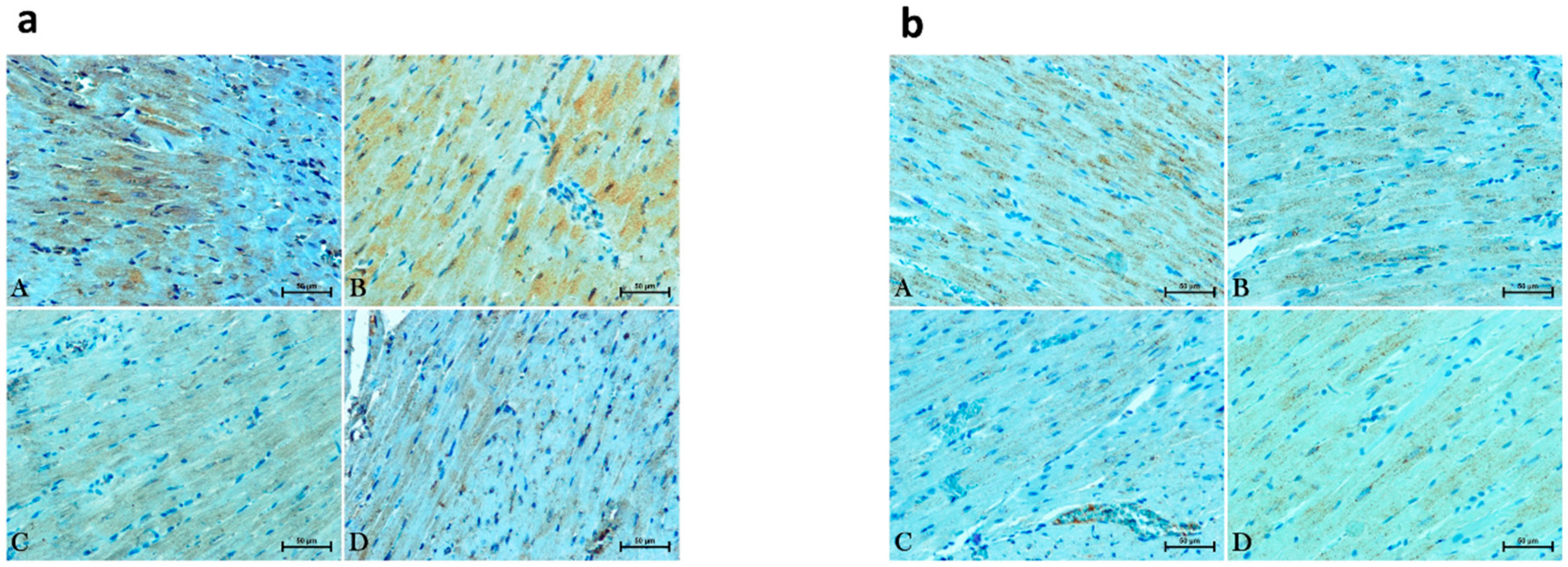
2.5. Immunohistochemical Analysis
2.6. Statistical Analyses
3. Results
3.1. Effect of Enhanced Activation of the Endocannabinoid System on Plasma Levels of Glucose and Insulin as Well as HOMA-IR in the Normotensive State and Primary Hypertension
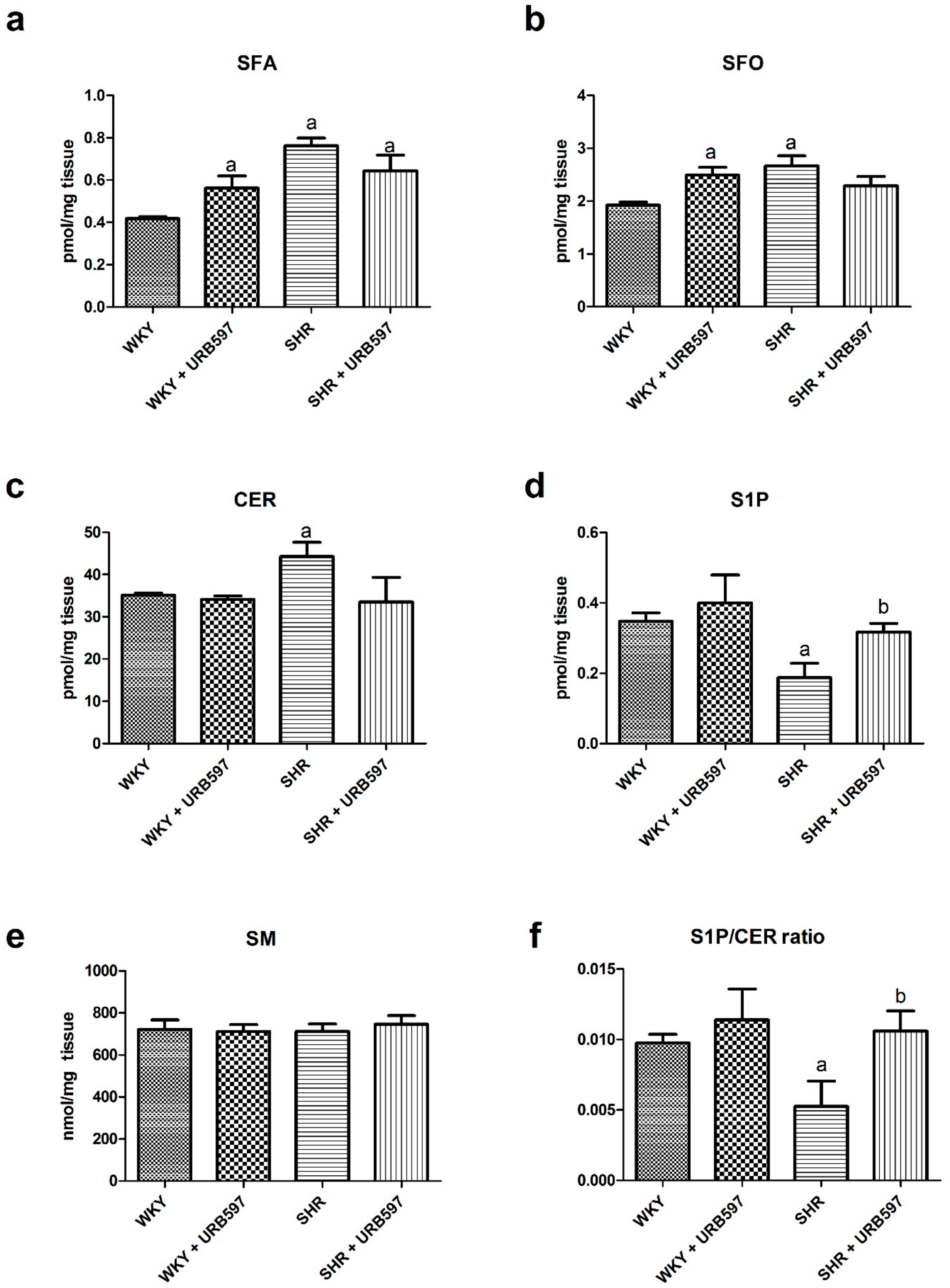
3.2. Effect of Enhanced Activation of the Endocannabinoid System on the Sphingolipid Pathway (Sphinganine, Ceramide, Sphingosine, and Shingosine-1-phosphate) in the Left Ventricle in Both Normotensive State and Primary Hypertension
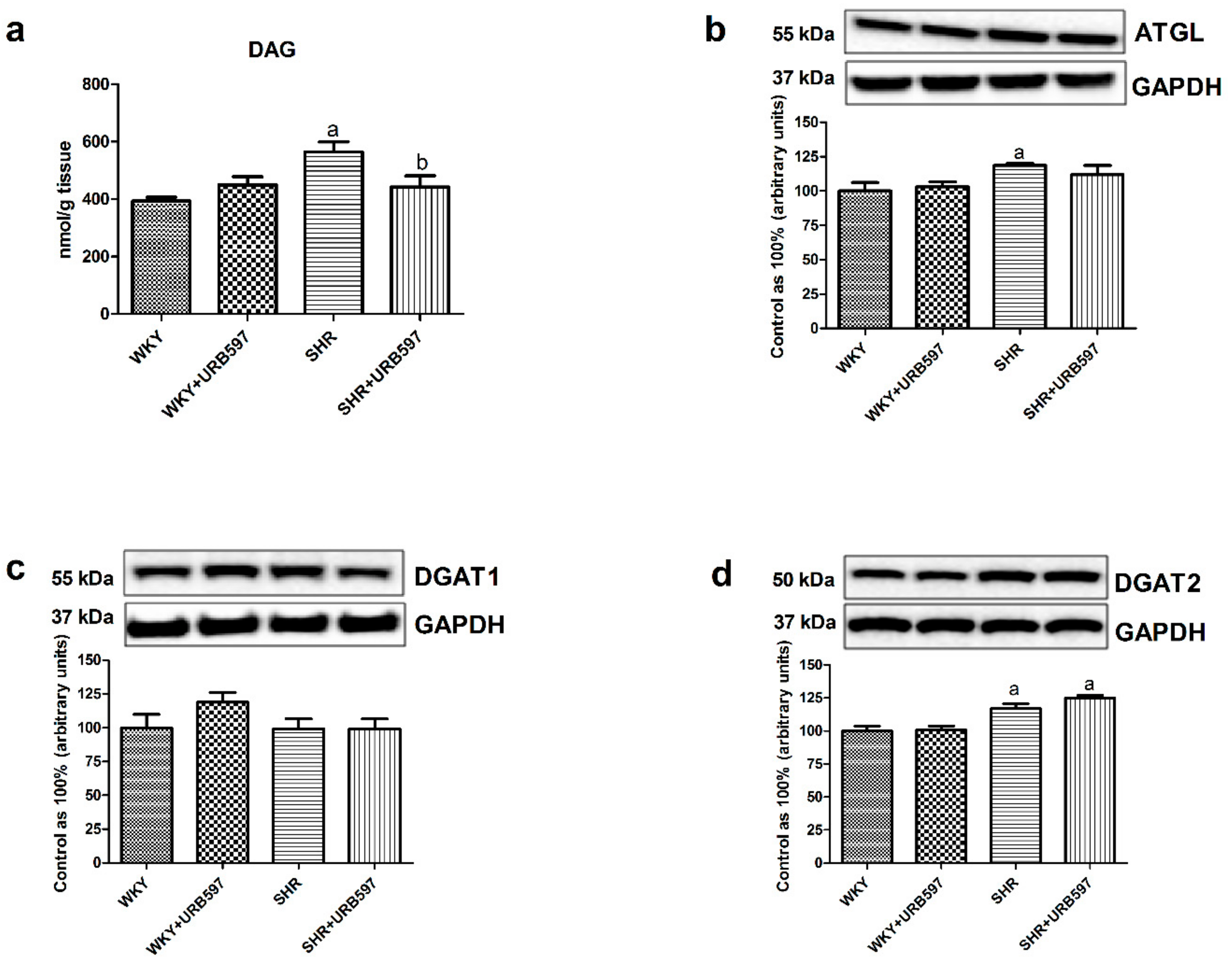
3.3. Effect of Enhanced Activation of the Endocannabinoid System on the Left Ventricle Content of Diacylglycerol and Total Expression of Enzymes Involved in Its Metabolism in the Normotensive State and Primary Hypertension
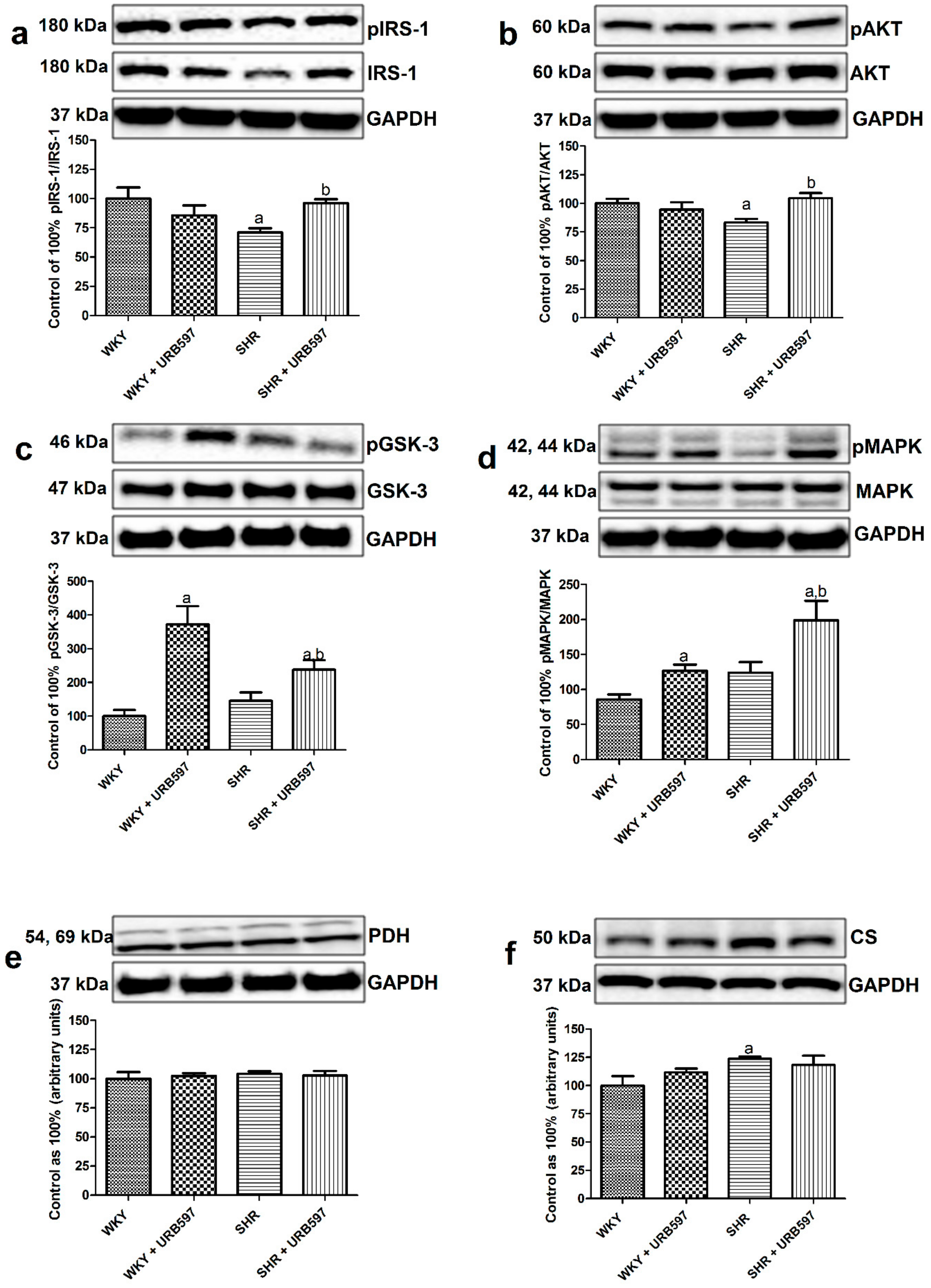
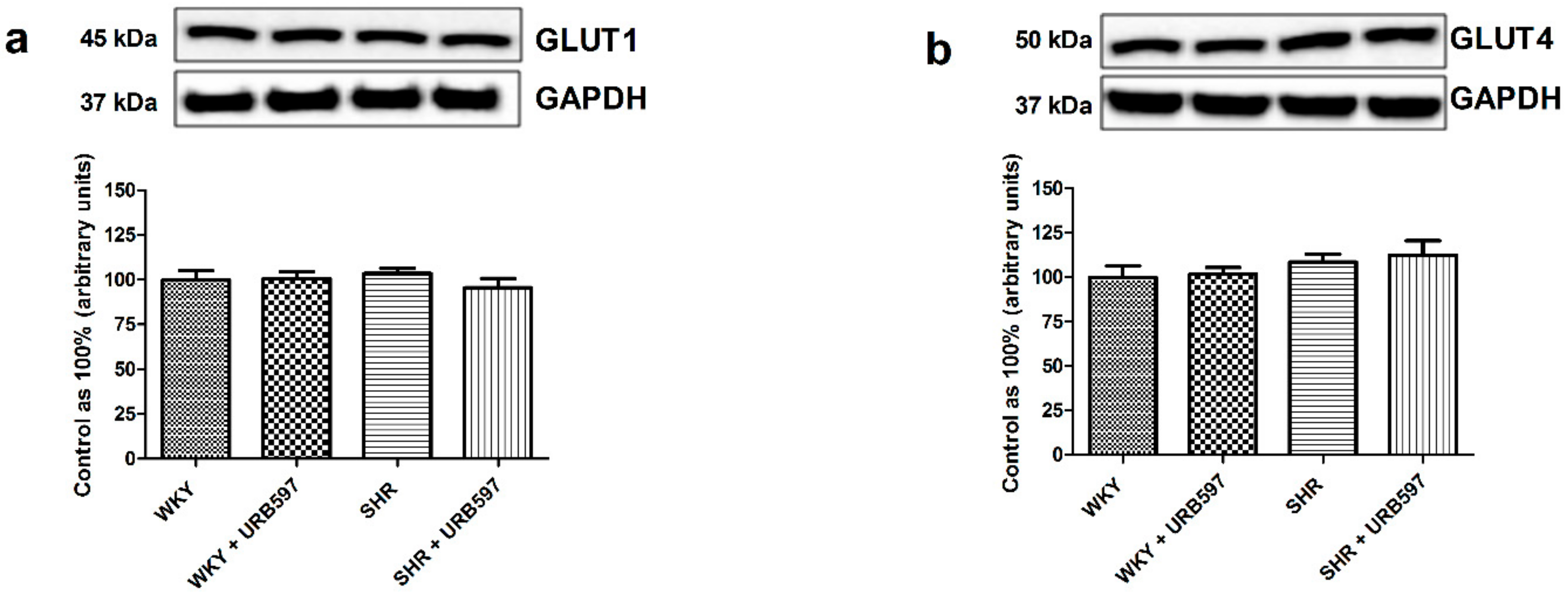
3.4. Effect of Enhanced Activation of the Endocannabinoid System on the Total Expression and Phosphorylation of Intramyocardial (Left Ventricle) Insulin Signaling Proteins and Proteins Involved in Glucose Metabolism in the Normotensive State and Primary Hypertension
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, V. Metabolic syndrome: What are the risks for humans? Biosci. Trends 2010, 4, 204–212. [Google Scholar] [PubMed]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 2012, 7, e52036. [Google Scholar] [CrossRef]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef]
- Zecchin, H.G.; Bezerra, R.M.; Carvalheira, J.B.; Carvalho-Filho, M.A.; Metze, K.; Franchini, K.G.; Saad, M.J. Insulin signalling pathways in aorta and muscle from two animal models of insulin resistance--the obese middle-aged and the spontaneously hypertensive rats. Diabetologia 2003, 46, 479–491. [Google Scholar] [CrossRef]
- Gouveia, L.M.; Kettelhut, I.C.; Foss, M.C. Abnormalities of glucose metabolism in spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2000, 33, 1357–1362. [Google Scholar] [CrossRef][Green Version]
- Lopaschuk, G.D. Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders. Ann. Nutr. Metab. 2016, 68 (Suppl. 3), 15–20. [Google Scholar] [CrossRef]
- Konstantynowicz-Nowicka, K.; Harasim, E.; Baranowski, M.; Chabowski, A. New evidence for the role of ceramide in the development of hepatic insulin resistance. PLoS ONE 2015, 10, e0116858. [Google Scholar] [CrossRef]
- Glatz, J.F.; Luiken, J.J.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef]
- De las Fuentes, L.; Herrero, P.; Peterson, L.R.; Kelly, D.P.; Gropler, R.J.; Dávila-Román, V.G. Myocardial fatty acid metabolism: Independent predictor of left ventricular mass in hypertensive heart disease. Hypertension 2003, 41, 83–87. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Wang, Y.; Zhang, J.; Zhao, W.; Fu, M.; Han, X.; Zhou, J.; Ge, J. Enhances Cardiac Glucose Metabolism and Improves Diastolic Function in Spontaneously Hypertensive Rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 3197320. [Google Scholar] [CrossRef] [PubMed]
- Hajri, T.; Ibrahimi, A.; Coburn, C.T.; Knapp, F.F.; Kurtz, T.; Pravenec, M.; Abumrad, N.A. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J. Biol. Chem. 2001, 276, 23661–23666. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Costa, M.A.; Almada, M.; Correia-da-Silva, G.; Teixeira, N.A. Endogenous cannabinoids revisited: A biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013, 102–103, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef]
- Toczek, M.; Baranowska-Kuczko, M.; Grzęda, E.; Pędzińska-Betiuk, A.; Weresa, J.; Malinowska, B. Age-specific influences of chronic administration of the fatty acid amide hydrolase inhibitor URB597 on cardiovascular parameters and organ hypertrophy in DOCA-salt hypertensive rats. Pharmacol. Rep. 2016, 68, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Pędzińska-Betiuk, A.; Weresa, J.; Toczek, M.; Baranowska-Kuczko, M.; Kasacka, I.; Harasim-Symbor, E.; Malinowska, B. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br. J. Pharmacol. 2017, 174, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Harasim-Symbor, E.; Polak, A.; Pędzińska-Betiuk, A.; Weresa, J.; Malinowska, B.; Lewandowska, A.; Kasacka, I.; Chabowski, A. Fatty acid amide hydrolase inhibitor (URB597) as a regulator of myocardial lipid metabolism in spontaneously hypertensive rats. Chem. Phys. Lipids 2019, 218, 141–148. [Google Scholar] [CrossRef]
- Polak, A.; Harasim-Symbor, E.; Malinowska, B.; Kasacka, I.; Pędzińska-Betiuk, A.; Weresa, J.; Chabowski, A. The effects of chronic FAAH inhibition on myocardial lipid metabolism in normotensive and DOCA-salt hypertensive rats. Life Sci. 2017, 183, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Harasim-Symbor, E.; Malinowska, B.; Kasacka, I.; Lewandowska, A.; Chabowski, A. The Endocannabinoid System Affects Myocardial Glucose Metabolism in the DOCA-Salt Model of Hypertension. Cell. Physiol. Biochem. 2018, 46, 727–739. [Google Scholar] [CrossRef] [PubMed]
- LaPier, T.L.; Rodnick, K.J. Changes in cardiac energy metabolism during early development of female SHR. Am. J. Hypertens. 2000, 13, 1074–1081. [Google Scholar] [CrossRef][Green Version]
- Rivera, P.; Bindila, L.; Pastor, A.; Pérez-Martín, M.; Pavón, F.J.; Serrano, A.; de la Torre, R.; Lutz, B.; Rodríguez de Fonseca, F.; Suárez, J. Pharmacological blockade of the fatty acid amide hydrolase (FAAH) alters neural proliferation, apoptosis and gliosis in the rat hippocampus, hypothalamus and striatum in a negative energy context. Front. Cell. Neurosci. 2015, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, G.; Alapafuja, S.O.; Bátkai, S.; Nikas, S.P.; Cinar, R.; Offertáler, L.; Osei-Hyiaman, D.; Liu, J.; Mukhopadhyay, B.; Harvey-White, J.; et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem. Biol. 2010, 17, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Van der Vusse, G.J.; Roemen, T.H.; Reneman, R.S. Assessment of fatty acids in dog left ventricular myocardium. Biochim. Biophys. Acta 1980, 617, 347–349. [Google Scholar] [CrossRef]
- Baranowski, M.; Zabielski, P.; Blachnio, A.; Gorski, J. Effect of exercise duration on ceramide metabolism in the rat heart. Acta Physiol. (Oxf.) 2008, 192, 519–529. [Google Scholar] [CrossRef]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef]
- Herman, G.E.; Elfont, E.A. The taming of immunohistochemistry: The new era of quality control. Biotech. Histochem. 1991, 66, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Polak-Iwaniuk, A.; Harasim-Symbor, E.; Gołaszewska, K.; Chabowski, A. How Hypertension Affects Heart Metabolism. Front. Physiol. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ussher, J.R.; Oka, T.; Cadete, V.J.; Wagg, C.; Lopaschuk, G.D. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc. Res. 2011, 89, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. (Lausanne) 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.K.; Jammula, S.; Panda, S.; Modi, K.D. Effect of diabetes on alteration of metabolism in cardiac myocytes: Therapeutic implications. Diabetes Technol. Ther. 2011, 13, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, Z.; Zhu, Q.; Thomas, C.; Kumar, R.; Feng, H.; Dostal, D.E.; White, M.F.; Baker, K.M.; Guo, S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes 2013, 62, 3887–3900. [Google Scholar] [CrossRef]
- Wende, A.R.; Abel, E.D. Lipotoxicity in the heart. Biochim. Biophys. Acta 2010, 1801, 311–319. [Google Scholar] [CrossRef]
- Nakata, M.; Yada, T. Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta-cells via CB1 receptors. Regul. Pept. 2008, 145, 49–53. [Google Scholar] [CrossRef]
- Juan-Picó, P.; Fuentes, E.; Bermúdez-Silva, F.J.; Javier Díaz-Molina, F.; Ripoll, C.; Rodríguez de Fonseca, F.; Nadal, A. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium 2006, 39, 155–162. [Google Scholar] [CrossRef]
- Anderson, R.L.; Randall, M.D.; Chan, S.L. The complex effects of cannabinoids on insulin secretion from rat isolated islets of Langerhans. Eur. J. Pharmacol. 2013, 706, 56–62. [Google Scholar] [CrossRef]
- Spijkers, L.J.; van den Akker, R.F.; Janssen, B.J.; Debets, J.J.; De Mey, J.G.; Stroes, E.S.; van den Born, B.J.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; et al. Hypertension is associated with marked alterations in sphingolipid biology: A potential role for ceramide. PLoS ONE 2011, 6, e21817. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M. Cardioprotective role of sphingosine-1-phosphate. J. Physiol. Pharmacol. 2011, 62, 601–607. [Google Scholar] [PubMed]
- Jin, Z.Q.; Zhou, H.Z.; Zhu, P.; Honbo, N.; Mochly-Rosen, D.; Messing, R.O.; Goetzl, E.J.; Karliner, J.S.; Gray, M.O. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1970–H1977. [Google Scholar] [CrossRef]
- Dantas, A.P.; Igarashi, J.; Michel, T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2045–H2052. [Google Scholar] [CrossRef]
- Igarashi, J.; Bernier, S.G.; Michel, T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001, 276, 12420–12426. [Google Scholar] [CrossRef] [PubMed]
| WKY | WKY + URB597 | SHR | SHR + URB597 | |
|---|---|---|---|---|
| Glucose (mg/dL) | 103 ± 12 | 96 ± 10 | 118 ± 10 a | 119 ± 15 a |
| Insulin (μg/L) | 1.91 ± 0.13 | 1.52 ± 0.22 | 2.21 ± 0.61 | 1.58 ± 0.31 b |
| HOMA-IR | 1.14 ± 0.13 | 0.81 ± 0.12 | 1.44 ± 0.41 | 1.03 ± 0.21 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harasim-Symbor, E.; Polak-Iwaniuk, A.; Konstantynowicz-Nowicka, K.; Bielawiec, P.; Malinowska, B.; Kasacka, I.; Chabowski, A. Experimental Activation of Endocannabinoid System Reveals Antilipotoxic Effects on Cardiac Myocytes. Molecules 2020, 25, 1932. https://doi.org/10.3390/molecules25081932
Harasim-Symbor E, Polak-Iwaniuk A, Konstantynowicz-Nowicka K, Bielawiec P, Malinowska B, Kasacka I, Chabowski A. Experimental Activation of Endocannabinoid System Reveals Antilipotoxic Effects on Cardiac Myocytes. Molecules. 2020; 25(8):1932. https://doi.org/10.3390/molecules25081932
Chicago/Turabian StyleHarasim-Symbor, Ewa, Agnieszka Polak-Iwaniuk, Karolina Konstantynowicz-Nowicka, Patrycja Bielawiec, Barbara Malinowska, Irena Kasacka, and Adrian Chabowski. 2020. "Experimental Activation of Endocannabinoid System Reveals Antilipotoxic Effects on Cardiac Myocytes" Molecules 25, no. 8: 1932. https://doi.org/10.3390/molecules25081932
APA StyleHarasim-Symbor, E., Polak-Iwaniuk, A., Konstantynowicz-Nowicka, K., Bielawiec, P., Malinowska, B., Kasacka, I., & Chabowski, A. (2020). Experimental Activation of Endocannabinoid System Reveals Antilipotoxic Effects on Cardiac Myocytes. Molecules, 25(8), 1932. https://doi.org/10.3390/molecules25081932





