Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View
Abstract
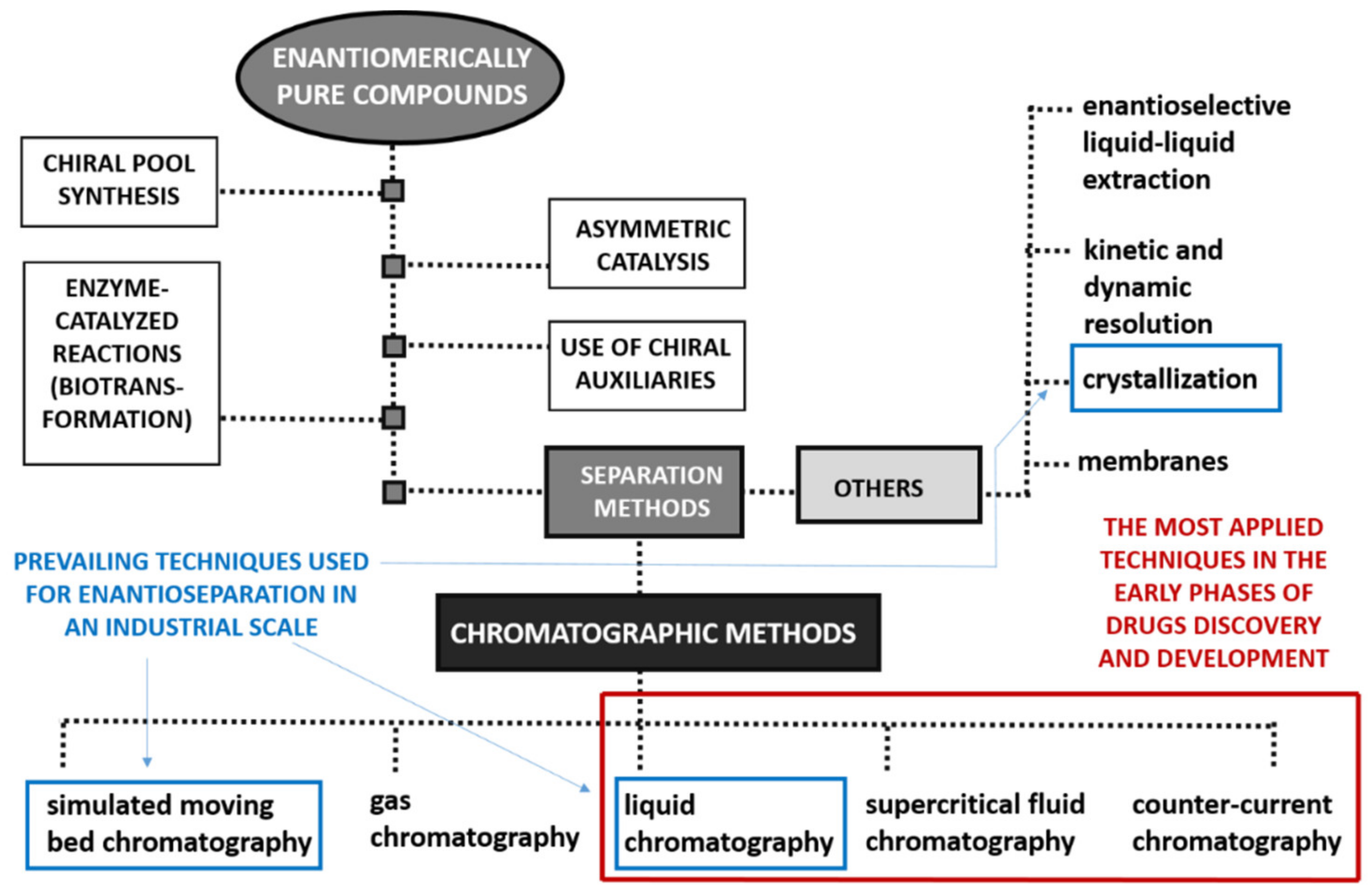
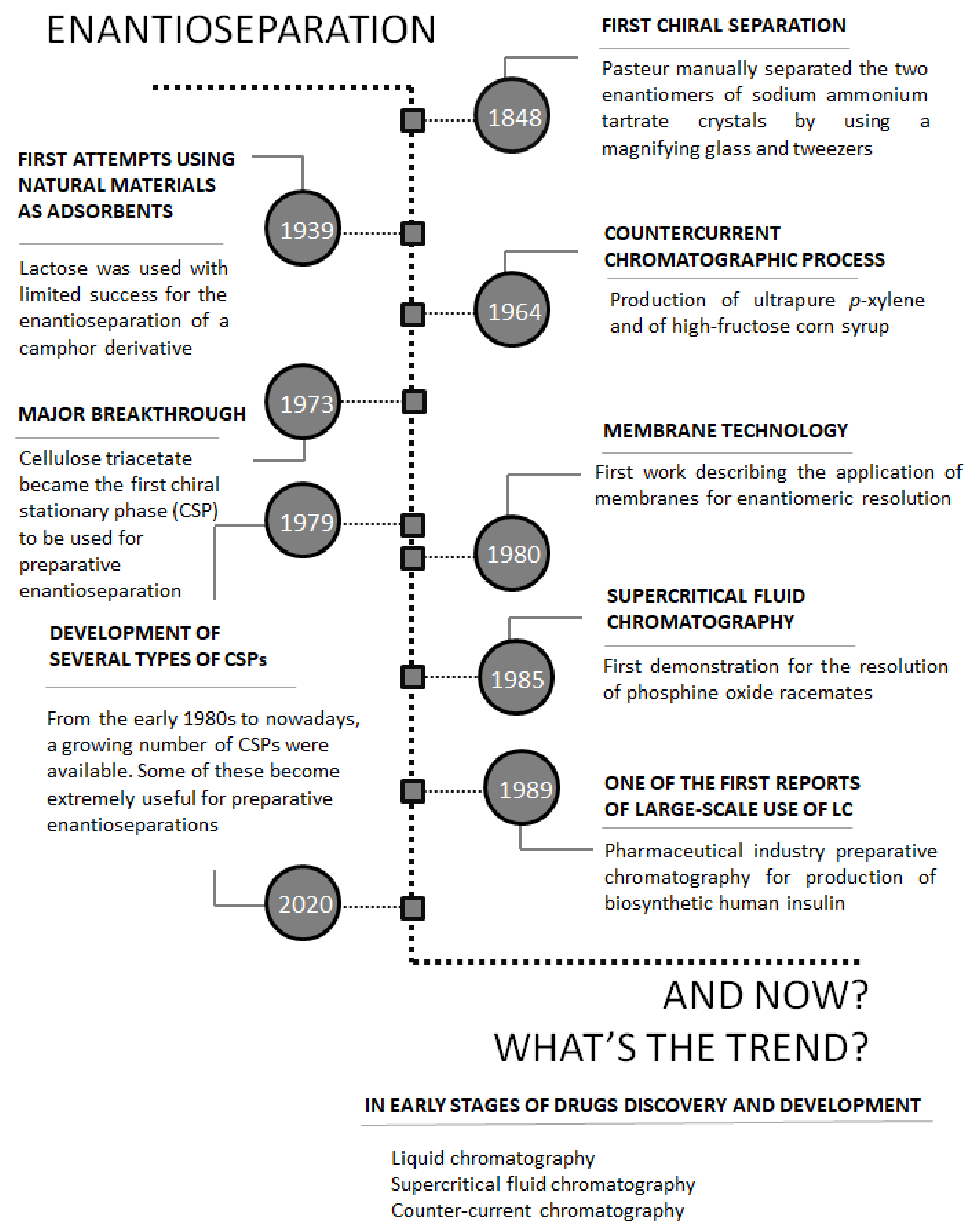
1. Introduction
2. Trends in Chiral Separations on Preparative Scale in Early Phases of Drug Discovery and Development
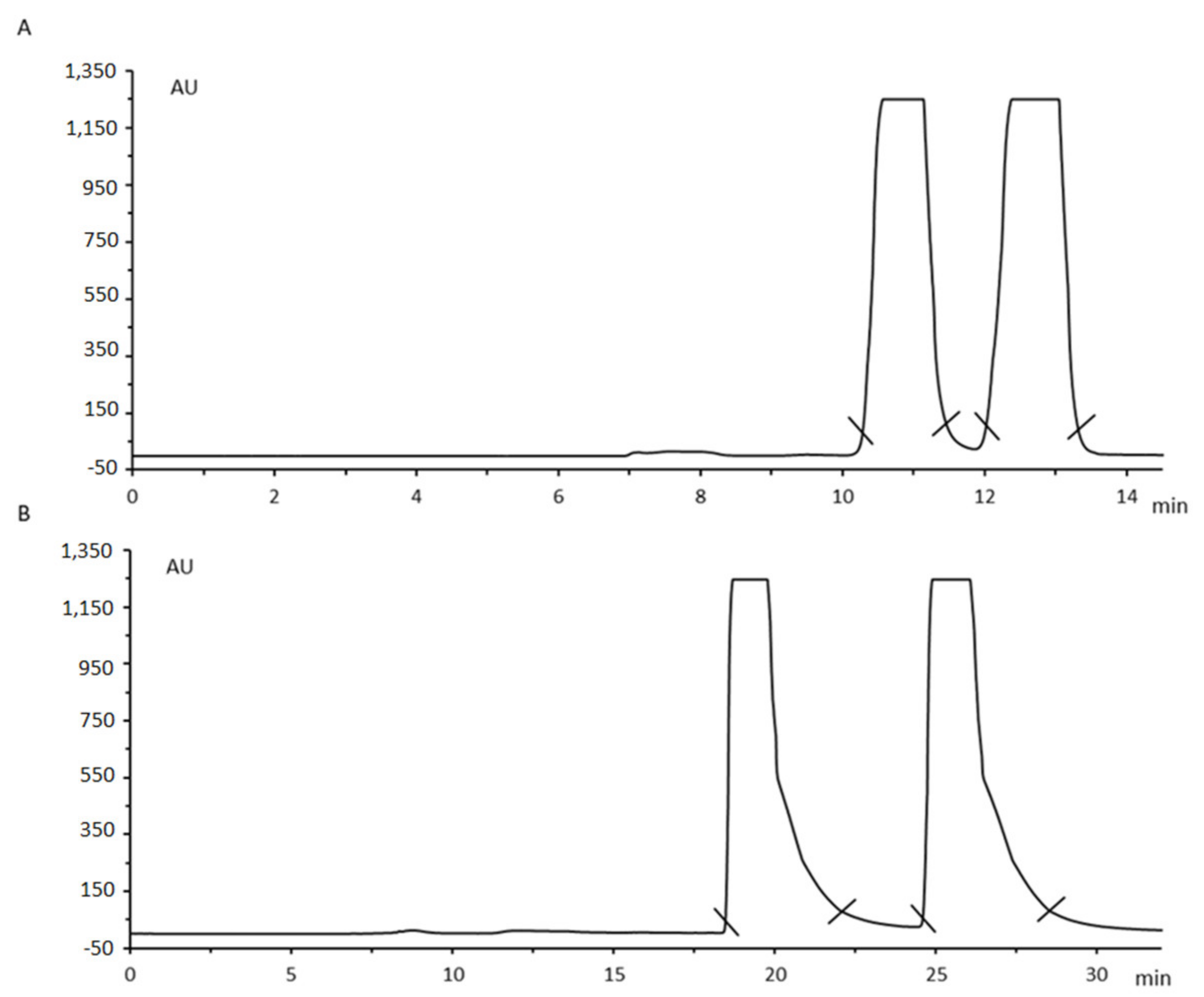
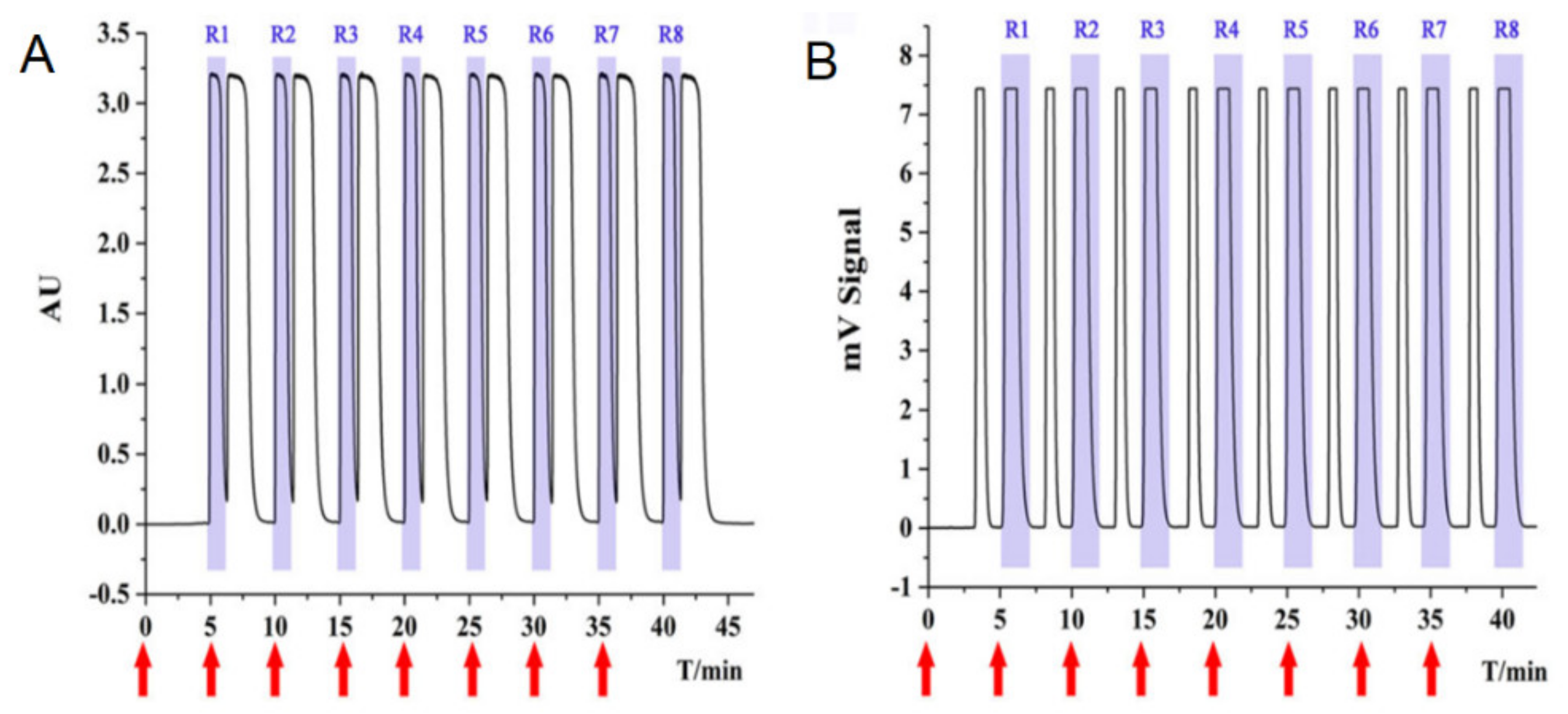
2.1. Liquid Chromatography
2.2. Supercritical Fluid Chromatography
2.3. Counter-Current Chromatography
2.4. Other Enantioseparation Methods
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wermuth, C.G.; Ganellin, C.R.; Lindberg, P.; Mitscher, L.A. Glossary of terms used in medicinal chemistry (iupac recommendations 1998). Pure Appl. Chem. 1998, 70, 1129–1143. [Google Scholar] [CrossRef]
- Lin, G.Q.; You, Q.D.; Cheng, J.F. Chiral Drugs: Chemistry and Biological Action; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Grecsó, N.; Kohout, M.; Carotti, A.; Sardella, R.; Natalini, B.; Fülöp, F.; Lindner, W.; Péter, A.; Ilisz, I. Mechanistic considerations of enantiorecognition on novel cinchona alkaloid-based zwitterionic chiral stationary phases from the aspect of the separation of trans-paroxetine enantiomers as model compounds. J. Pharm. Biomed. Anal. 2016, 124, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M. Chiral pharmaceuticals. In Kirk-Othmer Encyclopedia of Chemicl Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Brocks, D.R. Drug disposition in three dimensions: An update on stereoselectivity in pharmacokinetics. Biopharm. Drug Dispos. 2006, 27, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Brown, T. The 10 Most-Prescribed and Top-Selling Medications, Medscape Medical News. Available online: Http://www.Webmd.Com/news/20150508/most-prescribedtop-selling-drugs (accessed on 20 April 2020).
- Urquhart, L. Biobusiness briefs, top drugs and companies by sales in 2018. Nat. Rev. Drug Discov. 2019, 18, 245. [Google Scholar] [CrossRef]
- Biospace. Available online: https://www.Biospace.Com/article/top-10-drug-sales-projected-through-2024/ (accessed on 22 March 2020).
- Agranat, I.; Wainschtein, S.R. The strategy of enantiomer patents of drugs. Drug Discov. Today 2010, 15, 163–170. [Google Scholar] [CrossRef]
- Anonymous. Fda’s policy statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340. [Google Scholar] [CrossRef]
- Bhadra, S.; Yamamoto, H. Substrate directed asymmetric reactions. Chem. Rev. 2018, 118, 3391–3446. [Google Scholar] [CrossRef]
- Xue, Y.P.; Cao, C.H.; Zheng, Y.G. Enzymatic asymmetric synthesis of chiral amino acids. Chem. Soc. Rev. 2018, 47, 1516–1561. [Google Scholar] [CrossRef]
- Gumustas, M.; Ozkan, S.A.; Chankvetadze, B. Analytical and preparative scale separation of enantiomers of chiral drugs by chromatography and related methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef]
- Lorenz, H.; Seidel-Morgenstern, A. Processes to separate enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef] [PubMed]
- Francotte, E.R. Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Francotte, E. Isolation and production of optically pure drugs by enantioselective chromatography. In Chirality in Drug Research; Wiley-VCH Verlag: Weinheim, Germany, 2006; Volume 33, pp. 155–187. [Google Scholar]
- Leek, H.; Andersson, S. Preparative scale resolution of enantiomers enables accelerated drug discovery and development. Molecules 2017, 22, 158. [Google Scholar] [CrossRef] [PubMed]
- Francotte, E.; Junker-Buchheit, A. Preparative chromatographic separation of enantiomers. J. Chromatogr. B Biomed. Sci. Appl. 1992, 576, 1–45. [Google Scholar] [CrossRef]
- Cox, G.B. Preparative enantioselective Chromatography; John Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 1–330. [Google Scholar]
- Schmidt-Traub, H.; Schulte, M.; Seidel-Morgenstern, A. Preparative Chromatography, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Wu, D.; Pan, F.; Tan, W.; Gao, L.; Tao, Y.; Kong, Y. Recent progress of enantioseparation under scale production (2014–2019). J. Sep. Sci. 2020, 43, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.; Okamoto, Y. Enantioseparation using helical polyacetylene derivatives. TrAC Trends Anal. Chem. 2020, 123. [Google Scholar] [CrossRef]
- Deng, X.; Li, W.; Wang, Y.; Ding, G. Recognition and separation of enantiomers based on functionalized magnetic nanomaterials. TrAC Trends Anal. Chem. 2020, 124. [Google Scholar] [CrossRef]
- Majors, R.E. Developments in preparative-scale chromatography: Columns and accessories. LC-GC Eur. 2004, 17, 630–638. [Google Scholar]
- Speybrouck, D.; Lipka, E. Preparative supercritical fluid chromatography: A powerful tool for chiral separations. J. Chromatogr. A 2016, 1467, 33–55. [Google Scholar] [CrossRef]
- Sardella, R.; Ianni, F.; Marinozzi, M.; Macchiarulo, A.; Natalini, B. Laboratory-scale preparative enantioseparations of pharmaceutically relevant compounds on commercially available chiral stationary phases for hplc. Curr. Med. Chem. 2017, 24, 796–817. [Google Scholar] [CrossRef]
- Fanali, C. Enantiomers separation by capillary electrochromatography. TrAC Trends Anal. Chem. 2019, 120. [Google Scholar] [CrossRef]
- Kellogg, R.M.; Leeman, M. 9.16 crystallization as a tool in industrial applications of asymmetric synthesis. In Comprehensive Chirality; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 367–399. [Google Scholar]
- Saigo, K.; Sakai, K. Resolution of chiral drugs and drug intermediates by crystallisation. In Chirality in Drug Research; Wiley-VCH Verlag: Weinheim, Germany, 2006; Volume 33, pp. 125–154. [Google Scholar]
- Kim, K.M.; Lee, J.W.; Kim, S.; Santos da Silva, F.V.; Seidel-Morgenstern, A.; Lee, C.H. Advanced operating strategies to extend the applications of simulated moving bed chromatography. Chem. Eng. Technol. 2017, 40, 2163–2178. [Google Scholar] [CrossRef]
- Leek, H.; Thunberg, L.; Jonson, A.C.; Öhlén, K.; Klarqvist, M. Strategy for large-scale isolation of enantiomers in drug discovery. Drug Discov. Today 2017, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Jalapa, N.A.; Roque Ramires, M.A.; Toscano, R.A.; Djukic, J.P.; Le Lagadec, R. Preparative resolution of stable enantio-enriched pocop-based planar chiral pincer complexes. J. Organomet. Chem. 2017, 845, 125–134. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Xu, J.; Fan, J.; Yu, Y.; Zhang, W. Enantioseparation of metalaxyl racemate by simulated moving bed chromatography. Chin. J. Chromatogr. 2016, 34, 68–73. [Google Scholar] [CrossRef]
- Bléhaut, J.; Franco, P.; Zhang, T.; Lang, E.; Valéry, E.; Marcoux, J.F. 9.17 industrial applications of chiral chromatography. In Comprehensive Chirality; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 400–456. [Google Scholar]
- Francotte, E.R. Polysaccharide derivatives as unique chiral selectors for enantioselective chromatography. Chimia 2017, 71, 430–450. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral separations: A review of current topics and trends. Anal. Chem. 2012, 84, 626–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective chromatography in drug discovery. Drug Discov. Today 2005, 10, 571–577. [Google Scholar] [CrossRef]
- Samuelsson, J.; Leśko, M.; Enmark, M.; Högblom, J.; Karlsson, A.; Kaczmarski, K. Optimizing column length and particle size in preparative batch chromatography using enantiomeric separations of omeprazole and etiracetam as models: Feasibility of taguchi empirical optimization. Chromatographia 2018, 81, 851–860. [Google Scholar] [CrossRef]
- Tiritan, M.E.; Cass, Q.B.; Del Alamo, A.; Matlin, S.A.; Grieb, S.J. Preparative enantioseparation on polysaccharide phase using microporous silica as a support. Chirality 1998, 10, 573–577. [Google Scholar] [CrossRef]
- Sousa, M.E.; Tiritan, M.E.; Belaz, K.R.A.; Pedro, M.; Nascimento, M.S.J.; Cass, Q.B.; Pinto, M.M.M. Multimilligram enantioresolution of low-solubility xanthonolignoids on polysaccharide chiral stationary phases using a solid-phase injection system. J. Chromatogr. A 2006, 1120, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.C.; Bósio, G.C.; Cassiano, N.M.; Cass, Q.B.; Moreau, R.L.M. Chiral separation of 3,4-methylenedioxymethamphetamine (mdma) enantiomers using batch chromatography with peak shaving recycling and its effects on oxidative stress status in rat liver. J. Pharm. Biomed. Anal. 2013, 73, 13–17. [Google Scholar] [CrossRef]
- Perna, R.F.; Cremasco, M.A.; Santana, C.C. Chromatographic separation of verapamil racemate using a varicol continuous multicolumn process. Brazil J. Chem. Eng. 2015, 32, 929–939. [Google Scholar] [CrossRef]
- Lourenço, T.C.; Batista, J.M., Jr.; Furlan, M.; He, Y.; Nafie, L.A.; Santana, C.C.; Cass, Q.B. Albendazole sulfoxide enantiomers: Preparative chiral separation and absolute stereochemistry. J. Chromatogr. A 2012, 1230, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral stationary phases based on small molecules: An update of the last 17 years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography: Recent developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Chiral recognition in separation science – an update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Recognition mechanisms of chiral selectors: An overview. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1985, pp. 1–33. [Google Scholar]
- Sardella, R.; Ianni, F.; Macchiarulo, A.; Pucciarini, L.; Carotti, A.; Natalini, B. Elucidation of the chromatographic enantiomer elution order through computational studies. Mini-Rev. Med. Chem. 2018, 18, 88–97. [Google Scholar] [CrossRef]
- Francotte, E. Chiral stationary phases for preparative enantioselective chromatography. In Preparative Enantioselective Chromatography; John Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 48–77. [Google Scholar]
- Okamoto, Y.; Ikai, T. Chiral hplc for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef]
- Ribeiro, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography based on chitin- and chitosan-derived marine polysaccharides. Symmetry 2017, 9, 190. [Google Scholar] [CrossRef]
- Padró, J.M.; Keunchkarian, S. State-of-the-art and recent developments of immobilized polysaccharide-based chiral stationary phases for enantioseparations by high-performance liquid chromatography (2013–2017). Microchem. J. 2018, 140, 142–157. [Google Scholar] [CrossRef]
- Chi, Y.; Wu, Z.; Zhong, Y.; Dong, S. Enantiomeric resolution, stereochemical assignment and toxicity evaluation of tpa enantiomers. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Rullo, M.; Catto, M.; de Candia, M.; Carrieri, A.; Cellamare, S.; Altomare, C.D. Structure–property relationship study of the hplc enantioselective retention of neuroprotective 7-[(1-alkylpiperidin-3-yl)methoxy]coumarin derivatives on an amylose-based chiral stationary phase. J. Sep. Sci. 2018, 41, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.Q.; Fan, J.; Tan, Q.; Guo, D.; Chen, T.; He, R.J.; Li, D.; Zhang, H.; Zhang, W.G. Reliable hplc separation, vibrational circular dichroism spectra, and absolute configurations of isoborneol enantiomers. Chirality 2017, 29, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Onyameh, E.K.; Bricker, B.A.; Ofori, E.; Ablordeppey, S.Y. Enantioseparation of 5-chloro-2-{2-[3,4-dihydroisoquinoline-2(1h)-yl]ethyl}-2-methyl-2,3-dihydro-1h-inden-1-one (sya 40247), a high-affinity 5-ht7 receptor ligand, by hplc–pda using amylose tris-(3, 5- dimethylphenylcarbamate) as a chiral stationary phase. Biomed. Chromatogr. 2019. [Google Scholar] [CrossRef]
- Rosales-Hurtado, M.; Lebeau, A.; Bourouh, C.; Cebrian-Torrejon, G.; Albalat, M.; Jean, M.; Naubron, J.V.; Annicotte, J.S.; Benfodda, Z.; Meffre, P. Improved synthesis, resolution, absolute configuration determination and biological evaluation of hlm006474 enantiomers. Bioorg. Med. Chem. Lett. 2019, 29, 380–382. [Google Scholar] [CrossRef]
- Rui, M.; Marra, A.; Pace, V.; Juza, M.; Rossi, D.; Collina, S. Novel enantiopure sigma receptor modulators: Quick (semi-)preparative chiral resolution via hplc and absolute configuration assignment. Molecules 2016, 21, 1210. [Google Scholar] [CrossRef]
- Silva, B.; Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M.; Valente, M.J.; Carvalho, M.; de Pinho, P.G.; Remião, F. Chiral enantioresolution of cathinone derivatives present in “legal highs”, and enantioselectivity evaluation on cytotoxicity of 3,4-methylenedioxypyrovalerone (mdpv). Forensic Toxicol. 2016, 34, 372–385. [Google Scholar] [CrossRef]
- Silva, B.; Pereira, J.A.; Cravo, S.; Araújo, A.M.; Fernandes, C.; Pinto, M.M.M.; De Pinho, P.G.; Remião, F. Multi-milligram resolution and determination of absolute configuration of pentedrone and methylone enantiomers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1100–1101, 158–164. [Google Scholar] [CrossRef]
- Maier, N.M.; Lindner, W. Stereoselective chromatographic methods for drug analysis. In Chirality in Drug Research; Wiley-VCH Verlag: Weinheim, Germany, 2006; Volume 33, pp. 189–260. [Google Scholar]
- Armenta, S.; De la Guardia, M. Green chromatography for the analysis of foods of animal origin. TrAC Trends Anal. Chem. 2016, 80, 517–530. [Google Scholar] [CrossRef]
- Shaaban, H. New insights into liquid chromatography for more eco-friendly analysis of pharmaceuticals. Anal. Bioanal. Chem. 2016, 408, 6929–6944. [Google Scholar] [CrossRef] [PubMed]
- Speybrouck, D.; Lipka, E. Productivity and solvent waste in supercritical fluid chromatography for preparative chiral separations: A guide for a convenient strategy. J. Chromatogr. A 2020, 1610. [Google Scholar] [CrossRef] [PubMed]
- Mourier, P.A.; Eliot, E.; Caude, M.H.; Rosset, R.H.; Tambute, A.G. Supercritical and subcritical fluid chromatography on a chiral stationary phase for the resolution of phosphine oxide enantiomers. Anal. Chem. 1985, 57, 2819–2823. [Google Scholar] [CrossRef]
- Płotka, J.M.; Biziuk, M.; Morrison, C.; Namieśnik, J. Pharmaceutical and forensic drug applications of chiral supercritical fluid chromatography. TrAC Trends Anal. Chem. 2014, 56, 74–89. [Google Scholar] [CrossRef]
- West, C. Recent trends in chiral supercritical fluid chromatography. TrAC Trends Anal. Chem. 2019, 120. [Google Scholar] [CrossRef]
- Saito, M. History of supercritical fluid chromatography: Instrumental development. J. Biosci. Bioeng. 2013, 115, 590–599. [Google Scholar] [CrossRef]
- Ren-Qi, W.; Teng-Teng, O.; Siu-Choon, N.; Weihua, T. Recent advances in pharmaceutical separations with supercritical fluid chromatography using chiral stationary phases. TrAC Trends Anal. Chem. 2012, 37, 83–100. [Google Scholar] [CrossRef]
- Miller, L. Preparative enantioseparations using supercritical fluid chromatography. J. Chromatogr. A 2012, 1250, 250–255. [Google Scholar] [CrossRef]
- Hoguet, V.; Charton, J.; Hecquet, P.E.; Lakhmi, C.; Lipka, E. Supercritical fluid chromatography versus high performance liquid chromatography for enantiomeric and diastereoisomeric separations on coated polysaccharides-based stationary phases: Application to dihydropyridone derivatives. J. Chromatogr. A 2018, 1549, 39–50. [Google Scholar] [CrossRef]
- Wu, D.R.; Yip, S.H.; Li, P.; Sun, D.; Kempson, J.; Mathur, A. Additive free preparative chiral sfc separations of 2,2-dimethyl-3-aryl-propanoic acids. J. Pharm. Biomed. Anal. 2016, 131, 54–63. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, J.; Fu, Q.; Ke, Y. Efficient preparative separation of 6-(4-aminophenyl)-5-methyl-4, 5-dihydro-3(2h)-pyridazinone enantiomers on polysaccharide-based stationary phases in polar organic solvent chromatography and supercritical fluid chromatography. J. Sep. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kalíková, K.; Šlechtová, T.; Vozka, J.; Tesařová, E. Supercritical fluid chromatography as a tool for enantioselective separation; a review. Anal. Chim. Acta 2014, 821, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Zehani, Y.; Lemaire, L.; Ghinet, A.; Millet, R.; Chavatte, P.; Vaccher, C.; Lipka, E. Exploring chiral separation of 3-carboxamido-5-aryl isoxazole derivatives by supercritical fluid chromatography on amylose and cellulose tris dimethyl- and chloromethyl phenylcarbamate polysaccharide based stationary phases. J. Chromatogr. A 2016, 1467, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.H.; Wu, D.R.; Li, P.; Sun, D.; Watterson, S.H.; Zhao, R.; Tino, J.; Mathur, A. Separation of bruton’s tyrosine kinase inhibitor atropisomers by supercritical fluid chromatography. J. Chromatogr. A 2019, 1586, 106–115. [Google Scholar] [CrossRef]
- Balasubramanyam, P.; Ashraf-Khorassani, M.; Josan, J.S. Separation of stereoisomers of 7-oxa-bicyclo[2.2.1]heptene sulfonate (obhs), a selective estrogen receptor modulator (serm), via chiral stationary phases using sfc/uv and sfc/ms. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1092, 279–285. [Google Scholar] [CrossRef]
- West, C. Enantioselective separations with supercritical fluids - review. Curr. Anal. Chem. 2014, 10, 99–120. [Google Scholar] [CrossRef]
- Rajendran, A. Design of preparative-supercritical fluid chromatography. J. Chromatogr. A 2012, 1250, 227–249. [Google Scholar] [CrossRef]
- Akchich, A.; Charton, J.; Lipka, E. Application of tandem coupling of columns in supercritical fluid chromatography for stereoisomeric separation: Optimization and simulation. J. Chromatogr. A 2019, 1588, 115–126. [Google Scholar] [CrossRef]
- Yan, Y.; Fan, J.; Lai, Y.; He, J.; Guo, D.; Zhang, H.; Zhang, W. Efficient preparative separation of β-cypermethrin stereoisomers by supercritical fluid chromatography with a two-step combined strategy. J. Sep. Sci. 2018, 41, 1442–1449. [Google Scholar] [CrossRef]
- DaSilva, J.O.; Bennett, R.; Mann, B.F. Doing more with less: Evaluation of the use of high linear velocities in preparative supercritical fluid chromatography. J. Chromatogr. A 2019, 1595, 199–206. [Google Scholar] [CrossRef]
- Michaels, P.; Neef, J.; Galyan, K.; Ginsburg-Moraff, C.; Zhou, X.; Dunstan, D.; Poirier, J.; Reilly, J. Enabling chiral separations in discovery chemistry with open-access chiral supercritical fluid chromatography. Chirality 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Pei, D.; Liu, J.F.; Di, D.L. A review on chiral separation by counter-current chromatography: Development, applications and future outlook. J. Chromatogr. A 2018, 1531, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Minguillón, C. Countercurrent chromatography, scope and perspectives: Application to chirotechnology. Chem. Eng. Technol. 2012, 35, 35–45. [Google Scholar] [CrossRef]
- Yao, S.; Cao, Y.; Jia, C.M.; Wang, Y.; Song, H. Developments of instruments and methods related with high-speed countercurrent chromatography and their applications in research of natural medicines. Cent. Eur. J. Chem. 2012, 10, 417–432. [Google Scholar] [CrossRef]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [PubMed]
- Faure, K.; Mekaoui, N.; Berthod, A. Saving solvents in chromatographic purifications: The counter-current chromatography technique. In Green Chromatographic Techniques: Separation and Purification of Organic and Inorganic Analytes; Springer: Dodlek, The Netherlands, 2014; pp. 1–18. ISBN 9789400777354. [Google Scholar]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent chromatography in analytical chemistry (iupac technical report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Countercurrent chromatography: People and applications. J. Chromatogr. A 2009, 1216, 4206–4217. [Google Scholar] [CrossRef]
- Hu, R.; Pan, Y. Recent trends in counter-current chromatography. TrAC Trends Anal. Chem. 2012, 40, 15–27. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Elfakir, C. New advances in countercurrent chromatography and centrifugal partition chromatography: Focus on coupling strategy. Anal. Bioanal. Chem. 2014, 406, 957–969. [Google Scholar] [CrossRef]
- Huang, X.Y.; Di, D.L. Chiral separation by counter-current chromatography. TrAC Trends Anal. Chem. 2015, 67, 128–133. [Google Scholar] [CrossRef]
- Huang, X.Y.; Ignatova, S.; Hewitson, P.; Di, D.L. An overview of recent progress in elution mode of counter current chromatography. TrAC Trends Anal. Chem. 2016, 77, 214–225. [Google Scholar] [CrossRef]
- Han, C.; Wang, W.; Xue, G.; Xu, D.; Zhu, T.; Wang, S.; Cai, P.; Luo, J.; Kong, L. Metal ion-improved complexation countercurrent chromatography for enantioseparation of dihydroflavone enantiomers. J. Chromatogr. A 2018, 1532, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Cheng, Q.; Zhang, P.; Tang, K.; Huang, Y.; Xu, W. Preparative enantioseparation of 2-(4-hydroxyphenyl)propionic acid by high speed counter-current chromatography with hydroxyethyl-β-cyclodextrin as chiral selector. Sep. Sci. Technol. 2018, 53, 2981–2989. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, X.; Lv, L.; Sun, W.; Wang, C.; Yan, J.; Tong, S. Preparative enantioseparation of loxoprofen precursor by recycling countercurrent chromatography with hydroxypropyl-β-cyclodextrin as a chiral selector. J. Sep. Sci. 2018, 41, 2828–2836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xie, X.; Tang, K.; Xu, W.; Xiong, B.; Liu, Y. Optimizing two-phase system by experiment and simulation for high-speed counter-current chromatographic separation of 2-phenylbutyric acid enantiomer. Sep. Sci. Technol. 2017, 52, 1275–1282. [Google Scholar] [CrossRef]
- Qiu, X.; Sun, W.; Wang, C.; Yan, J.; Tong, S. Enantioseparation of acetyltropic acid by countercurrent chromatography with sulfobutyl ether-β-cyclodextrin as chiral selector. J. Sep. Sci. 2020, 43, 681–688. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Luo, J.; Lin, Y.; Xu, X.; Han, C.; Kong, L. Preparative enantioseparation of synephrine by conventional and ph-zone-refining counter-current chromatography. J. Chromatogr. A 2018, 1575, 122–127. [Google Scholar] [CrossRef]
- Sciarrone, D.; Pantò, S.; Ragonese, C.; Dugo, P.; Mondello, L. Evolution and status of preparative gas chromatography as a green sample-preparation technique. TrAC Trends Anal. Chem. 2015, 71, 65–73. [Google Scholar] [CrossRef]
- Zuo, H.L.; Yang, F.Q.; Huang, W.H.; Xia, Z.N. Preparative gas chromatography and its applications. J. Chromatogr. Sci. 2013, 51, 704–715. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral separation in preparative scale: A brief overview of membranes as tools for enantiomeric separation. Symmetry 2017, 9, 206. [Google Scholar] [CrossRef]
- Gössi, A.; Riedl, W.; Schuur, B. Enantioseparation with liquid membranes. J. Chem. Technol. Biotechnol. 2018, 93, 629–644. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Simon, G.P.; Wang, H. Polycrystalline advanced microporous framework membranes for efficient separation of small molecules and ions. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, Y.; Gao, J.; Dong, S.; Luo, G.; Li, H.; Zhao, L. Applications of hybrid organic–inorganic materials in chiral separation. TrAC Trends Anal. Chem. 2017, 95, 140–148. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Zhu, H.; Mao, Y.; Guo, Z.; Zhang, W.; Ren, Z. Selective separation of salbutamol enantiomers with simultaneously synergistic extraction and stripping method. J. Membr. Sci. 2016, 499, 343–351. [Google Scholar] [CrossRef]
- Salgın, S.; Salgın, U.; Tuzlalı, N. Enantiomeric separation of antidepressant drug fluoxetine based on chiral membranes. Desalin. Water Treat. 2018, 105, 245–249. [Google Scholar] [CrossRef]
- Chan, J.Y.; Zhang, H.; Nolvachai, Y.; Hu, Y.; Zhu, H.; Forsyth, M.; Gu, Q.; Hoke, D.E.; Zhang, X.; Marriot, P.J.; et al. Incorporation of homochirality into a zeolitic imidazolate framework membrane for efficient chiral separation. Angew. Chem. Int. Ed. 2018, 57, 17130–17134. [Google Scholar] [CrossRef]
- Anand, D.; Dhoke, G.V.; Gehrmann, J.; Garakani, T.M.; Davari, M.D.; Bocola, M.; Zhu, L.; Schwaneberg, U. Chiral separation of d/l-arginine with whole cells through an engineered fhua nanochannel. Chem. Commun. 2019, 55, 5431–5434. [Google Scholar] [CrossRef]
- Otmar, M.; Gaálová, J.; Žitka, J.; Brožová, L.; Cuřínová, P.; Kohout, M.; Hovorka, Š.; Bara, J.E.; Van der Bruggen, B.; Jirsák, J.; et al. Preparation of psebs membranes bearing (s)-(−)-methylbenzylamine as chiral selector. Eur. Polym. J. 2020, 122. [Google Scholar] [CrossRef]
- Li, P.; Feng, J.; Pan, K.; Deng, J. Preparation and chirality investigation of electrospun nanofibers from optically active helical substituted polyacetylenes. Macromolecules 2020, 53, 602–608. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Ingole, P.G.; Hazarika, S. Selective permeation of l-tyrosine through functionalized single-walled carbon nanotube thin film nanocomposite membrane. Sep. Purif. Technol. 2020, 233. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, M.M.M.; Fernandes, C.; Tiritan, M.E. Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules 2020, 25, 1931. https://doi.org/10.3390/molecules25081931
Pinto MMM, Fernandes C, Tiritan ME. Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules. 2020; 25(8):1931. https://doi.org/10.3390/molecules25081931
Chicago/Turabian StylePinto, Madalena M.M., Carla Fernandes, and Maria E. Tiritan. 2020. "Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View" Molecules 25, no. 8: 1931. https://doi.org/10.3390/molecules25081931
APA StylePinto, M. M. M., Fernandes, C., & Tiritan, M. E. (2020). Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules, 25(8), 1931. https://doi.org/10.3390/molecules25081931






