Computational Prediction of Chiral Iron Complexes for Asymmetric Transfer Hydrogenation of Pyruvic Acid to Lactic Acid
Abstract
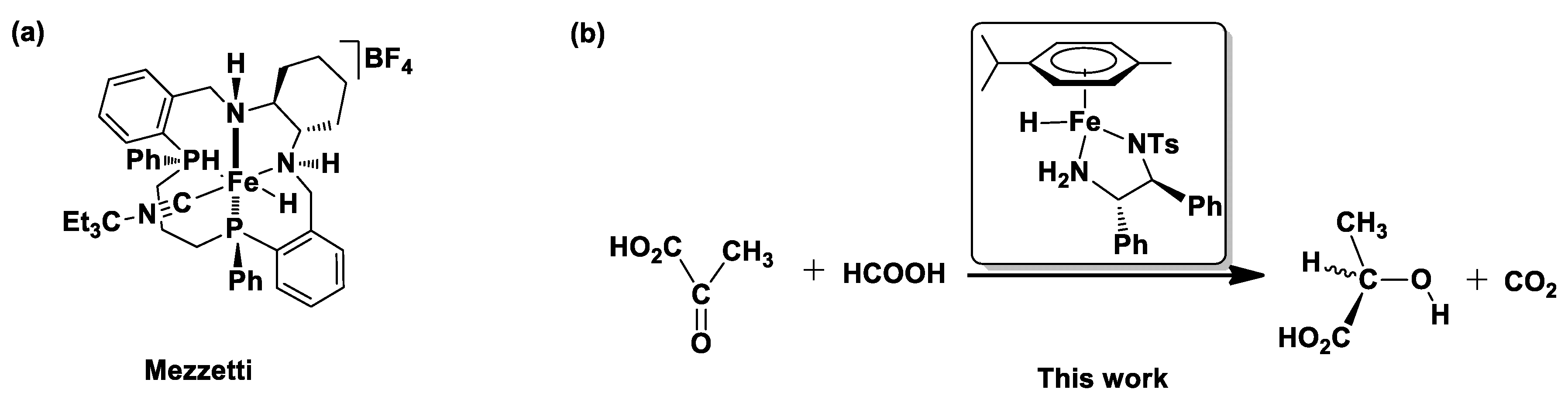
1. Introduction
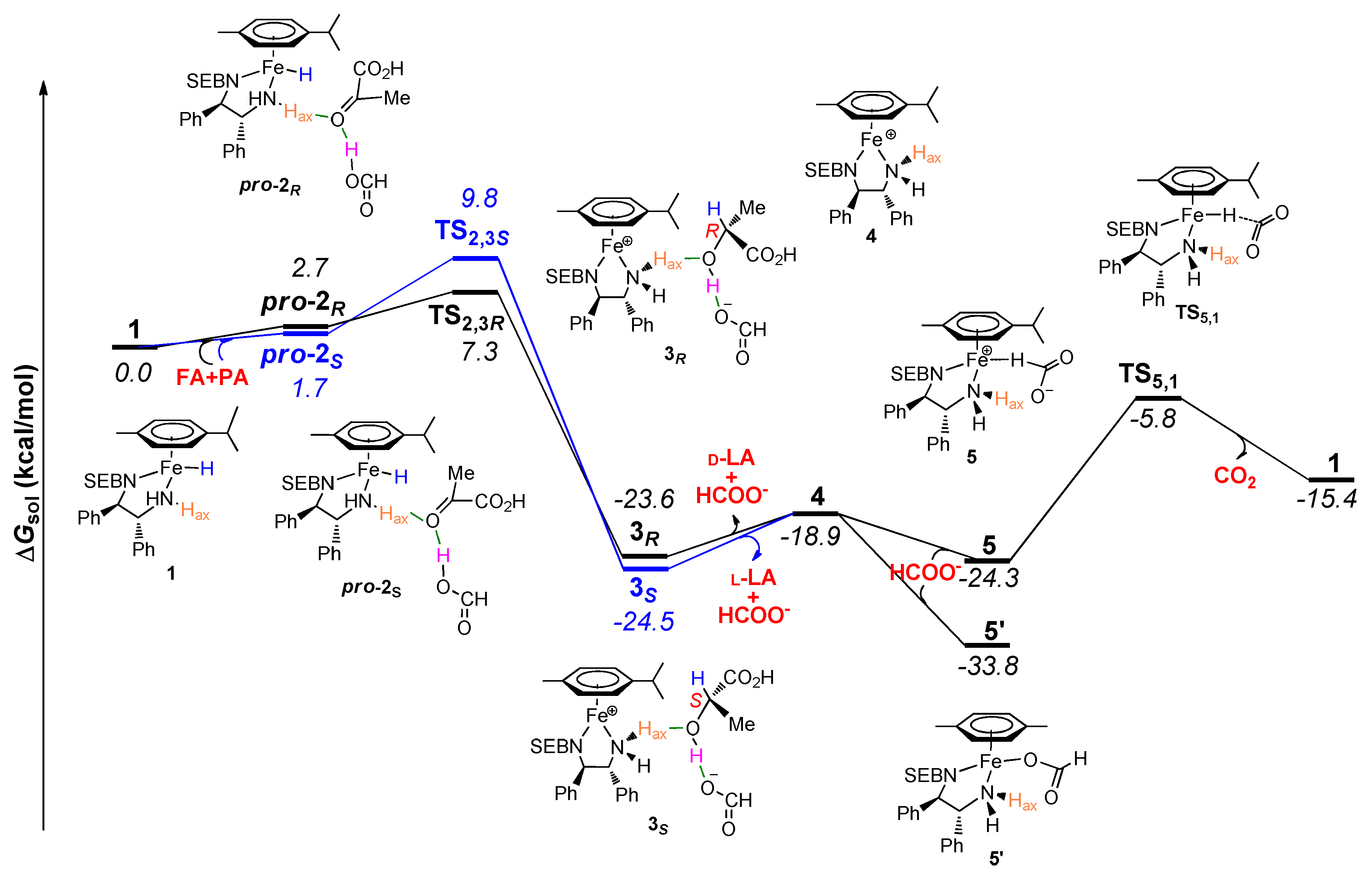
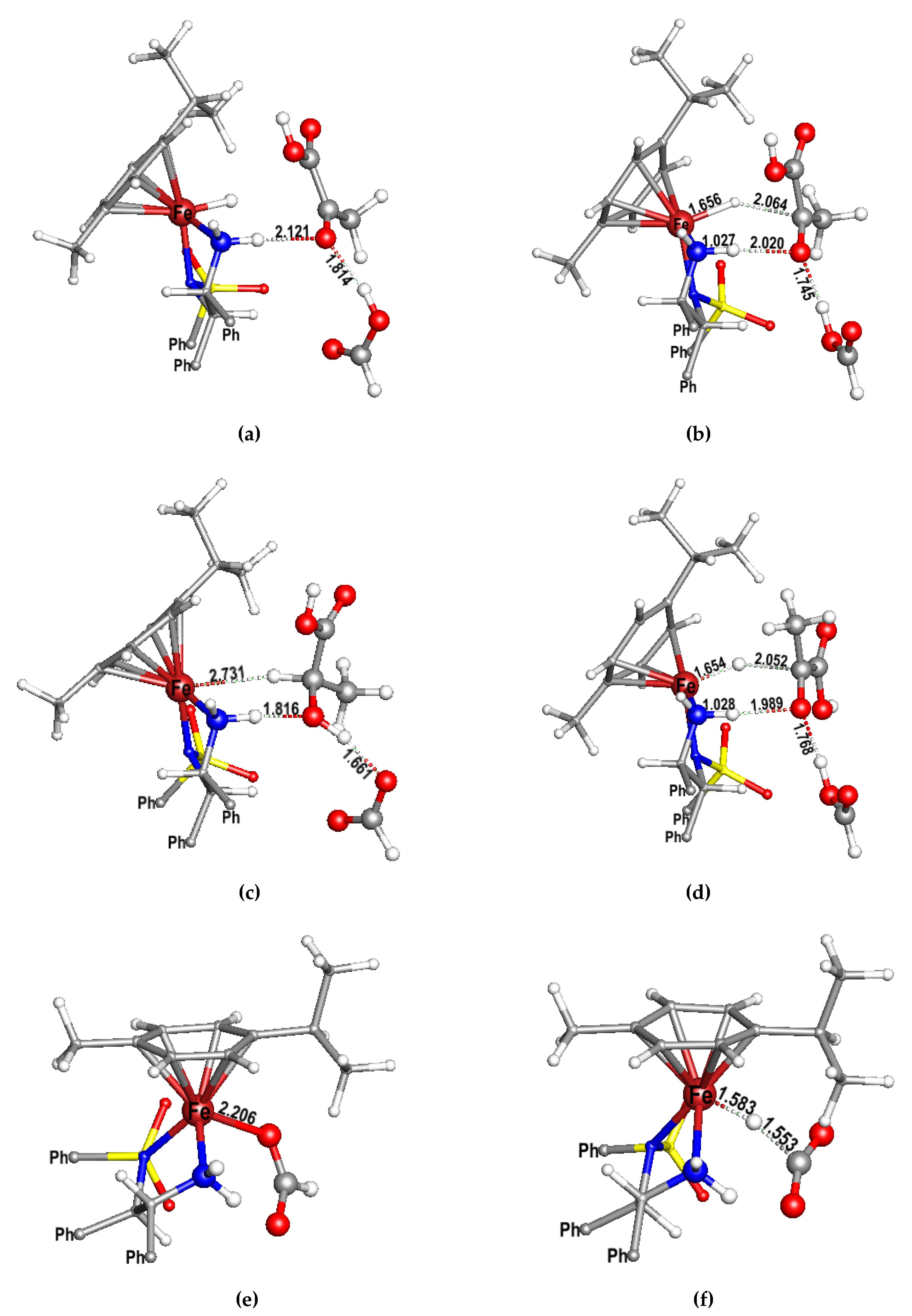
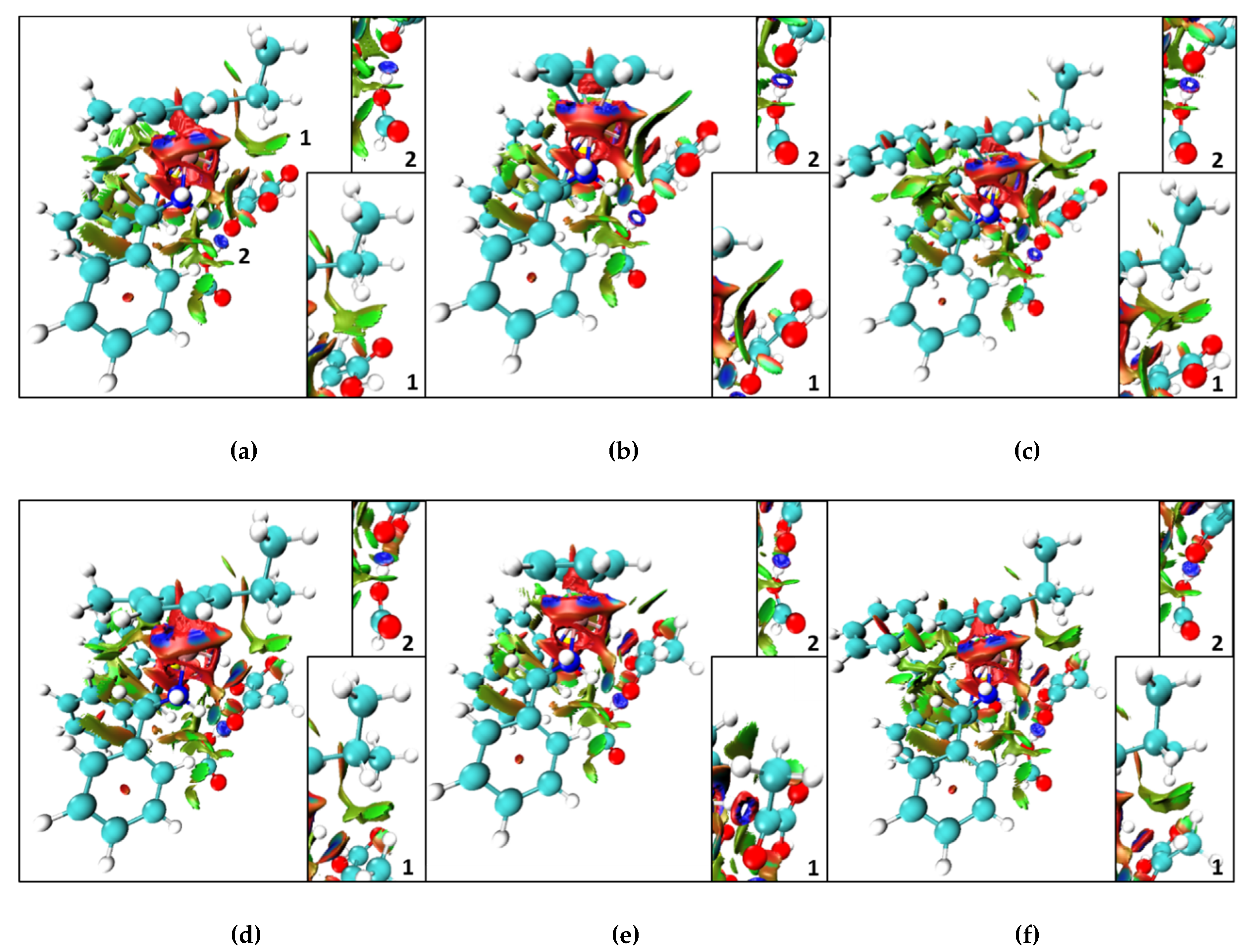
2. Results and Discussion
3. Computational Methods
4. Evaluation of Ground State Spin Multiplicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Virtanen, P.; Salminen, E.; Mäki-Arvela, P.; Mikkola, J.-P. Selective Hydrogenation for Fine Chemical Synthesis. Supported Ionic Liquids: Fundam. Appl. 2014, 251–262. [Google Scholar] [CrossRef]
- Coverdale, J.; Romero-Canelon, I.; Sanchez-Cano, C.; Clarkson, G.J.; Habtemariam, A.; Wills, M.; Sadler, P.J. Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells. Nat. Chem. 2018, 10, 347–354. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, X.; Luo, C.; Zhang, L.; Lei, M. Mechanisms of Ketone/Imine Hydrogenation Catalyzed by Transition-Metal Complexes. Energy Environ. Mater. 2019, 2, 292–312. [Google Scholar] [CrossRef]
- Zassinovich, G.; Mestroni, G.; Gladiali, S. Asymmetric hydrogen transfer reactions promoted by homogeneous transition metal catalysts. Chem. Rev. 1992, 92, 1051–1069. [Google Scholar] [CrossRef]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef]
- Samec, J.S.M.; Bäckvall, J.-E.; Andersson, P.G.; Brandt, P. Mechanistic aspects of transition metal-catalyzed hydrogen transfer reactions. Chem. Soc. Rev. 2006, 35, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.H. Asymmetric hydrogenation, transfer hydrogenation and hydrosilylation of ketones catalyzed by iron complexes. Chem. Soc. Rev. 2009, 38, 2282–2291. [Google Scholar] [CrossRef]
- Ager, D.J.; De Vries, A.H.M.; De Vries, J.G. Asymmetric homogeneous hydrogenations at scale. Chem. Soc. Rev. 2012, 41, 3340. [Google Scholar] [CrossRef]
- Fujii, A.; Hashiguchi, S.; Uematsu, N.; Ikariya, T.; Noyori, R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid−Triethylamine Mixture. J. Am. Chem. Soc. 1996, 118, 2521–2522. [Google Scholar] [CrossRef]
- Uematsu, N.; Fujii, A.; Hashiguchi, S.; Ikariya, T.; Noyori, R. Asymmetric Transfer Hydrogenation of Imines. J. Am. Chem. Soc. 1996, 118, 4916–4917. [Google Scholar] [CrossRef]
- Gao, J.-X.; Ikariya, T.; Noyori, R. A Ruthenium(II) Complex with aC2-Symmetric Diphosphine/Diamine Tetradentate Ligand for Asymmetric Transfer Hydrogenation of Aromatic Ketones†. Organometallics 1996, 15, 1087–1089. [Google Scholar] [CrossRef]
- Haack, K.-J.; Hashiguchi, S.; Fujii, A.; Ikariya, T.; Noyori, R. The catalyst precursor, catalyst, and intermediate in the ruii-promoted asymmetric hydrogen transfer between alcohols and ketones. Angew. Chem. Int. Ed. 1997, 36, 285–288. [Google Scholar] [CrossRef]
- Knighton, R.C.; Vyas, V.K.; Mailey, L.H.; Bhanage, B.M.; Wills, M. Asymmetric transfer hydrogenation of acetophenone derivatives using 2-benzyl-tethered ruthenium (II)/TsDPEN complexes bearing η6-(p-OR) (R = H, iPr, Bn, Ph) ligands. J. Organomet. Chem. 2018, 875, 72–79. [Google Scholar] [CrossRef]
- Dub, P.A.; Ikariya, T. Quantum Chemical Calculations with the Inclusion of Nonspecific and Specific Solvation: Asymmetric Transfer Hydrogenation with Bifunctional Ruthenium Catalysts. J. Am. Chem. Soc. 2013, 135, 2604–2619. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, X. Mechanistic insights into asymmetric transfer hydrogenation of pyruvic acid catalysed by chiral osmium complexes with formic acid assisted proton transfer. Chem. Commun. 2019, 55, 9633–9636. [Google Scholar] [CrossRef]
- Cortez, N.A.; Aguirre, G.; Parra-Hake, M.; Somanathan, R.; Cortez-Lemus, N.A. Ruthenium(II) and rhodium(III) catalyzed asymmetric transfer hydrogenation (ATH) of acetophenone in isopropanol and in aqueous sodium formate using new chiral substituted aromatic monosulfonamide ligands derived from (1R,2R)-diaminocyclohexane. Tetrahedron: Asymmetry 2008, 19, 1304–1309. [Google Scholar] [CrossRef]
- Ikariya, T.; Murata, K.; Noyori, R. Bifunctional transition metal-based molecular catalysts for asymmetric syntheses. Org. Biomol. Chem. 2006, 4, 393–406. [Google Scholar] [CrossRef]
- Xie, J.-H.; Liu, X.-Y.; Wang, L.-X.; Zhou, Q.-L. An Additional Coordination Group Leads to Extremely Efficient Chiral Iridium Catalysts for Asymmetric Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2011, 50, 7329–7332. [Google Scholar] [CrossRef]
- Sui-Seng, C.; Freutel, F.; Lough, A.J.; Morris, R.H. Highly efficient catalyst systems using iron complexes with a tetradentate PNNP ligand for the asymmetric hydrogenation of polar bonds. Angew. Chem. Int. Ed. 2008, 47, 940–943. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Yu, S.-L.; Shen, W.-Y.; Gao, J.-X. Iron-, Cobalt-, and Nickel-Catalyzed Asymmetric Transfer Hydrogenation and Asymmetric Hydrogenation of Ketones. Acc. Chem. Res. 2015, 48, 2587–2598. [Google Scholar] [CrossRef]
- Bigler, R.; Huber, R.; Mezzetti, A. Highly enantioselective transfer hydrogenation of ketones with chiral (NH)2P2 macrocyclic iron(II) complexes. Angew. Chem. Int. Ed. 2015, 54, 5171–5174. [Google Scholar] [CrossRef] [PubMed]
- Bigler, R.; Mezzetti, A. Isonitrile Iron(II) Complexes with Chiral N2P2 Macrocycles in the Enantioselective Transfer Hydrogenation of Ketones. Org. Lett. 2014, 16, 6460–6463. [Google Scholar] [CrossRef] [PubMed]
- Corberan, R.; Peris, E.V. An Unusual Example of Base-Free Catalyzed Reduction of C=O and C=NR Bonds by Transfer Hydrogenation and Some Useful Implications. Organometallics 2008, 27, 1954–1958. [Google Scholar] [CrossRef]
- De Luca, L.; Passera, A.; Mezzetti, A. Asymmetric Transfer Hydrogenation with a Bifunctional Iron(II) Hydride: Experiment Meets Computation. J. Am. Chem. Soc. 2019, 141, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.; Sanchez-Cano, C.; Clarkson, G.J.; Soni, R.; Wills, M.; Sadler, P.J. Easy To Synthesize, Robust Organo-osmium Asymmetric Transfer Hydrogenation Catalysts. Chem. A Eur. J. 2015, 21, 8043–8046. [Google Scholar] [CrossRef]
- Maeda, H.; Yamamoto, K.; Kohno, I.; Hafsi, L.; Itoh, N.; Nakagawa, S.; Kanagawa, N.; Suzuki, K.; Uno, T. Design of a Practical Fluorescent Probe for Superoxide Based on Protection–Deprotection Chemistry of Fluoresceins with Benzenesulfonyl Protecting Groups. Chem. A Eur. J. 2007, 13, 1946–1954. [Google Scholar] [CrossRef]
- Macchioni, A. Ion pairing in transition-metal organometallic chemistry. Chem. Rev. 2005, 105, 2039–2074. [Google Scholar] [CrossRef]
- Kozuch, S.; Shaik, S. How to Conceptualize Catalytic Cycles? The Energetic Span Model. Acc. Chem. Res. 2011, 44, 101–110. [Google Scholar] [CrossRef]
- Noyori, R.; Hashiguchi, S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997, 30, 97–102. [Google Scholar] [CrossRef]
- Masashi, Y.; Issaku, Y.; Ryoji, N. CH/π attraction: The origin of enantioselectivity in transfer hydrogenation of aromatic carbonyl compounds catalyzed by chiral η6-arene-ruthenium(II) complexes. Angew. Chem. Int. Ed. 2001, 40, 2818–2821. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-Garcia, J.; Cohen, A.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Acc. 2008, 119, 525. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted abinitio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Manson, J.; Webster, C.E.; Hall, M.B. JIMP2, Version 0.091, A Free Program for Visualizing and Manipulating Molecules; Texas A&M University: College Station, TX, USA, 2006. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Straathof, A.; Jongejan, J. The enantiomeric ratio: origin, determination and prediction. Enzym. Microb. Technol. 1997, 21, 559–571. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
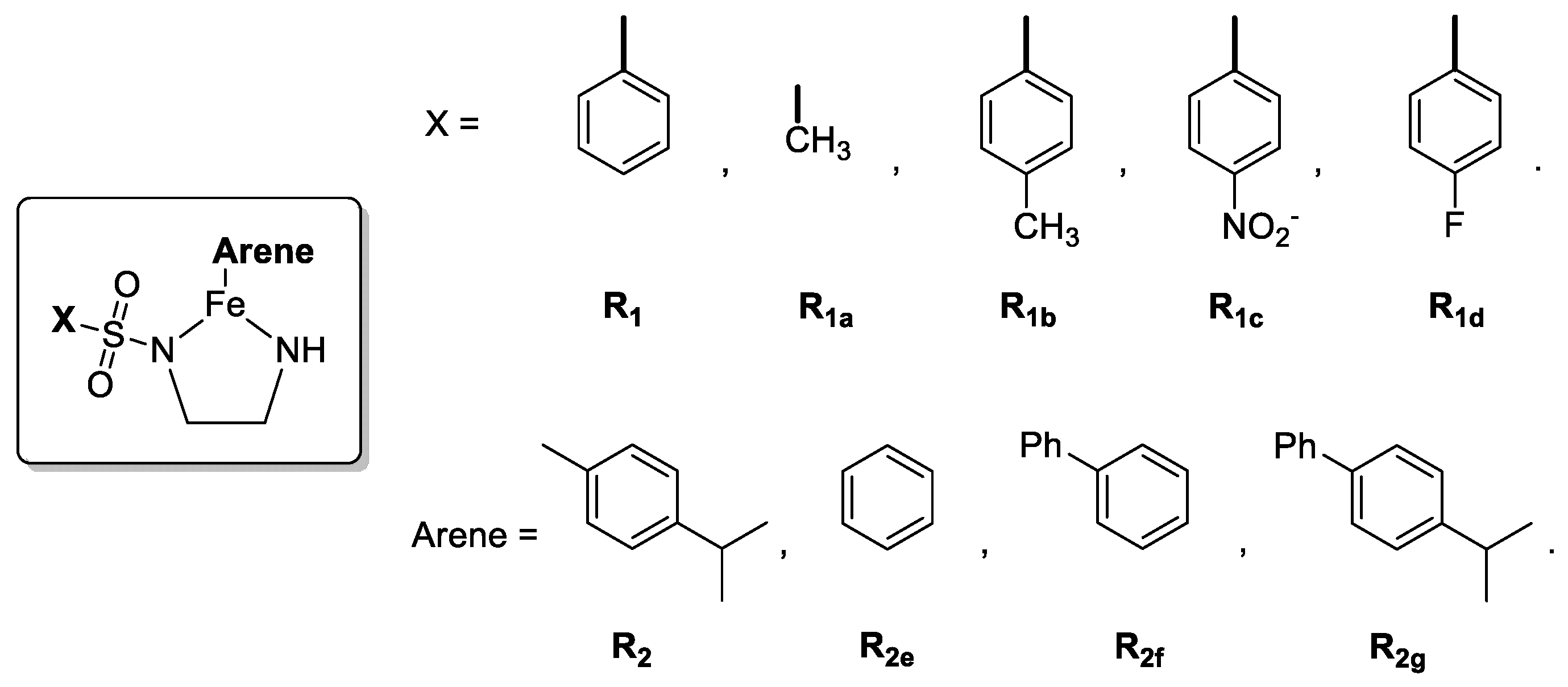
| Complexes | X | Arene | TS2,3-R→TS2,3-S | 5′→TS5,1 |
|---|---|---|---|---|
| ΔG (kcal mol−1) | ||||
| 1 | R1 | R2 | 3.2 | 28.0 |
| 1a | R1a | R2 | 0.6 | 27.5 |
| 1b | R1b | R2 | 3.8 | 27.9 |
| 1c | R1c | R2 | 3.0 | 28.8 |
| 1d | R1d | R2 | 4.1 | 27.5 |
| 1e | R1 | R2e | 4.9 | 27.8 |
| 1f | R1 | R2f | 3.7 | 28.5 |
| 1g | R1 | R2g | 2.9 | 26.2 |
| Complexes | Singlet (a.u.) | Triplet (a.u.) | ΔG (kcal mol−1) |
|---|---|---|---|
| 1 | −1944.102697 | −1944.118152 | −9.7 |
| 2R | −2475.926296 | −2475.940345 | −8.8 |
| 2S | −2475.924832 | −2475.941943 | −10.7 |
| 3R | −2475.943887 | −2475.982223 | −24.1 |
| 3S | −2475.946197 | −2475.983624 | −23.5 |
| 4 | −1943.384036 | −1943.421081 | −23.2 |
| 5 | −2132.591160 | −2132.625404 | −21.5 |
| 5′ | −2132.620516 | −2132.640419 | −12.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yang, X. Computational Prediction of Chiral Iron Complexes for Asymmetric Transfer Hydrogenation of Pyruvic Acid to Lactic Acid. Molecules 2020, 25, 1892. https://doi.org/10.3390/molecules25081892
Wang W, Yang X. Computational Prediction of Chiral Iron Complexes for Asymmetric Transfer Hydrogenation of Pyruvic Acid to Lactic Acid. Molecules. 2020; 25(8):1892. https://doi.org/10.3390/molecules25081892
Chicago/Turabian StyleWang, Wan, and Xinzheng Yang. 2020. "Computational Prediction of Chiral Iron Complexes for Asymmetric Transfer Hydrogenation of Pyruvic Acid to Lactic Acid" Molecules 25, no. 8: 1892. https://doi.org/10.3390/molecules25081892
APA StyleWang, W., & Yang, X. (2020). Computational Prediction of Chiral Iron Complexes for Asymmetric Transfer Hydrogenation of Pyruvic Acid to Lactic Acid. Molecules, 25(8), 1892. https://doi.org/10.3390/molecules25081892






