Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, iPBS Profiles and Antioxidant and Antimicrobial Activities
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Materials
3.2. Microbiological Analysis
3.3. Identification of Microorganisms Using MALDI TOF MS Biotyper
3.4. Preparation of Plant Extracts
3.5. Assessment of Antimicrobial Activity with Disc Diffusion Method Against Selected Bacteria Species
3.6. Detection of Antibacterial Activity with Minimal Inhibition Concentration (MIC)
3.7. Sample Preparation for the Assessment of Bioactive Compounds
3.8. Radical Scavenging Activity—DPPH Method
3.9. Total Polyphenol Contents
3.10. Total Flavonoid Contents
3.11. Total Phenolic Acid Contents
3.12. Total Carotenoid Contents
3.13. Analysis of Genome Size, Ploidy, and iPBS Polymorphism
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lodewyckx, C.; Mergeay, M.; Vangronsveld, J.; Clijsters, H.; Van Der Lelie, D. Isolation, characterization, and identification of bacteria associated with the zinc hyperaccumulator Thlaspi caerulescens subsp. calaminaria. Int. J. Phytoremediation 2002, 4, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mastretta, C.; Barac, T.; Vangronsveld, J.; Newman, L.; Taghavi, S.; Lelie, D. van der Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotechnol. Genet. Eng. Rev. 2006, 23, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, B.; Bazaid, S.; Gherbawy, Y.; Elhariry, H. Characterization of endophytic bacteria associated with rose plant Rosa damascena trigintipeta) during flowering stage and their plant growth promoting traits. J. Plant Interact. 2012, 7, 248–253. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Nair, R.; Kalariya, T.; Sumitra, C. Antibacterial activity of some selected Indian medicinal flora. Turkish J. Biol. 2005, 29, 41–47. [Google Scholar]
- Astal, Z.Y.; Ashour, A.E.R.A.; Kerrit, A.A.M. Antimicrobial activity of some medicinal plant extracts in Palestine. Pakistan J. Med. Sci. 2005, 21, 187–193. [Google Scholar]
- Khan, A.A.; Bacha, N.; Ahmad, B.; Lutfullah, G.; Farooq, U.; Cox, R.J. Fungi as chemical industries and genetic engineering for the production of biologically active secondary metabolites. Asian Pac. J. Trop. Biomed. 2014, 4, 859–870. [Google Scholar] [CrossRef]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Joo, S.-S.; Kim, Y.-B.; Lee, D.-I. Antimicrobial and Antioxidant Properties of Secondary Metabolites from White Rose Flower. Plant Pathol. J. 2010, 26, 57–62. [Google Scholar] [CrossRef]
- Hirulkar, N.B.; Agrawal, M. Antimicrobial Activity of Rose petals Extract Against Some Pathogenic Bacteria. Int. J. Pharm. Biol. Arch. 2010, 1, 478–484. [Google Scholar]
- Tahirović, A.; Bašić, N. Determination of phenolic content and antioxidant activity of Rosa canina L. fruits in different extraction systems. Work. Fac. For. Univ. Sarajev. 2017, 1, 47–59. [Google Scholar]
- Das, M.; Royer, T.V.; Leff, L.G. Diversity of Fungi, Bacteria, and Actinomycetes on Leaves Decomposing in a Stream. Appl. Environ. Microbiol. 2007, 73, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.C.Y.; Fu, X.-Q.; Guo, H.; Li, T.; Wu, Z.-Z.; Chan, K.; Yu, Z.-L. The genus Rosa and arthritis: Overview on pharmacological perspectives. Pharmacol. Res. 2016, 114, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, A.; Werlemark, G.; Leitch, A.R.; Souckova-Skalicka, K.; Lim, Y.K.; Khaitová, L.; Koukalova, B.; Nybom, H. The asymmetric meiosis in pentaploid dogroses (Rosa sect. Caninae) is associated with a skewed distribution of rRNA gene families in the gametes. Heredity 2008, 101, 359–367. [Google Scholar] [CrossRef]
- Ferus, P.; Pachl, Š.; Ďurišová, Ľ.; Bartošová-Krajčovičová, D.; Rovná, K. Is there any relation between quantitative traits interesting for ornamental breeding and genome size in dog-roses (Rosa sect. Caninae)? Folia Oecologica 2013, 40, 11–21. [Google Scholar]
- McInroy, J.A.; Kloepper, J.W. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 1995, 173, 337–342. [Google Scholar] [CrossRef]
- Rovná, K.; Bakay, L.; Petrová, J.; Terentjeva, M.; Černá, J.; Kačániová, M. Characterization of endophytic microflora of Rosa canina fruits. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 65–68. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. CRC. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Mundt, J.O.; Hinkle, N.F. Bacteria within ovules and seeds. Appl. Environ. Microbiol. 1976, 32, 694–698. [Google Scholar] [CrossRef]
- Seghers, D.; Wittebolle, L.; Top, E.M.; Verstraete, W.; Siciliano, S.D. Impact of Agricultural Practices on the Zea mays L. Endophytic Community. Appl. Environ. Microbiol. 2004, 70, 1475–1482. [Google Scholar] [CrossRef]
- van Peer, R.; Punte, H.L.M.; de Weger, L.A.; Schippers, B. Characterization of Root Surface and Endorhizosphere Pseudomonads in Relation to Their Colonization of Roots. Appl. Environ. Microbiol. 1990, 56, 2462–2470. [Google Scholar] [CrossRef]
- Hashidoko, Y.; Itoh, E.; Yokota, K.; Yoshida, T.; Tahara, S. Characterization of Five Phyllosphere Bacteria Isolated from Rosa rugosa Leaves, and Their Phenotypic and Metabolic Properties. Biosci. Biotechnol. Biochem. 2002, 66, 2474–2478. [Google Scholar] [CrossRef]
- Isaeva, O.V.; Glushakova, A.M.; Yurkov, A.M.; Chernov, I.Y. The yeast Candida railenensis in the fruits of English oak (Quercus robur L.). Microbiology 2009, 78, 355–359. [Google Scholar] [CrossRef]
- Ozturk Yilmaz, S.; Ercisli, S. Antibacterial and antioxidant activity of fruits of some rose species from Turkey. Rom. Biotechnol. Lett. 2011, 16, 6407–6411. [Google Scholar]
- Živković, J.; Stojković, D.; Petrović, J.; Zdunić, G.; Glamočlija, J.; Soković, M. Rosa canina L.—New possibilities for an old medicinal herb. Food Funct. 2015, 6, 3687–3692. [Google Scholar] [CrossRef]
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Oyedemi, B.O.; Prieto, J.M.; Coopoosamy, R.M.; Stapleton, P.; Gibbons, S. In vitro assessment of antibiotic-resistance reversal of a methanol extract from Rosa canina L. S. Afr. J. Bot. 2016, 105, 337–342. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, Traditional Uses and Pharmacological Profile of Rose Hip: A Review. Curr. Pharm. Des. 2019, 24, 4101–4124. [Google Scholar] [CrossRef]
- Gruenwald, J.; Uebelhack, R.; Moré, M.I. Rosa canina – Rose hip pharmacological ingredients and molecular mechanics counteracting osteoarthritis – A systematic review. Phytomedicine 2019, 60, 152958. [Google Scholar] [CrossRef]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterisation of rose hip (Rosa canina L.) fruits extracts and evaluation of their in vitro antioxidant activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Fattahi, S.; Jamei, R.; Sarghein, S.H. Antioxidant and antiradical activities of Rosa canina and Rosa pimpinellifolia fruits from west Azerbaiyan. Iran. J. Plant Phys. 2012, 2, 523–529. [Google Scholar]
- Tekeli, A. Effect of Rosehip fruit (Rosa canina l.) supplementation to rations of broilers grown under cold stress conditions on some performance, blood, morphological, carcass and meat quality characteristics. Eur. Poult. Sci. 2014, 78, 1–14. [Google Scholar]
- Soare, R.; Babeanu, C.; Bonea, D.; Panipa, O. The content of total phenols, flavonoids and antioxidant activity in rosehip from the spontaneous flora from south Romania. Sci. Pap. Ser. A. Agron. 2015, LVIII, 307–314. [Google Scholar]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Influence of agro-ecological production areas on antioxidant activity, reducing sugar content, and selected phytonutrients of orange-fleshed sweet potato cultivars. Food Sci. Technol. 2015, 35, 32–37. [Google Scholar] [CrossRef]
- Oh, M.-M.; Trick, H.N.; Rajashekar, C.B. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J. Factors affecting the variation of bioactive compounds in Hypericum species. Biol. Futur. 2019, 70, 198–209. [Google Scholar] [CrossRef]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L.biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef]
- Adamczak, A.; Buchwald, W.; Zieliński, J.; Mielcarek, S. Flavonoid and Organic Acid Content in Rose Hips (Rosa L., Sect. Caninae Dc. Em. Christ.). Acta Biol. Cracoviensia Ser. Bot. 2012, 54. [Google Scholar] [CrossRef]
- Bhave, A.; Schulzova, V.; Chmelarova, H.; Mrnka, L.; Hajslova, J. Assessment of rosehips based on the content of their biologically active compounds. J. Food Drug Anal. 2017, 25, 681–690. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Elmastaş, M.; Demir, A.; Genç, N.; Dölek, Ü.; Güneş, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef]
- Ropciuc, S. The content of carotenoids pigments in Rosa canina L. fruit. Food Environ. Saf. J. 2017, 10, 108–111. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef]
- Nybom, H.; Esselink, G.D.; Werlemark, G.; Vosman, B. Microsatellite DNA marker inheritance indicates preferential pairing between two highly homologous genomes in polyploid and hemisexual dog-roses, Rosa L. Sect. Caninae DC. Heredity 2004, 92, 139–150. [Google Scholar] [CrossRef]
- Pecrix, Y.; Rallo, G.; Folzer, H.; Cigna, M.; Gudin, S.; Le Bris, M. Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J. Exp. Bot. 2011, 62, 3587–3597. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Ma, J.X.; Devos, K.M. Mechanisms of Recent Genome Size Variation in Flowering Plants. Ann. Bot. 2005, 95, 127–132. [Google Scholar] [CrossRef]
- Wissemann, V. Conventional Taxonomy (Wild Roses). In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Lim, K.Y.; Werlemark, G.; Matyasek, R.; Bringloe, J.B.; Sieber, V.; El Mokadem, H.; Meynet, J.; Hemming, J.; Leitch, A.R.; Roberts, A. V Evolutionary implications of permanent odd polyploidy in the stable sexual, pentaploid of Rosa canina L. Heredity 2005, 94, 501–506. [Google Scholar] [CrossRef]
- Marhold, K.; Mártongi, P.; Mereďa, P.; Mráz, P. Chromosome Number Survey of the Ferns and Flowering Plants of Slovakia; Veda: Bratislava, Slovakia, 2007. [Google Scholar]
- Roberts, A.V.; Gladis, T.; Brumme, H. DNA amounts of roses (Rosa L.) and their use in attributing ploidy levels. Plant Cell Rep. 2009, 28, 61–71. [Google Scholar] [CrossRef]
- Roberts, A.V. The use of bead beating to prepare suspensions of nuclei for flow cytometry from fresh leaves, herbarium leaves, petals and pollen. Cytom. Part A 2007, 71A, 1039–1044. [Google Scholar] [CrossRef]
- Deák, T. Molekuláris markerek alkalmazása a szolo magvatlanságának követésére és Rosa L. taxonok rokonsági viszonyainak vizsgálatára. Ph.D. Thesis, Corvinus University of Budapest, Budapest, Hungary, 2010. [Google Scholar]
- Herklotz, V.; Ritz, C.M. Multiple and asymmetrical origin of polyploid dog rose hybrids ( Rosa L. sect. Caninae (DC.) Ser.) involving unreduced gametes. Ann. Bot. 2016, mcw217. [Google Scholar] [CrossRef]
- Nybom, H.; Werlemark, G.; Esselink, G.D.; Vosman, B. Mac-pr (microsatellite dna allele counting using peak ratios) reveals unique genomic configuration in polyploid dogroses. Acta Hortic. 2004, 563–568. [Google Scholar] [CrossRef]
- Kellner, A.; Ritz, C.M.; Wissemann, V. Hybridization with invasive Rosa rugosa threatens the genetic integrity of native Rosa mollis. Bot. J. Linn. Soc. 2012, 170, 472–484. [Google Scholar] [CrossRef][Green Version]
- Větvička, V.; Bertová, L. Rosa L. Flora of Slovakia IV/3; Veda: Bratislava, Slovakia, 1992; ISBN 80-224-0077-7. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 296, 695–698. [Google Scholar] [CrossRef]
- Farmakopea Polska XI; Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych: Warsaw, Poland, 2018; ISBN 978-83-7887-87-6.
- STN 56 0053. Determination of Vitamin A and Its Provitamins; ÚNN: Prague, Czech Republic, 1986. [Google Scholar]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Rosa canina are available from the authors. |
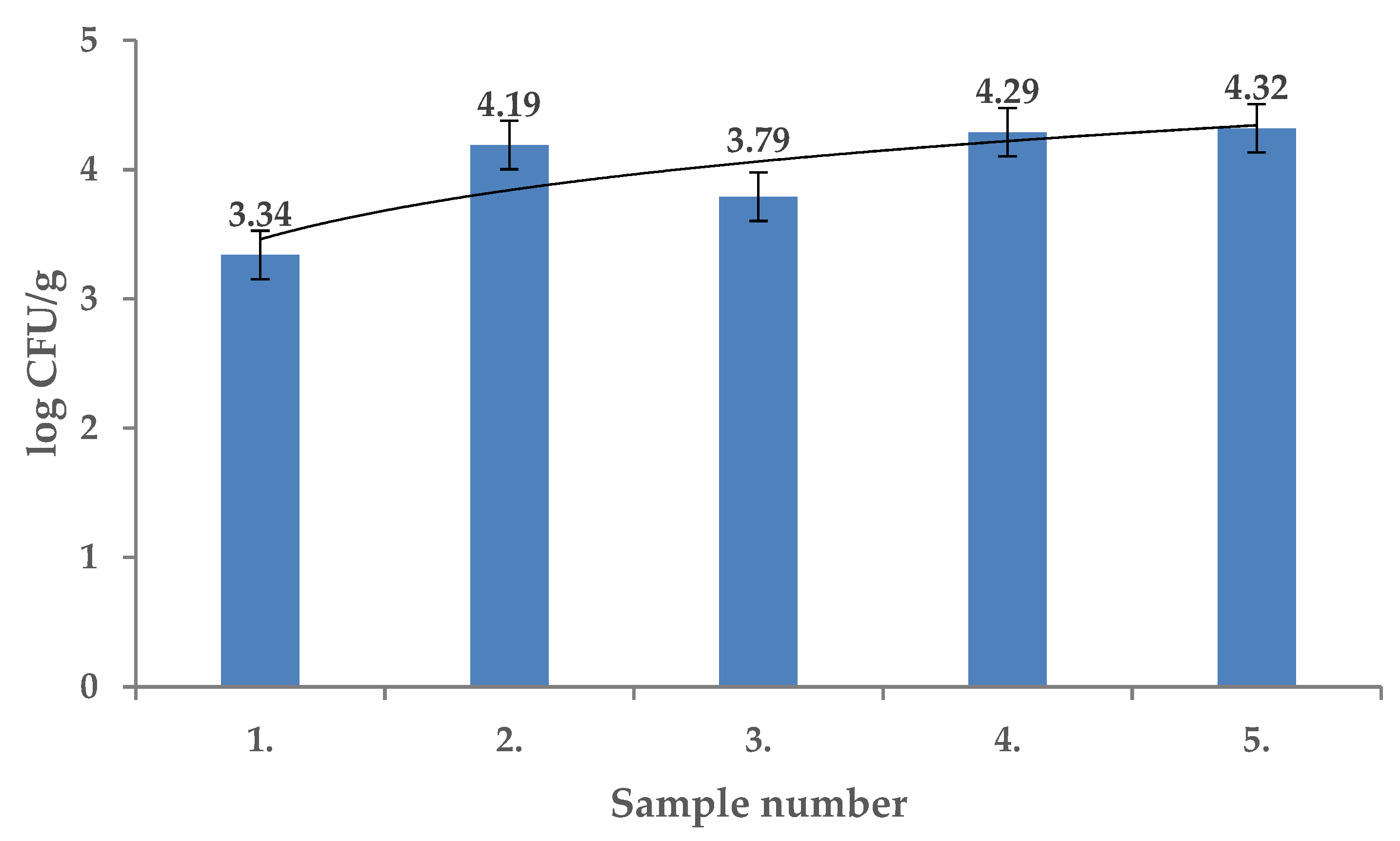
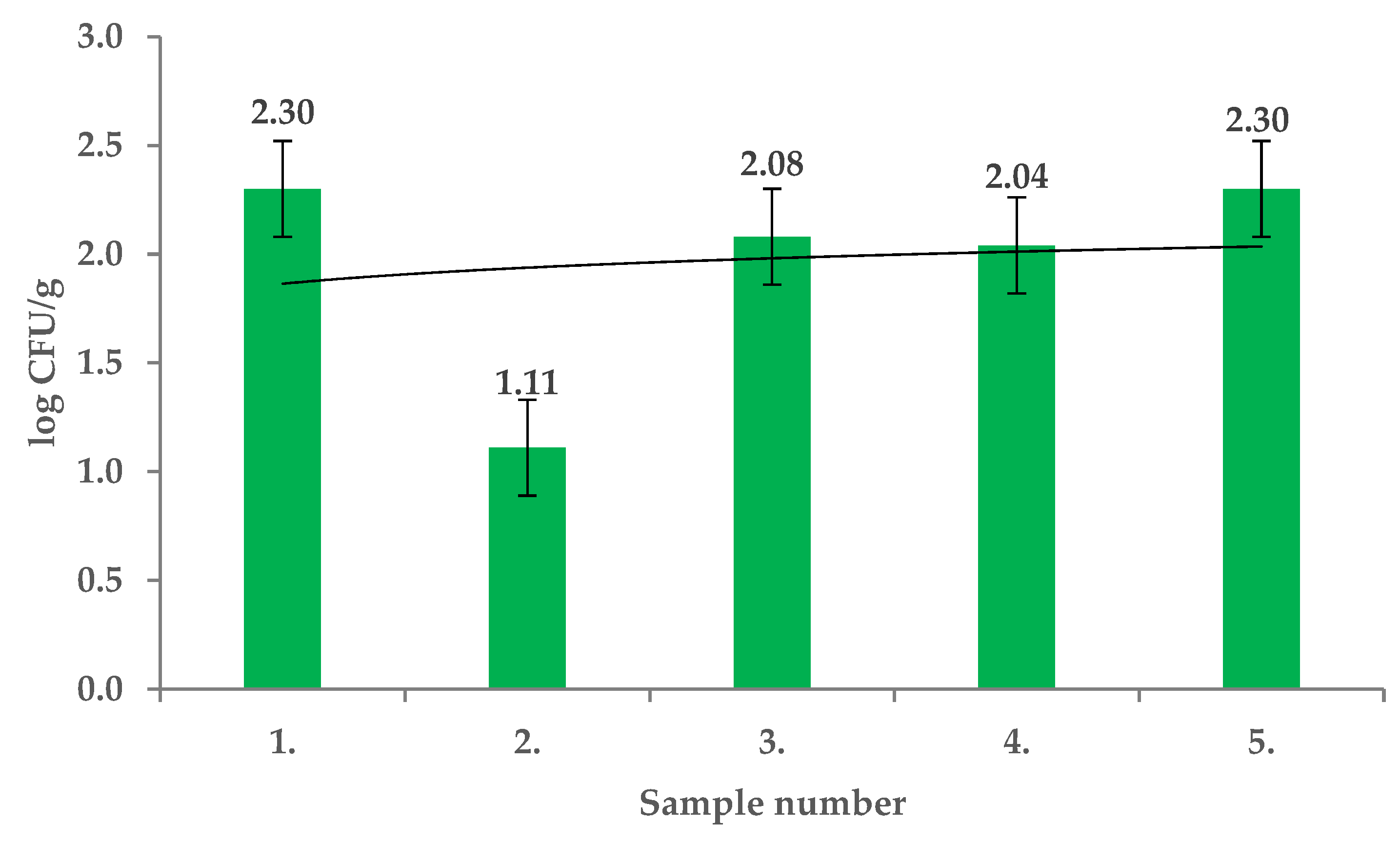
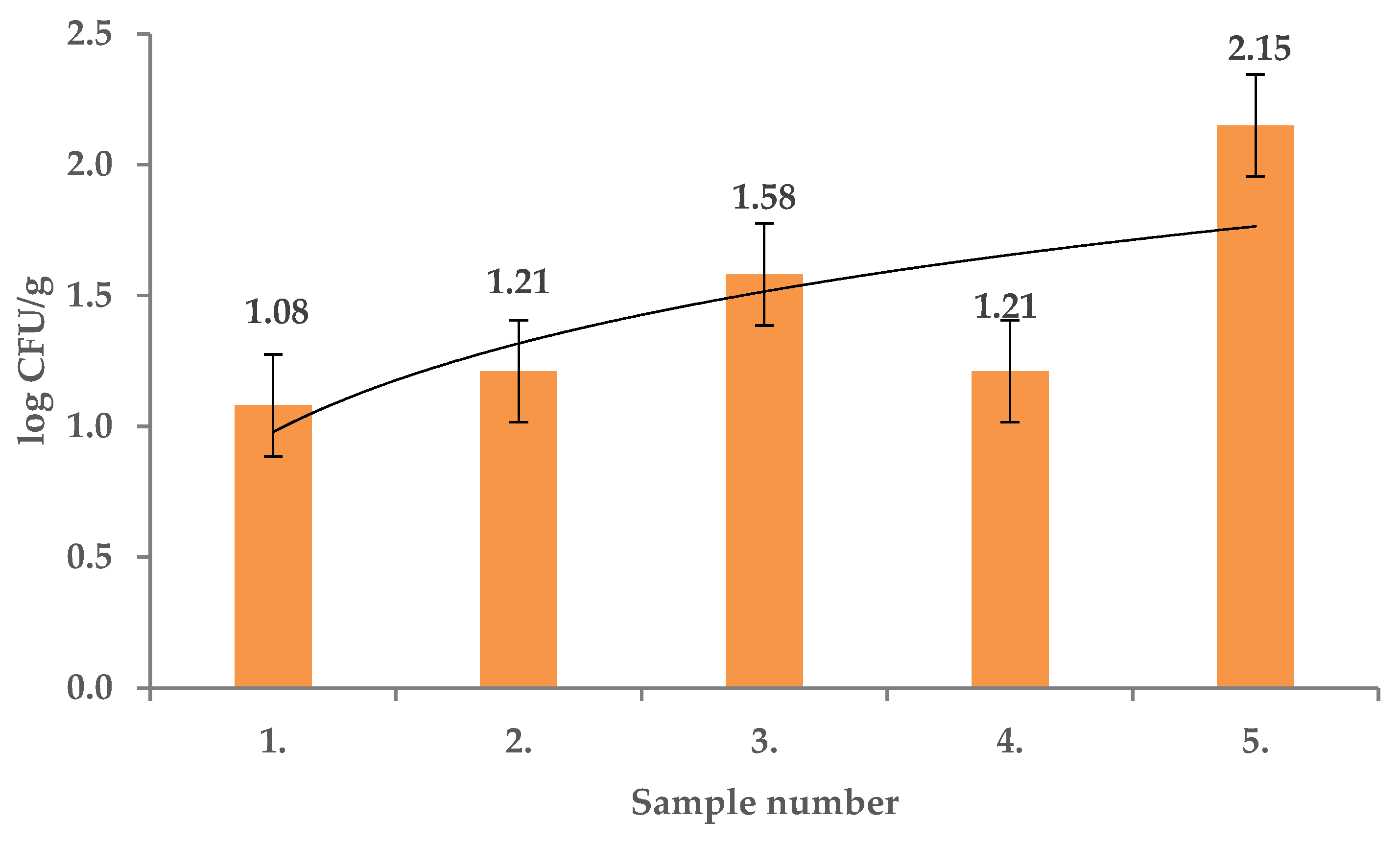
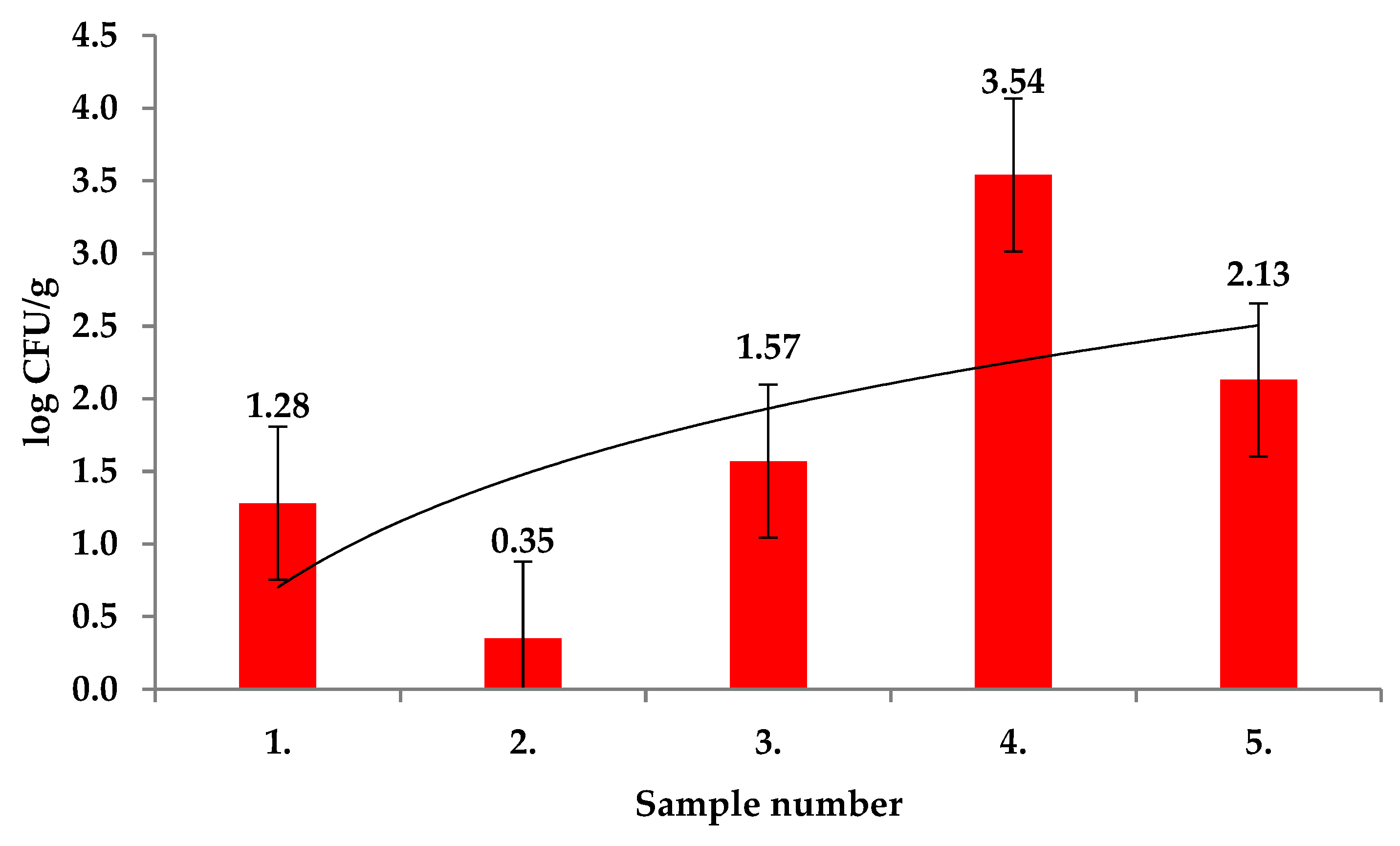
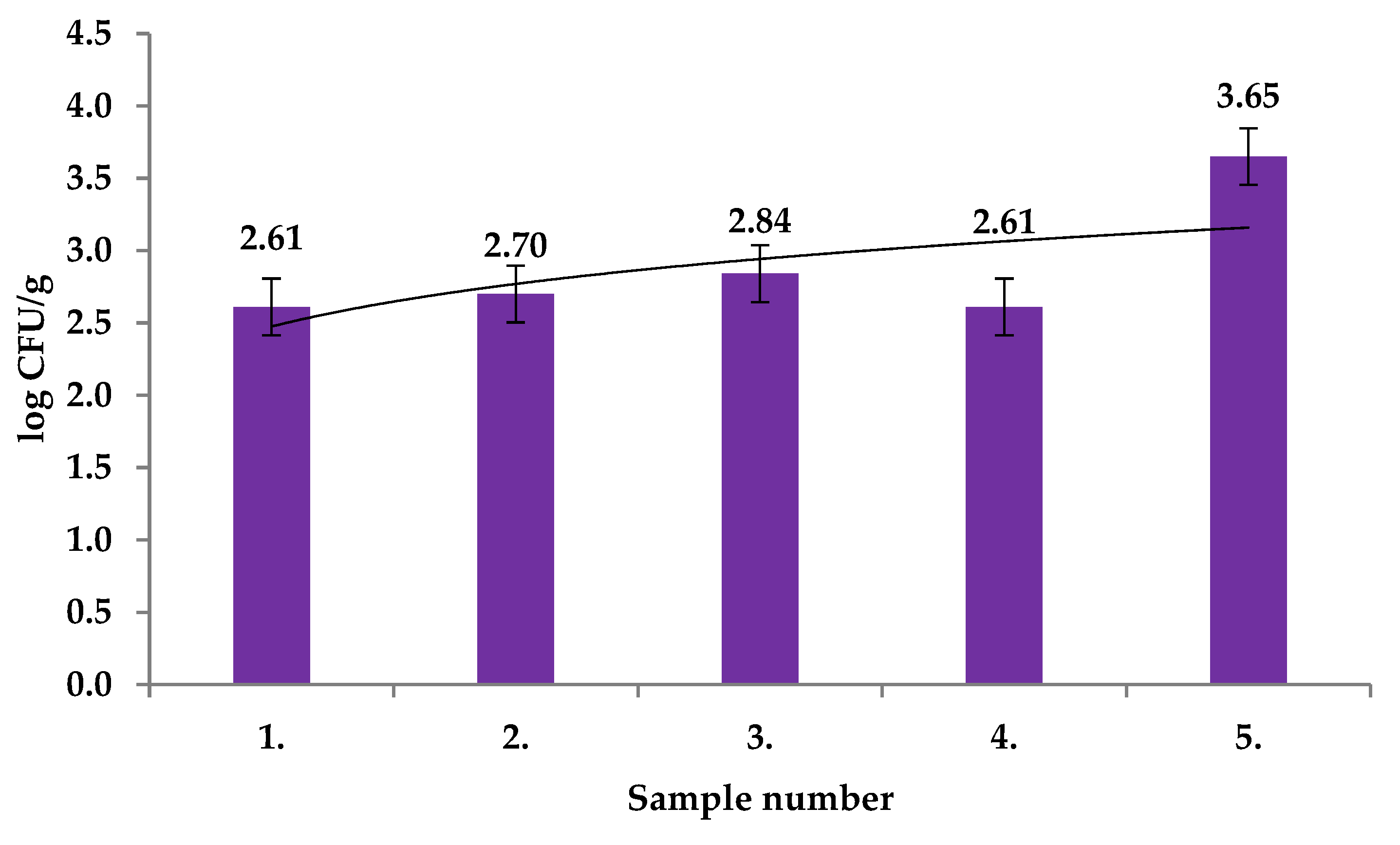
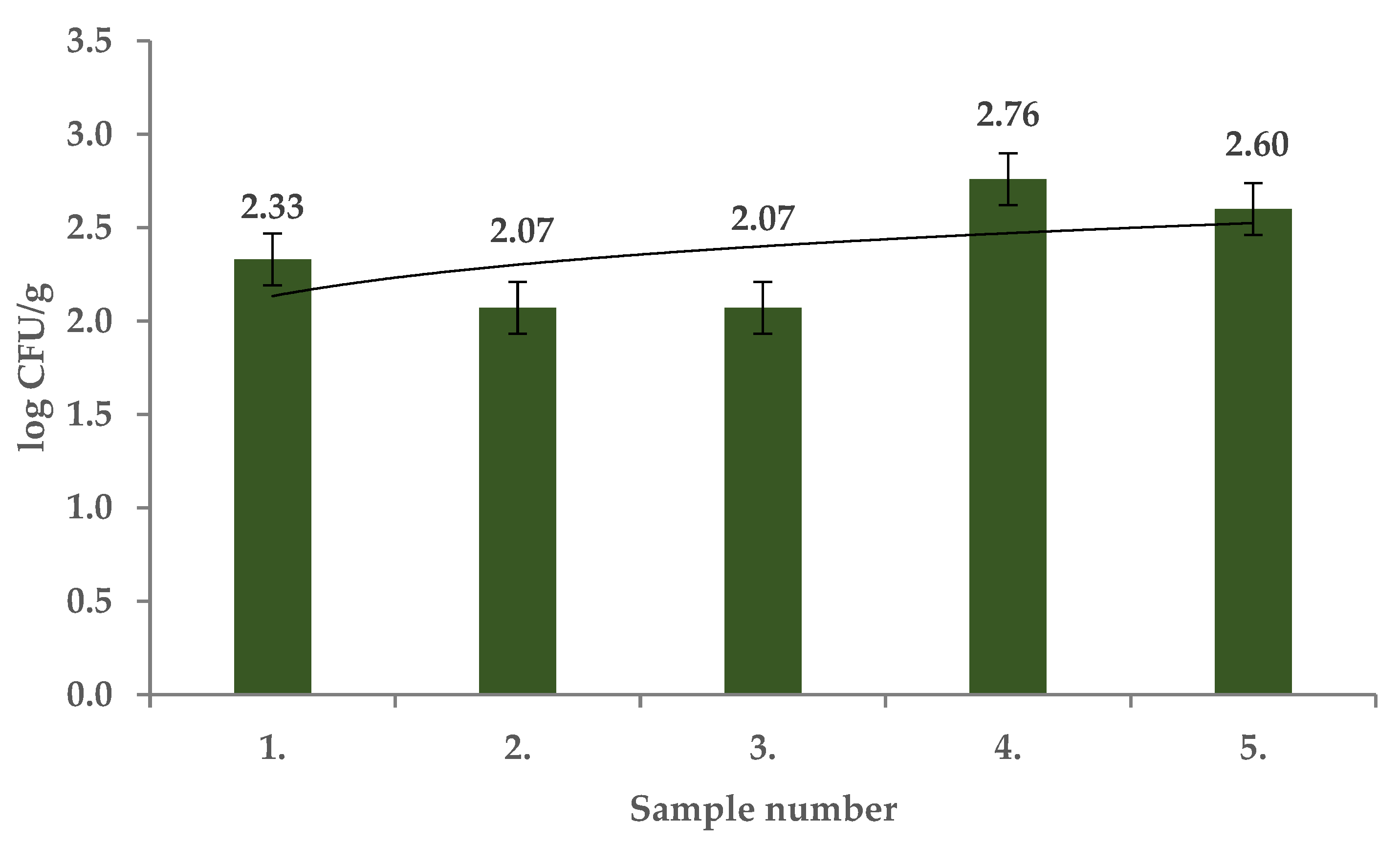
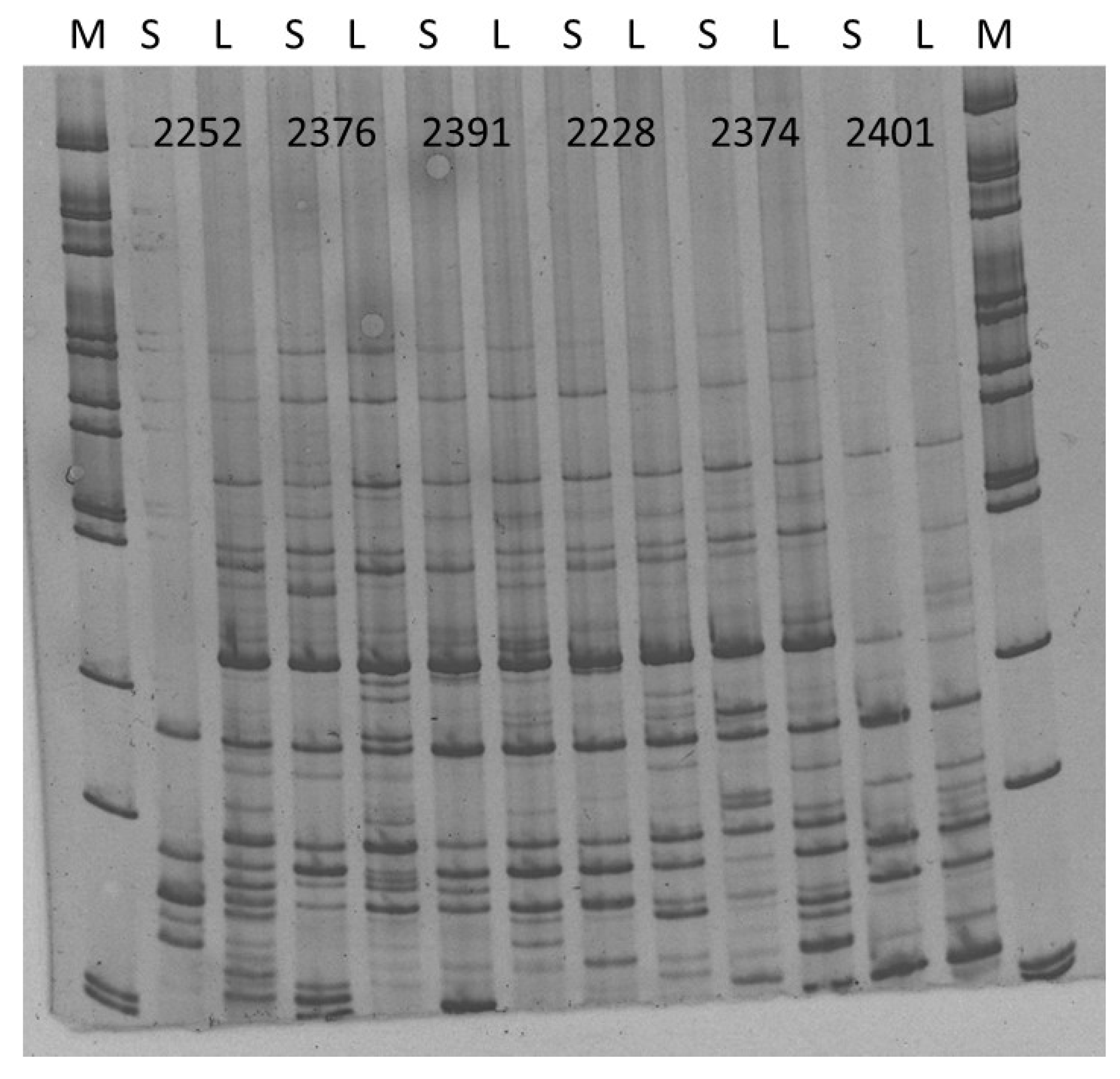
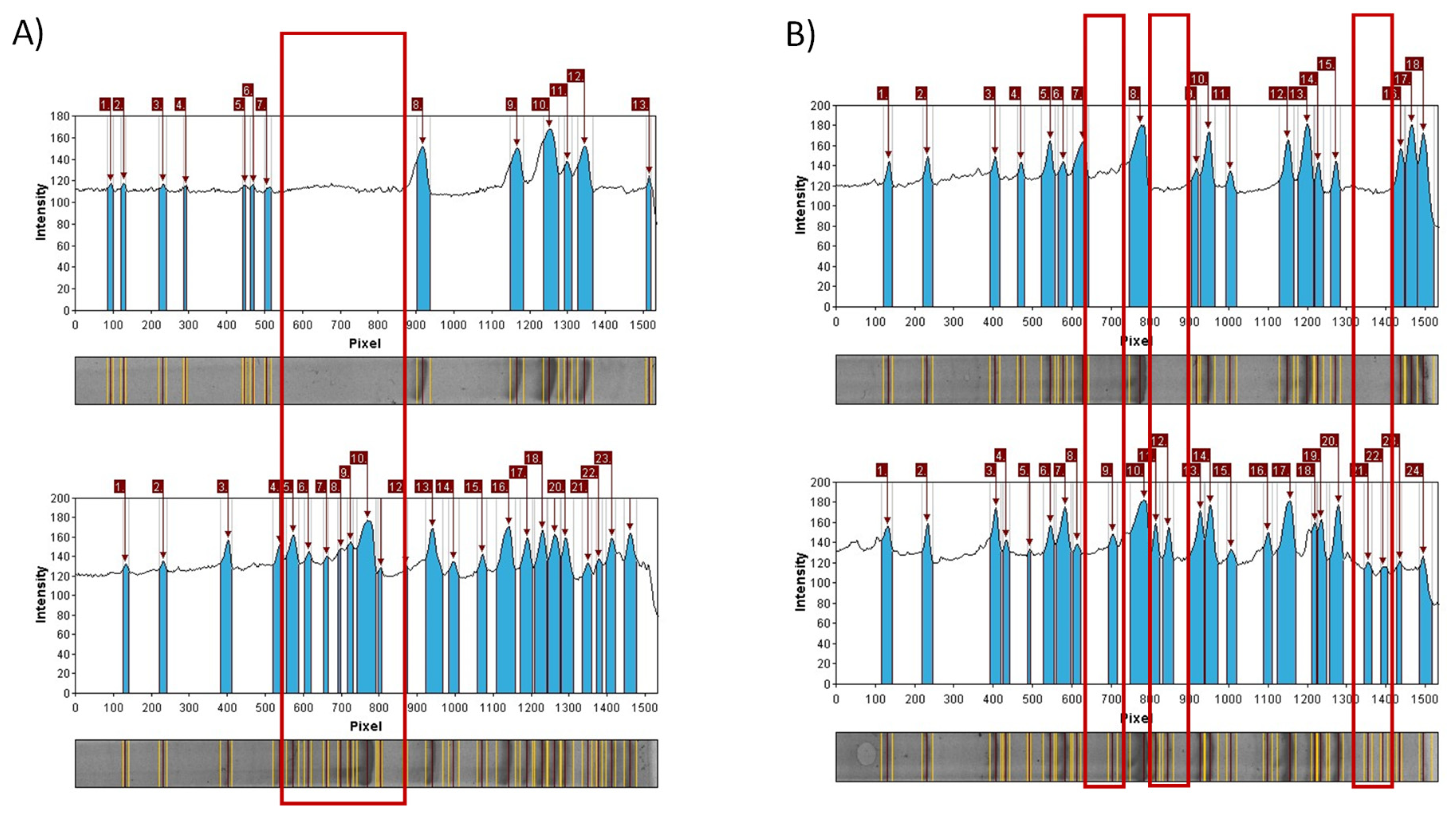
| Extract Activity in Mm | |||||
|---|---|---|---|---|---|
| Microorganisms | 1. | 2. | 3. | 4. | 5. |
| EC | 14.67 ± 0.58 | 15.33 ± 0.58 | 15.67 ± 1.15 | 15.33 ± 1.15 | 14.33 ± 0.58 |
| KO | 11.33 ± 0.58 | 11.33 ± 1.15 | 11.67 ± 0.58 | 10.67 ± 0.58 | 12.00 ± 1.00 |
| PA | 10.67 ± 0.58 | 10.33 ± 0.58 | 10.33 ± 1.15 | 9.33 ± 0.58 | 9.67 ± 0.58 |
| BC | 8.67 ± 2.31 | 8.33 ± 0.58 | 8.67 ± 1.53 | 8.00 ± 1.00 | 8.33 ± 0.58 |
| CP | 4.67 ± 0.58 | 5.33 ± 1.53 | 4.33 ± 0.58 | 4.67 ± 1.53 | 4.00 ± 1.00 |
| LM | 6.67 ± 1.53 | 6.33 ± 0.58 | 5.67 ± 1.15 | 5.67 ± 0.58 | 6.00 ± 1.00 |
| Extract | |||||
|---|---|---|---|---|---|
| Microorganisms | 1. | 2. | 3. | 4. | 5. |
| EC | 32 | 32 | 32 | 32 | 32 |
| KO | 64 | 64 | 64 | 64 | 64 |
| PA | 64 | 64 | 64 | 128 | 128 |
| BC | 128 | 128 | 128 | 128 | 128 |
| CP | 512 | 512 | 512 | 512 | 512 |
| LM | 256 | 256 | 256 | 256 | 256 |
| Sample | AA [mg TEAC/g] | TPC [mg GAE/g] | TFC [mg QE/g] | TPAC [mg CAE/g] | TCC [µg/g] |
|---|---|---|---|---|---|
| 1 | 6.99 ± 0.14 c | 6.33 ± 0.35 a | 2.03 ± 0.11 a | 1.15 ± 0.09 a | 37.12 ± 0.18 e |
| 2 | 7.63 ± 0.21 a,b | 2.95 ± 0.17 c,d | 0.35 ± 0.02 d | 0.37 ± 0.02 b | 57.57 ± 0.22 d |
| 3 | 7.73 ± 0.11 a | 2.61 ± 0.13 d | 0.08 ± 0.01 e | 0.18 ± 0.01 c | 61.07 ± 0.09 c |
| 4 | 7.41 ± 0.09 b | 5.08 ± 0.31 b | 0.92 ± 0.05 b | 0.26 ± 0.03 c | 99.62 ± 0.15 a |
| 5 | 7.28 ± 0.05 b | 3.21 ± 0.12 c | 0.71 ± 0.03 c | 0.23 ± 0.02 c | 75.14 ± 0.31 b |
| Specie | Locality | Genome Size | Ploidy |
|---|---|---|---|
| Rosa canina var. canina | Modra-Pažite (accession 1) | 2.4–2.72 pg | pentapoid (2n = 35) |
| Modra-Pažite (accession 2) | 2.43–2.71 pg | hexaploid (2n = 42) | |
| Vrbové-Baraní dvor | 2.57–2.96 pg | hexaploid (2n = 42) | |
| Rosa canina | Zobor –Lyžiarska lúka | 2.76–2.80 pg | tetraploid (2n = 28) |
| Rosa canina var. dumalis | Modra-Pažite | 2.42–2.66 pg | hexaploid (2n = 42) |
| Vrbové-Baraní dvor (sample 1) | 2.3–2.65 pg | oktaploid (2n = 56) | |
| Vrbové-Baraní dvor (sample 2) | 2.52–2.70 pg | pentaploid (2n = 35) | |
| Rosa canina var. squarosa A.Rau | Zobor –Lyžiarska lúka | 2.31–2.51 pg | pentapoid (2n = 35) |
| Vrbové-Baraní dvor | 2.45–2.67 pg | octaploid (2n = 56) | |
| Rosa canina var. lapidicola Heinr. Braun | Zobor –Lyžiarska lúka | 2.2–2.51 pg | pentapoid (2n = 35) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.Ł.; Kačániová, M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, iPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. https://doi.org/10.3390/molecules25081888
Rovná K, Ivanišová E, Žiarovská J, Ferus P, Terentjeva M, Kowalczewski PŁ, Kačániová M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, iPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules. 2020; 25(8):1888. https://doi.org/10.3390/molecules25081888
Chicago/Turabian StyleRovná, Katarína, Eva Ivanišová, Jana Žiarovská, Peter Ferus, Margarita Terentjeva, Przemysław Łukasz Kowalczewski, and Miroslava Kačániová. 2020. "Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, iPBS Profiles and Antioxidant and Antimicrobial Activities" Molecules 25, no. 8: 1888. https://doi.org/10.3390/molecules25081888
APA StyleRovná, K., Ivanišová, E., Žiarovská, J., Ferus, P., Terentjeva, M., Kowalczewski, P. Ł., & Kačániová, M. (2020). Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, iPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules, 25(8), 1888. https://doi.org/10.3390/molecules25081888









