In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania braziliensis and Leishmania panamensis Promastigotes Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Singlet Oxygen Quantum Yield
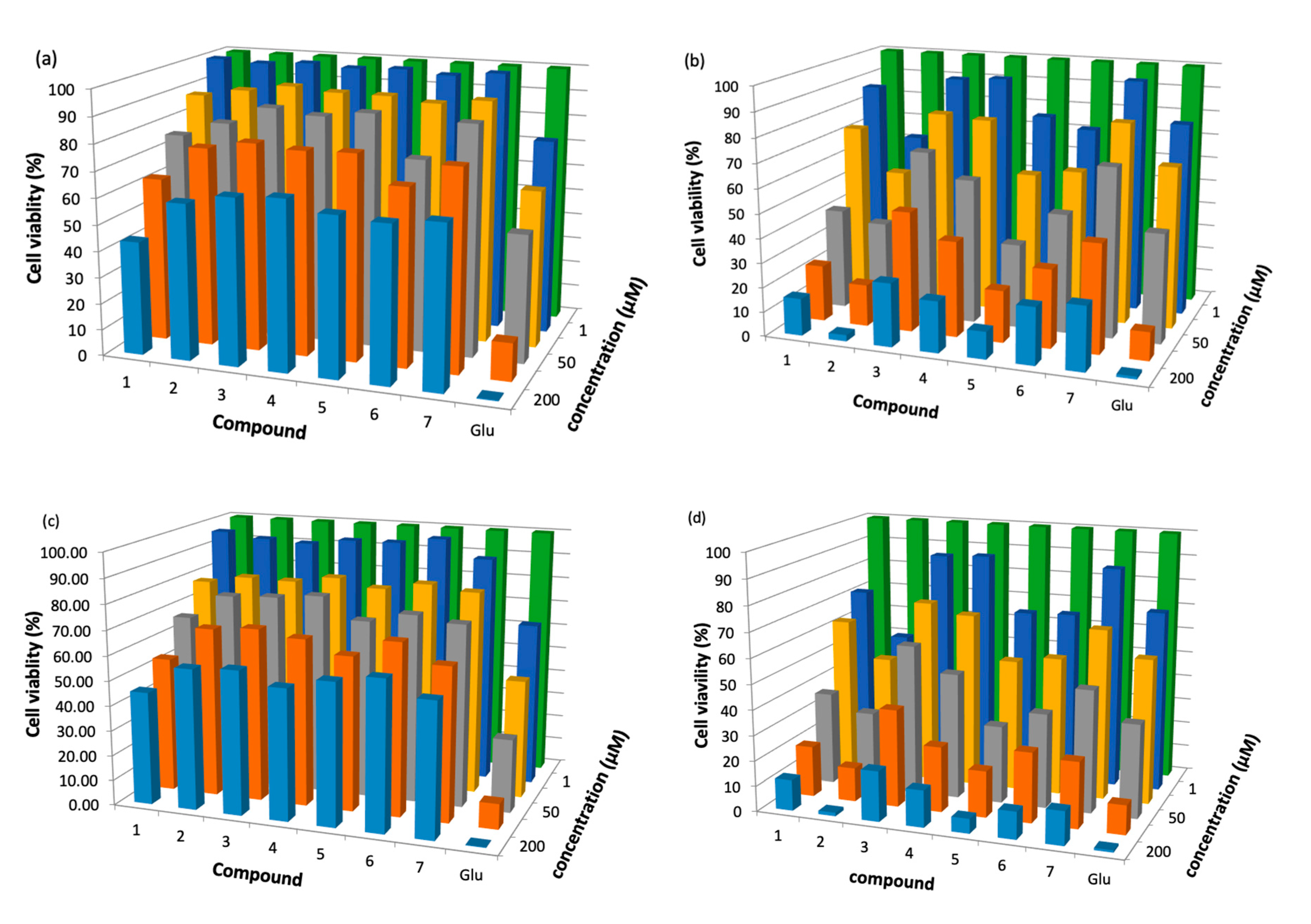
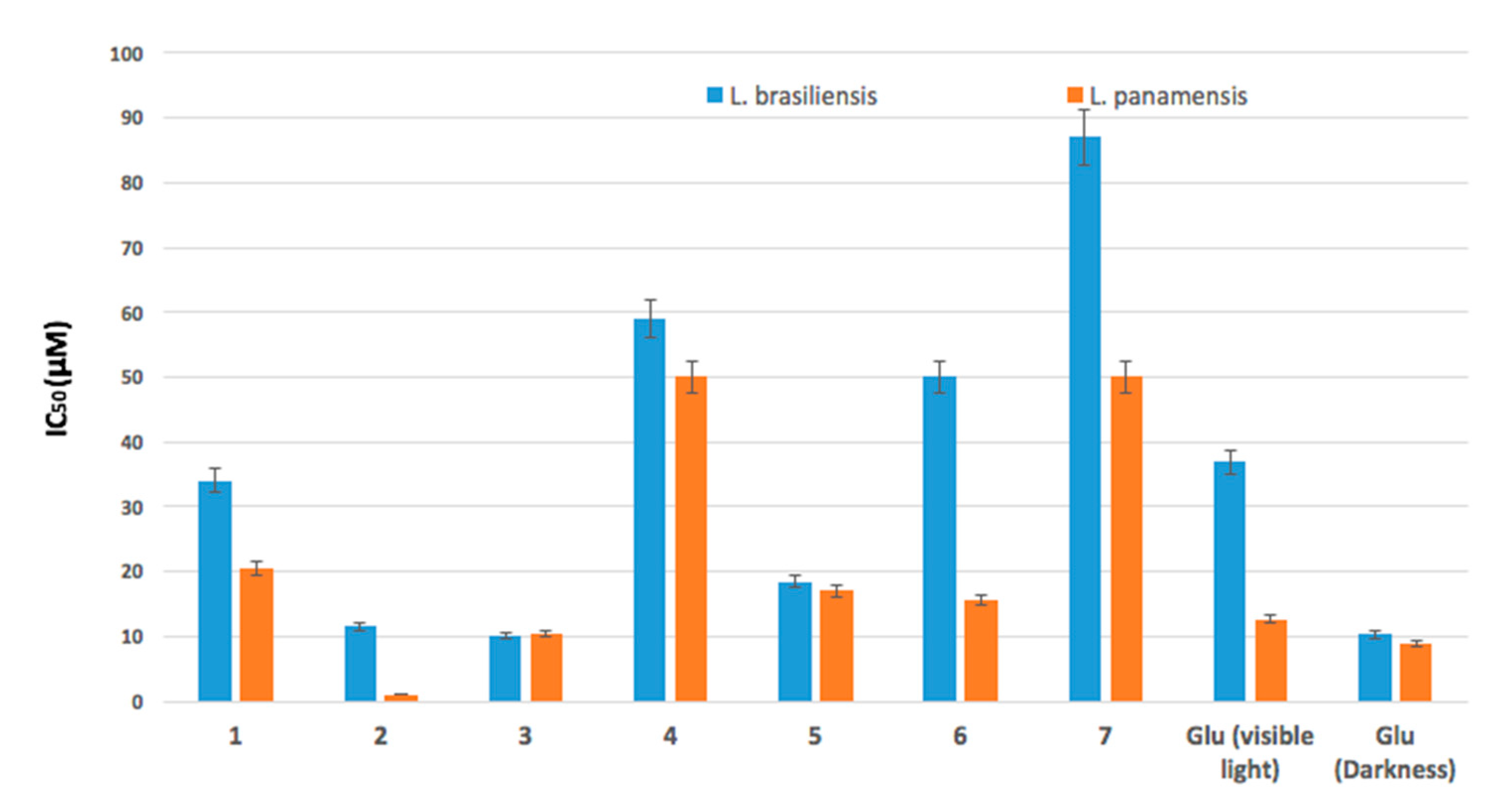
2.2. Antileishmanicidal Activity
3. Materials and Methods
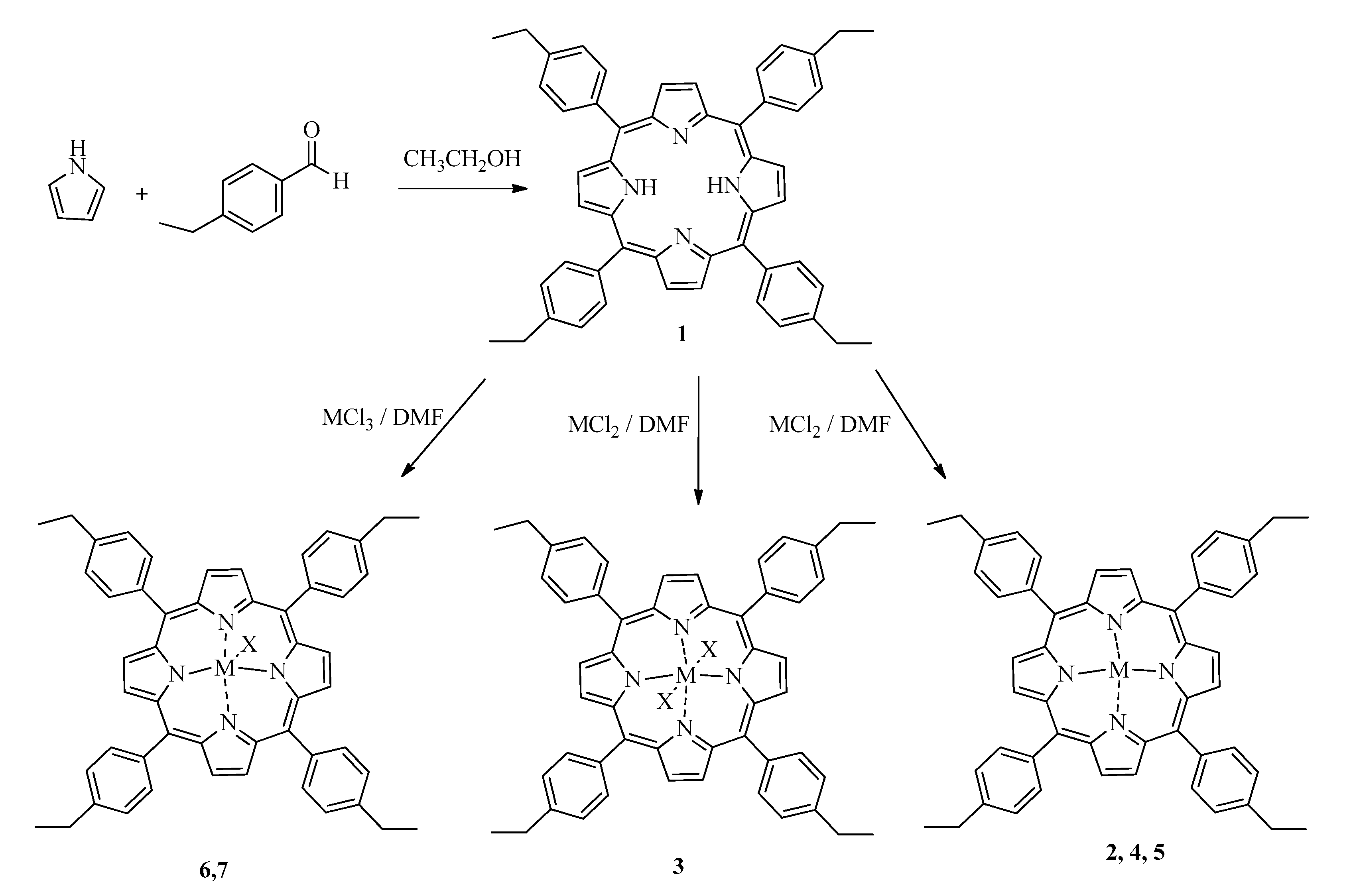
3.1. Synthesis
3.2. Singlet Oxygen Quantum Yield
3.3. Fluorescence Quantum Yield
3.4. Parasites
3.5. Parasite Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 12 February 2019).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Khademvatan, S.; Salmanzadeh, S.; Foroutan-Rad, M.; Bigdeli, S.; Hedayati-Rad, F.; Saki, J.; Heydari-Gorji, E. Spatial distribution and epidemiological features of cutaneous leishmaniasis in southwest of Iran. Alex. J. Med. 2017, 53, 93–98. [Google Scholar] [CrossRef]
- Steverding, D. The history of leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Londoño, S.Y.; Salazar, L.M.; Acero, C.T.; Bernal, I.D.V. Aspectos socioepidemiológicos y culturales de la leishmaniasis cutánea concepciones, actitudes y prácticas en las poblaciones de Tierralta y Valencia, (Córdoba, Colombia). Salud Colect. 2017, 13, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Gore Saravia, N.; Nicholls, R.S. Leishmaniasis: Un reto para la salud pública que exige concertación de voluntades y esfuerzos. Biomédica 2012, 26, 5. [Google Scholar] [CrossRef]
- Singh, S.; Sivakumar, R. Challenges and new discoveries in the treatment of leishmaniasis. J. Infect. Chemother. 2004, 10, 307–315. [Google Scholar] [CrossRef]
- Lockwood, D.; Moore, E. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151. [Google Scholar] [CrossRef]
- Tuon, F.F.; Amato, V.S.; Graf, M.E.; Siqueira, A.M.; Nicodemo, A.C.; Neto, V.A. Treatment of New World cutaneous leishmaniasis - a systematic review with a meta-analysis. Int. J. Dermatol. 2008, 47, 109–124. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; López-Vélez, R. Therapeutic Options for Old World Cutaneous Leishmaniasis and New World Cutaneous and Mucocutaneous Leishmaniasis. Drugs 2013, 73, 1889–1920. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Melo, M.H.; Meneses, A.M.; Schubach, A.O.; Moreira, J.S.; Conceição-Silva, F.; Salgueiro, M.M.; Pimentel, M.I.F.; Araújo-Silva, M.; Oliveira, R.V.C.; Carmo, C.N.; et al. Risk factors associated with dizziness during treatment of mucosal leishmaniasis with meglumine antimoniate: 16-year retrospective study of cases from Rio de Janeiro, Brazil. J. Laryngol. Otol. 2010, 124, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Berman, J.D. Chemotherapy for leishmaniasis: Biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988, 10, 560–586. [Google Scholar] [CrossRef] [PubMed]
- Heruti, R.J.; Sharabi, Y.; Arbel, Y.; Shochat, T.; Swartzon, M.; Brenner, G.; Justo, D. ORIGINAL RESEARCH—EPIDEMIOLOGY: The Prevalence of Erectile Dysfunction Among Hypertensive and Prehypertensive Men Aged 25–40 Years. J. Sex. Med. 2007, 4, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Guerin, P.J.; Gerstl, S.; Haaskjold, A.A.; Rottingen, J.-A.; Sundar, S. Treatment options for visceral leishmaniasis: A systematic review of clinical studies done in India, 1980–2004. Lancet Infect. Dis. 2005, 5, 763–774. [Google Scholar] [CrossRef]
- Renslo, A.R.; McKerrow, J.H. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2006, 2, 701–710. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; Alkahtani, S.; Kolli, B.; Tripathi, P.; Dutta, S.; Al-Kahtane, A.A.; Jiang, X.J.; Ng, D.K.P.; Chang, K.P. Aminophthalocyanine-mediated photodynamic inactivation of Leishmania tropica. Antimicrob. Agents Chemother. 2016, 60, 2003–2011. [Google Scholar] [CrossRef]
- Peloi, L.S.; Biondo, C.E.G.; Kimura, E.; Politi, M.J.; Lonardoni, M.V.C.; Aristides, S.M.A.; Dorea, R.C.C.; Hioka, N.; Silveira, T.G.V. Photodynamic therapy for American cutaneous leishmaniasis: The efficacy of methylene blue in hamsters experimentally infected with Leishmania (Leishmania) amazonensis. Exp. Parasitol. 2011, 128, 353–356. [Google Scholar] [CrossRef]
- Jeong, H.-G.; Choi, M.-S. Design and Properties of Porphyrin-based Singlet Oxygen Generator. Isr. J. Chem. 2016, 56, 110–118. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met. Based Drugs 2008, 2008. [Google Scholar] [CrossRef]
- Skwor, T.A.; Klemm, S.; Zhang, H.; Schardt, B.; Blaszczyk, S.; Bork, M.A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus and Escherichia coli: A metalloporphyrin comparison. J. Photochem. Photobiol. B Biol. 2016, 165, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.; Smith, T.A.; Ghiggino, K.P. Singlet oxygen quantum yields of potential porphyrin-based photosensitisers for photodynamic therapy. Photochem. Photobiol. Sci. 2007, 6, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Ormond, A.B.; Freeman, H.S. Effects of substituents on the photophysical properties of symmetrical porphyrins. Dye Pigment. 2013, 96, 440–448. [Google Scholar] [CrossRef]
- Cauzzo, G.; Gennari, C.; Jori, G.; Spikes, J.D. The effect of chemical structure on the photosensitizing efficiencies of porphyrins. Photochem. Photobiol. 1977, 25, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Lavi, A.; Weitman, H.; Holmes, R.T.; Smith, K.M.; Ehrenberg, B. The depth of porphyrin in a membrane and the membrane’s physical properties affect the photosensitizing efficiency. Biophys. J. 2002, 82, 2101–2110. [Google Scholar] [CrossRef]
- Lesar, A.; Begić, G.; Malatesti, N.; Gobin, I. Innovative approach in Legionella water treatment with photodynamic cationic amphiphilic porphyrin. Water Sci. Technol. Water Supply 2019, 19, 1473–1479. [Google Scholar] [CrossRef]
- Rojkiewicz, M.; Kuś, P.; Kozub, P.; Kempa, M. The synthesis of new potential photosensitizers. Dye Pigment. 2013, 99, 627–635. [Google Scholar] [CrossRef]
- Thomas, M.; Craik, J.D.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Amphiphilic cationic Zn-porphyrins with high photodynamic antimicrobial activity. Future Microbiol. 2015, 10, 709–724. [Google Scholar] [CrossRef]
- Ezzeddine, R.; Al-Banaw, A.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Effect of molecular characteristics on cellular uptake, subcellular localization, and phototoxicity of Zn(2) N-Alkylpyridylporphyrins. J. Biol. Chem. 2013, 288, 36579–36588. [Google Scholar] [CrossRef]
- Hosomizu, K.; Oodoi, M.; Umeyama, T.; Matano, Y.; Yoshida, K.; Isoda, S.; Isosomppi, M.; Tkachenko, N.V.; Lemmetyinen, H.; Imahori, H. Substituent effects of porphyrins on structures and photophysical properties of amphiphilic porphyrin aggregates. J. Phys. Chem. B 2008, 112, 16517–16524. [Google Scholar] [CrossRef] [PubMed]
- Stasheuski, A.S.; Galievsky, V.A.; Knyukshto, V.N.; Ghazaryan, R.K.; Gyulkhandanyan, A.G.; Gyulkhandanyan, G.V.; Dzhagarov, B.M. Water-Soluble Pyridyl Porphyrins with Amphiphilic N-Substituents: Fluorescent Properties and Photosensitized Formation of Singlet Oxygen. J. Appl. Spectrosc. 2014, 80, 813–823. [Google Scholar] [CrossRef]
- Gardner, D.M.; Taylor, V.M.; Cedeño, D.L.; Padhee, S.; Robledo, S.M.; Jones, M.A.; Lash, T.D.; Vélez, I.D. Association of Acenaphthoporphyrins with Liposomes for the Photodynamic Treatment of Leishmaniasis. Photochem. Photobiol. 2010, 86, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.G.; Pereira, A.H.C.; de Oliveira, M.A.; Kurachi, C.; Raniero, L.J.; Ferreira-Strixino, J. Chlorin E6 phototoxicity in L. major and L. braziliensis promastigotes—In vitro study. Photodiagn. Photodyn. Ther. 2016, 15, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bristow, C.-A.; Hudson, R.; Paget, T.A.; Boyle, R.W. Potential of cationic porphyrins for photodynamic treatment of cutaneous Leishmaniasis. Photodiagn. Photodyn. Ther. 2006, 3, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.L.; DeFreitas-Silva, G.; dos Reis, P.G.; Melo, M.N.; Frézard, F.; Demicheli, C.; Idemori, Y.M. Synthesis and characterization of bismuth(III) and antimony(V) porphyrins: High antileishmanial activity against antimony-resistant parasite. JBIC J. Biol. Inorg. Chem. 2015, 20, 771–779. [Google Scholar] [CrossRef]
- Andrade, C.G.; Figueiredo, R.C.B.Q.; Ribeiro, K.R.C.; Souza, L.I.O.; Sarmento-Neto, J.F.; Rebouças, J.S.; Santos, B.S.; Ribeiro, M.S.; Carvalho, L.B.; Fontes, A. Photodynamic effect of zinc porphyrin on the promastigote and amastigote forms of Leishmania braziliensis. Photochem. Photobiol. Sci. 2018, 17, 482–490. [Google Scholar] [CrossRef]
- Espitia-Almeida, F.; Díaz-Uribe, C.; Vallejo, W.; Gómez-Camargo, D.; Romero-Bohorquez, A.R.; Schott, E.; Zarate, X. Synthesis and Characterization of 5,10,15,20-Tetrakis(4-ethylphenyl)porphyrin and (Zn2+, Mn2+, Sn2+, Ni2+, Al3+, V3+)-Derivatives: Photophysical and DFT study. ChemistrySelect 2019, 4, 6290–6294. [Google Scholar] [CrossRef]
- Dube, E.; Nwaji, N.; Oluwole, D.O.; Mack, J.; Nyokong, T. Investigation of photophysicochemical properties of zinc phthalocyanines conjugated to metallic nanoparticles. J. Photochem. Photobiol. A Chem. 2017, 349, 148–161. [Google Scholar] [CrossRef]
- Zoltan, T.; Vargas, F.; López, V.; Chávez, V.; Rivas, C.; Ramírez, Á.H. Influence of charge and metal coordination of meso-substituted porphyrins on bacterial photoinactivation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 747–756. [Google Scholar] [CrossRef]
- Guillaumot, D.; Issawi, M.; Da Silva, A.; Leroy-Lhez, S.; Sol, V.; Riou, C. Synergistic enhancement of tolerance mechanisms in response to photoactivation of cationic tetra (N-methylpyridyl) porphyrins in tomato plantlets. JPB 2016, 156, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482296952. [Google Scholar]
- Pummer, A.; Knüttel, H.; Hiller, K.-A.; Buchalla, W.; Cieplik, F.; Maisch, T. Antimicrobial efficacy of irradiation with visible light on oral bacteria in vitro: A systematic review. Future Med. Chem. 2017, 9, 1557–1574. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.D.; Andrade, M.C.; Bagnato, V.S.; Vergani, C.E.; Primo, F.L.; Tedesco, A.C.; Pavarina, A.C. Antimicrobial photodynamic therapy against pathogenic bacterial suspensions and biofilms using chloro-aluminum phthalocyanine encapsulated in nanoemulsions. Lasers Med. Sci. 2015, 30, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lindoso, J.A.L.; Oyafuso, L.K.; Kanashiro, E.H.Y.; Cardoso, J.L.; Uchoa, A.F.; Tardivo, J.P.; Baptista, M.S. Photodynamic Therapy Using Methylene Blue to Treat Cutaneous Leishmaniasis. Photomed. Laser Surg. 2011, 29, 711–715. [Google Scholar] [CrossRef]
- Hernández, I.P.; Montanari, J.; Valdivieso, W.; Morilla, M.J.; Romero, E.L.; Escobar, P. In vitro phototoxicity of ultradeformable liposomes containing chloroaluminum phthalocyanine against New World Leishmania species. J. Photochem. Photobiol. B Biol. 2012, 117, 157–163. [Google Scholar] [CrossRef]
- Piñero, J.E.; Jiménez, I.A.; Valladares, B.; Ravelo, Á.G. Advances in leishmaniasis chemotherapy and new relevant patents. Expert Opin. Ther. Pat. 2004, 14, 1113–1123. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Drug Resistance in Leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 167. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Ouellette, M.; Papadopoulou, B. Mechanisms of drug resistance in Leishmania. Parasitol. Today 1993, 9, 150–153. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chesm. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed.; Oxford University Press: New York, NY, USA, 2015; ISBN 9780198717485. [Google Scholar]
- Breitenbach, T.; Kuimova, M.K.; Gbur, P.; Hatz, S.; Schack, N.B.; Pedersen, B.W.; Lambert, J.D.C.; Poulsen, L.; Ogilby, P.R. Photosensitized production of singlet oxygen: Spatially-resolved optical studies in single cells. Photochem. Photobiol. Sci. 2009, 8, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.D.; Longo, F.R.; Shergalis, W. Mechanistic Investigations of Porphyrin Syntheses. I. Preliminary Studies on ms-Tetraphenylporphin. J. Am. Chem. Soc. 1964, 86, 3145–3149. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Taylor, V.M.; Muñoz, D.L.; Cedeño, D.L.; Vélez, I.D.; Jones, M.A.; Robledo, S.M. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol. 2010, 126, 471–475. [Google Scholar] [CrossRef]
- Akilov, O.E.; Kosaka, S.; O’Riordan, K.; Hasan, T. Parasiticidal effect of δ-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp. Dermatol. 2007, 16, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kiderlen, A.F.; Kaye, P.M. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J. Immunol. Methods 1990, 127, 11–18. [Google Scholar] [CrossRef]
- Durmuş, M.; Nyokong, T. Photophysicochemical and fluorescence quenching studies of benzyloxyphenoxy-substituted zinc phthalocyanines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 1170–1177. [Google Scholar] [CrossRef]
- Khodov, I.A.; Nikiforov, M.Y.; Alper, G.A.; Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Synthesis and spectroscopic characterization of Ru(II) and Sn(IV)-porphyrins supramolecular complexes. J. Mol. Struct. 2015, 1081, 426–430. [Google Scholar] [CrossRef]
- Moreira, M.E.C.; Del Portillo, H.A.; Milder, R.V.; Balanco, J.M.F.; Barcinski, M.A. Heat shock induction of apoptosis in promastigotes of the unicellular organismLeishmania (Leishmania) amazonensis. J. Cell. Physiol. 1996, 167, 305–313. [Google Scholar] [CrossRef]
Sample Availability: Samples of the freeze-dried powders are available from the authors. |
| Compound | ε (M−1cm−1 × 104) | Φf | ΦΔ |
|---|---|---|---|
| 1, Ph | 5.0 | 0.11 ± 0.02 | 0.76 ± 0.09 |
| 2, Ph-Zn | 2.5 | 0.11 ± 0.03 | 0.90 ± 0.03 |
| 3, Ph-Sn | 6.0 | 0.32 ± 0.02 | 0.76 ± 0.05 |
| 4, Ph-Mn | 4.0 | 0.17 ± 0.02 | 0.83 ± 0.03 |
| 5, Ph-Ni | 7.0 | 0.08 ± 0.02 | 0.68 ± 0.02 |
| 6, Ph-Al | 3.4 | 0.010 ± 0.005 | 0.84 ± 0.04 |
| 7, Ph-V | 3.1 | 0.0020 ± 0.0005 | 0.86 ± 0.01 |
| Compound | L. braziliensis | L. panamensis | ||
|---|---|---|---|---|
| IC50 (μM) Under Irradiation | IC50 (μM) in the Dark | IC50 (μM) under Irradiation | IC50 (μM) in the Dark | |
| 1, Ph | 34.1 ± 1.8 | 117 | 20.6 ± 1.3 | 105 |
| 2, Ph-Zn | 11.6 ± 1.0 | >200 | 1.2 ± 0.2 | >200 |
| 3, Ph-Sn | 10.1 ± 0.7 | >200 | 10.4 ± 0.8 | >200 |
| 4, Ph-Mn | 59.0 ± 2.5 | >200 | 50.0 ± 1.3 | >200 |
| 5, Ph-Ni | 18.4 ± 2.5 | >200 | 17.0 ± 1.0 | >200 |
| 6, Ph-Al | 50 ± 1.2 | >200 | 15.6 ± 0.9 | >200 |
| 7, Ph-V | 87 ± 3.5 | >200 | 50.0 ± 2.1 | >200 |
| Glucantime | -- | 10.3 ± 0.9 | -- | 8.8 ± 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espitia-Almeida, F.; Díaz-Uribe, C.; Vallejo, W.; Gómez-Camargo, D.; Romero Bohórquez, A.R. In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania braziliensis and Leishmania panamensis Promastigotes Properties. Molecules 2020, 25, 1887. https://doi.org/10.3390/molecules25081887
Espitia-Almeida F, Díaz-Uribe C, Vallejo W, Gómez-Camargo D, Romero Bohórquez AR. In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania braziliensis and Leishmania panamensis Promastigotes Properties. Molecules. 2020; 25(8):1887. https://doi.org/10.3390/molecules25081887
Chicago/Turabian StyleEspitia-Almeida, Fabian, Carlos Díaz-Uribe, William Vallejo, Doris Gómez-Camargo, and Arnold R. Romero Bohórquez. 2020. "In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania braziliensis and Leishmania panamensis Promastigotes Properties" Molecules 25, no. 8: 1887. https://doi.org/10.3390/molecules25081887
APA StyleEspitia-Almeida, F., Díaz-Uribe, C., Vallejo, W., Gómez-Camargo, D., & Romero Bohórquez, A. R. (2020). In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania braziliensis and Leishmania panamensis Promastigotes Properties. Molecules, 25(8), 1887. https://doi.org/10.3390/molecules25081887







