Julbernardia paniculata and Pterocarpus angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Ethnobotanical Surveys
2.2. Phytochemical Characterization and Phenolic Profile
2.3. Antioxidant Activity
2.4. Anti-Inflammatory Activity and Cytotoxicity
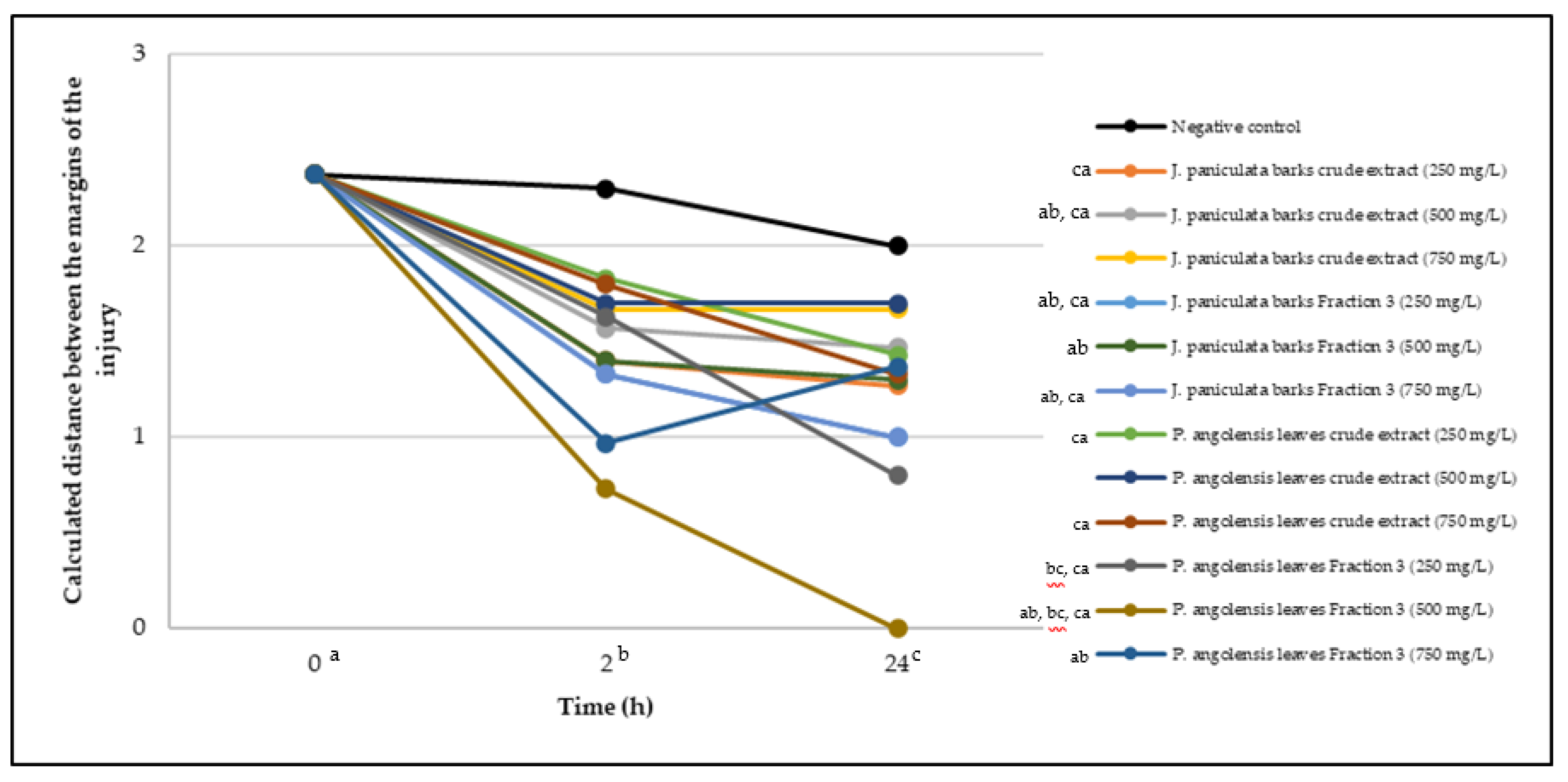
2.5. Wound-Healing Activity
- Between 0 and 2 h after the incubation with the samples: J. paniculata crude methanolic extract of barks (500 mg/L) and the respective Fraction 3 (250, 500 and 750 mg/L) and P. angolensis Fraction 3 of leaves (500 and 750 mg/L);
- Between 0 and 24 h after the incubation with the samples: J. paniculata crude methanolic extract of barks (500 mg/L) and the respective Fraction 3 (250 and 750 mg/L), P. angolensis crude methanolic extract of leaves (250 and 750 mg/L) and the respective Fraction 3 (500 mg/L);
- Between 2 and 24 h after the incubation with the samples: P. angolensis Fraction 3 of leaves (250 and 500 mg/L).
3. Materials and Methods
3.1. Ethnobotanical Surveys
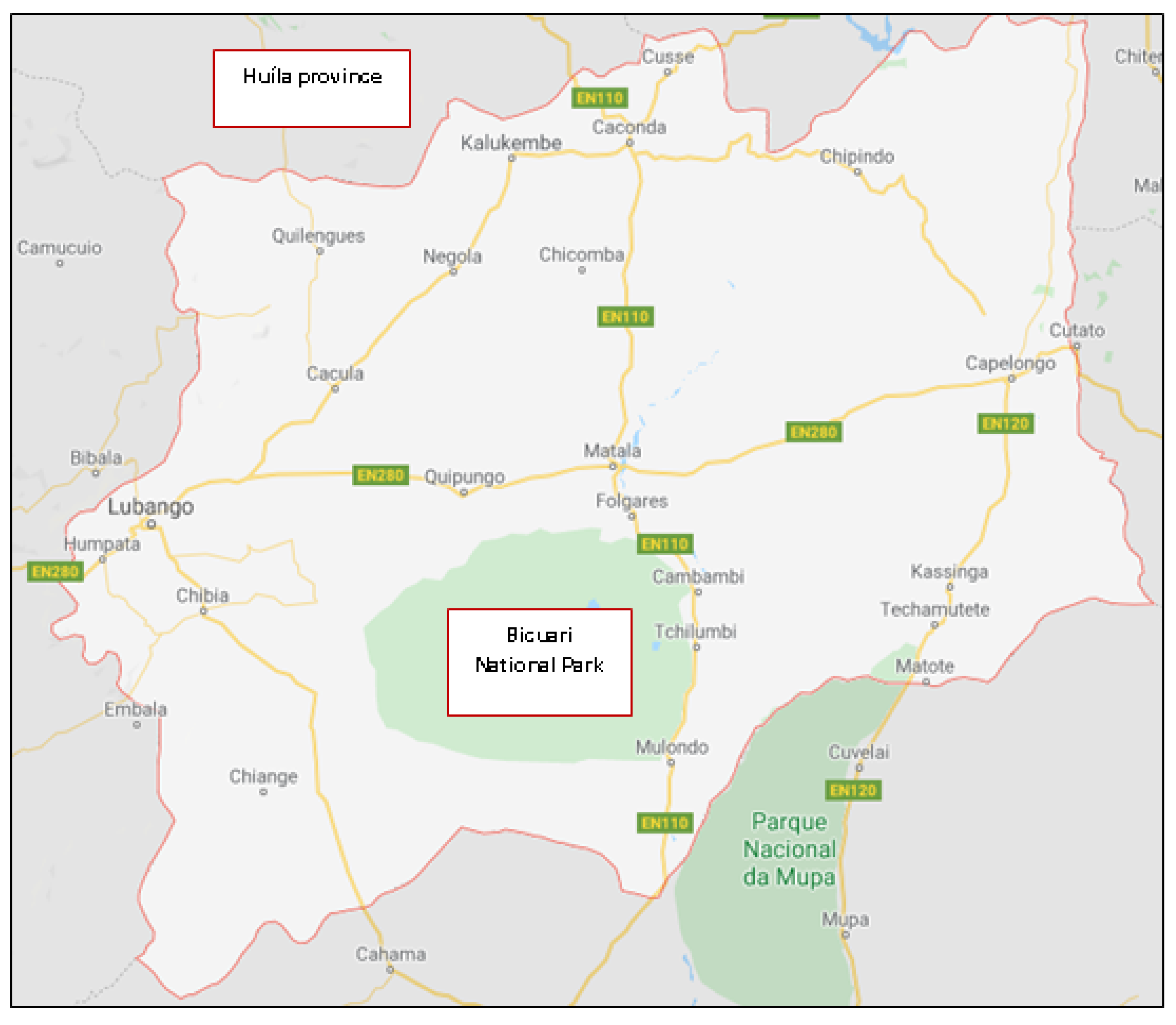
3.1.1. Huíla Province Geo-Ethnographical Profile
3.1.2. Field Interviews
3.1.3. Collection and Identification of Plant Materials
3.1.4. Quantitative Analysis of the Ethnobotanical Results
Informant Consensus Factor
Fidelity Level
Relative Frequency Citation
Use Value
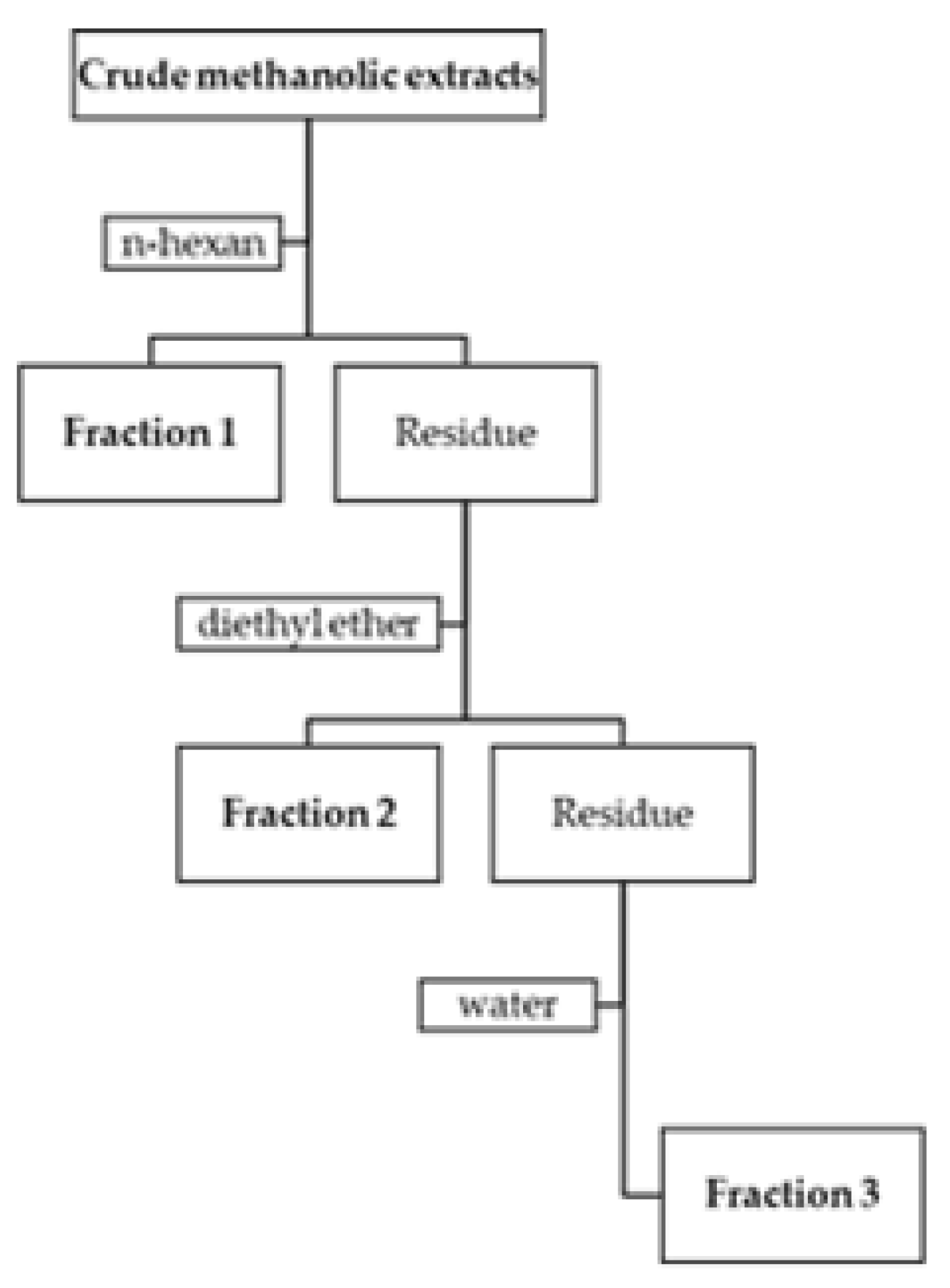
3.2. Extraction and Fractionation
3.3. Phytochemical Characterization and Phenolic Profile
3.3.1. Total Phenolic Compounds Determination
3.3.2. Flavonoids Determination
3.3.3. HPLC-DAD Analysis
3.3.4. GC-MS Analysis
3.4. Biological Activities Evaluation
3.4.1. Antioxidant Activity
DPPH Free Radical Scavenging Assay
β-Carotene Bleaching Test
3.4.2. Anti-Inflammatory Activity
3.4.3. Cytotoxicity
3.4.4. Wound-Healing Activity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bibi, T.; Ahmad, M.; Tareen, R.B.; Tareen, N.M.; Jabeen, R.; Rehman, S.U.; Sultana, S.; Zafar, M.; Yaseen, G. Ethnobotany of medicinal plants in district Mastung of Balochistan province-Pakistan. J. Ethnopharmacol. 2014, 157, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chipinga, J.V.; Kamanula, J.F.; Moyo, P.B.B. Efficacy of pterocarpus angolensis crude extracts against Candida krusei, Staphylococcus aureus, Streptococcus agalactiae and Escherichia coli. Malawi Med. J. 2018, 30, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bouasla, A.; Bouasla, I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine 2017, 36, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Camejo-Rodrigues, J.; Ascensão, L.; Bonet, M.À.; Vallès, J. An ethnobotanical study of medicinal and aromatic plants in the Natural Park of ‘Serra de São Mamede’ (Portugal). J. Ethnopharmacol. 2003, 89, 199–209. [Google Scholar] [CrossRef]
- Scartezzini, P.; Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar] [CrossRef]
- Mbosso, E.J.T.; Ngouela, S.; Nguedia, J.C.A.; Beng, V.P.; Rohmer, M.; Tsamo, E. In vitro antimicrobial activity of extracts and compounds of some selected medicinal plants from Cameroon. J. Ethnopharmacol. 2010, 128, 476–481. [Google Scholar] [CrossRef]
- Soares, M.O.; Vinha, A.F.; Coutinho, F.; Pires, P.C. Antimicrobial natural products. Formatex 2013, 2, 946–950. [Google Scholar]
- Chidumayo, E.N. Biotic interactions, climate and disturbance underlie the distribution of two Julbernardia tree species in miombo woodlands of Africa. J. Trop. Ecol. 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Shewry, P.R.; Fowden, L. 4,5-Dihydrpxypipecolic acids in the seed of Julbernardia, Isoberlinia and Brachystegia. Phytochemistry 1976, 15, 1981–1983. [Google Scholar] [CrossRef]
- Chungu, D.; Muimba-Kankolongo, A.; Roux, J.; Malambo, F.M. Bark removal for medicinal use predisposes indigenous forest trees to wood degradation in Zambia. South. Hemisph. J. 2007, 69, 157–163. [Google Scholar] [CrossRef]
- Abubakar, M.; Majinda, R. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.M.I.; Gonçalves, T.A.P.; Muñiz, G.I.B.; Matos, J.L.M.; Nisgoski, S. Mozambiques’s charcoals: Anatomy of nine native species. Bosque 2015, 36, 105–112. [Google Scholar] [CrossRef]
- Lumbile, A.U.; Kwerepe, B.C.; Kelathilwe, M. The Characteristics and Economic Importance of Pterocarpus angulensis in D. C. Botswana. Pak. J. Biol. Sci. 2007, 10, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Chichinye, A.; Geldenhuys, C.J.; Chirwa, P.W. Land-use impacts on the composition and diversity of the Baikiaea–Guibourtia–Pterocarpus woodlands of north-western Zimbabwe. South. For. J. For. Sci. 2019, 81, 151–165. [Google Scholar] [CrossRef]
- Sadiki, T.S.; Tshisikhawe, M.P. The ethnobotany of Pterocarpus angolensis DC.: A reflection of rural Venda speaking community of Gundani in Limpopo Province, South Africa. South Afr. J. Bot. 2018, 115, 308. [Google Scholar] [CrossRef]
- Samie, A.; Housein, A.; Lall, N.; Meyer, J.J.M. Crude extracts of, and purified compounds from, Pterocarpus angolensis, and the essential oil of Lippia javanica: Their in vitro cytotoxicities and activities against selected bacteria and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 2009, 103, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Lv, H.; Cao, C.; Zhang, L.; Cao, R.; Xu, B. Evaluation of antimicrobial activity of Pterocarpus extracts. Ind. Crop. Prod. 2019, 140, 111668. [Google Scholar] [CrossRef]
- Zininga, T.; Anokwuru, C.; Sigidi, M.; Tshisikhawe, M.; Ramaite, I.; Traoré, A.; Heinrich, H.; Shondai, A.; Potgieter, N. Extracts Obtained from Pterocarpus angolensis DC and Ziziphus mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function. Molecules 2017, 22, 1224. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J. Trifolium species-derived substances and extracts—Biological activity and prospects for medicinal applications. J. Ethnopharmacol. 2012, 143, 14–23. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yessoufou, K.; El-Settawy, A.A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Ereifej, K.I.; Esoh, R.B.; Al-u’datt, M.H.; Alrababah, M.A.; Yang, W. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Nat. Prod. Res. 2011, 25, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Luís, Â.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a Source of Antioxidant and Antimicrobial Polyphenols. Molecules 2014, 19, 6428–16446. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 2013. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm368107.pdf (accessed on 15 January 2020).
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Seely, K.; Lapoint, J.; Moran, J.; Fattore, L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 234–243. [Google Scholar] [CrossRef]
- Javanmardi, J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: Experiments with BHT used as standard antioxidant. Eur. Food Res. Technol. 2010, 231, 835–840. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.; Kumar, R.B.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P.K. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S976–S980. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Tiang, T.; Qi, W.Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. Engl. Ed. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Talekar, Y.P.; Apte, K.G.; Paygude, S.V.; Tondare, P.R.; Parab, P.B. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J. Ayurveda Integr. Med. 2017, 8, 73–81. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound Healing Study of Eucalyptus Essential Oil Containing Nanoemulsion in Rat Model. J. Oleo Sci. 2018, 67, 957–968. [Google Scholar] [CrossRef]
- Province Profile. Governo de Angola. 2015. Available online: http://www.huila.gov.ao/InformacoesProvinciais.aspx?tipo=Perfil (accessed on 26 February 2020).
- Parekh, J.; Jadeja, D.; Chanda, S. Efficacy of Aqueous and Methanol Extracts of Some Medicinal Plants for Potential Antibacterial Activity. Turk. J. Biol. 2005, 29, 203–210. [Google Scholar]
- Qi, G.; Yang, L.; Xiao, C.; Shi, J.; Mi, Y.; Liu, X. Nutrient values and bioactivities of the extracts from three fern species in China: A comparative assessment. Food Funct. 2015, 6, 2918–2929. [Google Scholar] [CrossRef]
- Pinho, P.M.; Naegchomnong, W.; Kijoa, A.; Nazareth, N.; Silva, A.M.S.; Eaton, G.; Herz, W. An unusual glucoside from Cleistanthus gracilis. Phytochemistry 2006, 67, 1789–1792. [Google Scholar] [CrossRef]
- Silva, O.; Viegas, S.; Mello-Sampayo, C.; Costa, M.J.P.; Serrano, R.; Cabrita, J.; Gomes, E.T. Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia 2012, 83, 872–876. [Google Scholar] [CrossRef]
- Luís, Â.; Sousa, S.; Duarte, A.P.; Pereira, L.; Domingues, F. Phytochemical characterization, and evaluation of rheological and antioxidant properties of commercially available juices of berries. J. Berry Res. 2018, 8, 11–23. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Luís, Â.; Sousa, S.; Wackerlig, J.; Dobusch, D.; Duarte, A.P.; Pereira, L.; Domingues, F. Star anise (Illicium verum Hook. f.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019, 34, 260–270. [Google Scholar]
- Luís, Â.; Breitenfeld, L.; Ferreira, S.; Duarte, A.P.; Domingues, F. Antimicrobial, antibiofilm and cytotoxic activities of Hakea sericea Schrader extracts. Pharm. Mag. 2014, 10, S6–S13. [Google Scholar]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: No compounds are available from the authors. |
| Plant Species | Plant Part | Samples | Phenolic Compounds (mg GAE/g Sample) 1 | Flavonoids (mg QE/g Sample) 1 |
|---|---|---|---|---|
| J. paniculata | Barks | Crude extract | 410.93 ± 16.72 g | 1.64 ± 0.19 a |
| Fraction 1 | 19.88 ± 2.72 a | 57.69 ± 3.25 f | ||
| Fraction 2 | 366.13 ± 7.94 f | 3.07 ± 0.18 a | ||
| Fraction 3 | 703.73 ± 13.29 h | 2.76 ± 0.02 a | ||
| Leaves | Crude extract | 77.60 ± 6.80 b | 18.65 ± 0.79 c | |
| Fraction 1 | 18.89 ± 0.84 a | 62.21 ± 0.82 g | ||
| Fraction 2 | 39.20 ± 2.26 a | 61.89 ± 2.81 f,g | ||
| Fraction 3 | 188.67 ± 4.41 c | 12.68 ± 0.18 b | ||
| P. angolensis | Barks | Crude extract | 258.40 ± 5.81 d | 2.25 ± 0.20 a |
| Fraction 1 | 6.83 ± 1.00 a | 71.97 ± 2.17 h | ||
| Fraction 2 | 173.07 ± 13.48 c | 10.79 ± 0.31 b | ||
| Fraction 3 | 305.73 ± 13.71 e | 4.47 ± 0.21 a | ||
| Leaves | Crude extract | 262.00 ± 1.70 d | 28.48 ± 1.26 d | |
| Fraction 1 | 93.33 ± 6.07 b | 68.10 ± 3.05 h | ||
| Fraction 2 | 257.00 ± 18.95 d | 45.78 ± 3.18 e | ||
| Fraction 3 | 360.93 ± 12.17 f | 23.41 ± 0.90 c,d |
| Phenolic Compounds (µg/mg Sample) | Barks | Leaves | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude Extract | Fraction 1 | Fraction 2 | Fraction 3 | Crude Extract | Fraction 1 | Fraction 2 | Fraction 3 | |
| Gallic acid | N.D. | N.D. | N.D. | N.D. | 0.13 ± 0.04 | 0.11 ± 0.03 | N.D. | 0.16 ± 0.01 |
| Chlorogenic acid | 0.21 ± 0.05 | N.D. | 0.20 ± 0.06 | 0.20 ± 0.05 | N.D. | N.D. | N.D. | N.D. |
| Caffeic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.16 ± 0.01 | N.D. |
| Vanillic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.96 ± 0.04 | N.D. |
| Syringic acid | 0.14 ± 0.04 | N.D. | N.D. | N.D. | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.15 ± 0.02 | N.D. |
| p-Coumaric acid | 0.16 ± 0.01 | N.D. | 0.20 ± 0.02 | 0.15 ± 0.01 | N.D. | N.D. | 0.54 ± 0.03 | 0.18 ± 0.02 |
| Taxifolin | 0.07 ± 0.01 | N.D. | 0.16 ± 0.04 | 0.11 ± 0.01 | 0.12 ± 0.02 | N.D. | N.D. | N.D. |
| Rutin | N.D. | N.D. | 0.11 ± 0.02 | N.D. | N.D. | N.D. | 0.90 ± 0.15 | 0.39 ± 0.03 |
| Ferulic acid | 0.28 ± 0.02 | N.D. | 0.28 ± 0.02 | N.D. | 0.32 ± 0.03 | 0.28 ± 0.01 | 0.87 ± 0.02 | 0.38 ± 0.02 |
| Ellagic acid | N.D. | N.D. | 0.09 ± 0.01 | N.D. | 0.15 ± 0.03 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.14 ± 0.02 |
| Rosmarinic acid | 0.08 ± 0.01 | N.D. | 0.12 ± 0.03 | 0.08 ± 0.01 | N.D. | 0.07 ± 0.01 | 0.12 ± 0.02 | 0.07 ± 0.01 |
| Quercetin | 0.25 ± 0.03 | N.D. | 0.20 ± 0.01 | 0.19 ± 0.02 | N.D. | 0.18 ± 0.02 | N.D. | N.D. |
| Total | 1.18 ± 0.04 | 0.00 ± 0.00 | 1.36 ± 0.01 | 0.73 ± 0.09 | 0.84 ± 0.01 | 0.81 ± 0.08 | 3.78 ± 0.22 | 1.33 ± 0.09 |
| Phenolic Compounds (µg/mg Sample) | Barks | Leaves | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude Extract | Fraction 1 | Fraction 2 | Fraction 3 | Crude Extract | Fraction 1 | Fraction 2 | Fraction 3 | |
| Gallic acid | 0.13 ± 0.01 | N.D. | 0.15 ± 0.03 | 0.12 ± 0.01 | N.D. | N.D. | N.D. | N.D. |
| Chlorogenic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Caffeic acid | 0.13 ± 0.02 | N.D. | 0.21 ± 0.01 | 0.13 ± 0.02 | 0.15 ± 0.01 | N.D. | N.D. | N.D. |
| Vanillic acid | N.D. | N.D. | N.D. | N.D. | 0.07 ± 0.01 | N.D. | 0.91 ± 0.07 | N.D. |
| Syringic acid | 0.11 ± 0.03 | N.D. | 0.14 ± 0.03 | N.D. | N.D. | N.D. | N.D. | N.D. |
| p-Coumaric acid | N.D. | N.D. | 0.17 ± 0.03 | N.D. | 0.34 ± 0.04 | N.D. | 0.39 ± 0.03 | 0.28 ± 0.04 |
| Taxifolin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Rutin | N.D. | N.D. | N.D. | N.D. | 39.87 ± 4.43 | 12.07 ± 1.44 | 14.50 ± 0.28 | 17.83 ± 2.64 |
| Ferulic acid | 0.27 ± 0.04 | 0.28 ± 0.05 | 0.34 ± 0.05 | N.D. | N.D. | N.D. | 0.57 ± 0.03 | N.D. |
| Ellagic acid | N.D. | N.D. | N.D. | N.D. | N.D. | 0.11 ± 0.01 | 0.28 ± 0.02 | N.D. |
| Rosmarinic acid | N.D. | N.D. | 0.18 ± 0.01 | 0.06 ± 0.01 | 0.43 ± 0.01 | N.D. | 0.71 ± 0.06 | N.D. |
| Quercetin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Total | 0.65 ± 0.01 | 0.28 ± 0.05 | 1.18 ± 0.03 | 0.31 ± 0.05 | 40.87 ± 4.47 | 12.18 ± 1.42 | 17.35 ± 0.12 | 18.12 ± 2.60 |
| Retention Time (min) | Compounds | Peak Area (% of Total) |
|---|---|---|
| 3.416 | Linalool oxide | 0.331 |
| 4.276 | 2,6-Di(t-butyl)-4-hydroxy-4-methyl-2,5-cyclohexadiene-1-one | 2.638 |
| 4.792 | Phenol | 3.076 |
| 5.223 | 2(4H)-Benzofuranone | 0.348 |
| 6.458 | 1-Hexadecene | 0.637 |
| 8.129 | 1-(P-Methoxyphenyl)-2-methoxyprop-1-ene | 0.729 |
| 8.597 | Loliolide | 1.849 |
| 8.890 | Atropine | 0.651 |
| 8.939 | Megastigma-5,7-diene-3,4,9-triol | 0.580 |
| 9.594 | Neophytadiene | 6.087 |
| 10.726 | 1,4-Dihydrophenanthrene | 0.506 |
| 11.085 | Hexadecanoic acid | 0.479 |
| 11.793 | 1,2-Benzenedicarboxylic acid | 0.317 |
| 13.224 | 4-Oxazolecarboxylic acid | 0.135 |
| 13.447 | Menthol | 0.545 |
| 14.164 | Linolenic acid methyl ester | 0.253 |
| 14.367 | Phytol | 1.996 |
| 15.501 | Dodecanamide | 0.383 |
| 18.427 | Linoleic acid | 0.485 |
| 18.521 | 9-Octadecenamide | 3.376 |
| 21.848 | Medicarpin | 0.690 |
| 22.021 | 6H-Benzofuro [3,2-c][1]benzopyran-6a(11aH)-ol | 0.377 |
| 26.436 | 1-Docosene | 0.407 |
| 31.523 | Stigmasta-5,22-dien-3-ol | 1.003 |
| 32.557 | 23S-Ethylcholest-5-en-3-β-ol | 6.932 |
| 32.721 | 3-Keto-urs-12-ene | 3.758 |
| 32.830 | Alnulin | 1.112 |
| 33.171 | β-Amyrin | 5.714 |
| 33.646 | D:C-Friedoolean-8-en-3-one | 17.602 |
| 34.114 | Lup-20(29)-en-3β-ol | 28.860 |
| Retention Time (min) | Compounds | Peak Area (% of Total) |
|---|---|---|
| 9.590 | Neophytadiene | 0.584 |
| 11.087 | 7,9-Di-tert-butyl-1-oxaspiro [4.5]deca-6,9-diene-2,8-dione | 0.275 |
| 12.092 | Eicosamethylcyclodecasiloxane | 0.901 |
| 15.483 | Hexadecanamide | 0.257 |
| 17.246 | Eseroline | 1.191 |
| 18.518 | 9-Octadecenamide | 3.303 |
| 20.845 | Hexadecanoic acid | 1.409 |
| 32.534 | Pregn-5-en-3-ol | 1.578 |
| 34.079 | Lup-20(29)-en-3β-ol | 10.332 |
| Plant Species | Plant Part | Samples | DPPH Free Radical Scavenging Assay | β-Carotene Bleaching Test | ||
|---|---|---|---|---|---|---|
| IC50 (mg/L) 1 | AAI 1 | Antioxidant Activity | IC50 (mg/L) 1 | |||
| J. paniculata | Barks | Crude extract | 5.51 ± 0.93 a | 8.21 ± 0.19 e | Very strong | 422.67 ± 18.53 a |
| Fraction 1 | 39.06 ± 7.91 a | 1.15 ± 0.19 a | Strong | 1350.79 ± 287.89 a,b,c | ||
| Fraction 2 | 6.60 ± 1.56 a | 6.52 ± 0.52 d | Very strong | 493.19 ± 34.15 a,b | ||
| Fraction 3 | 7.35 ± 1.20 a | 6.86 ± 0.44 d | Very strong | 533.83 ± 1.55 a,b | ||
| Leaves | Crude extract | 57.62 ± 7.41 ab | 0.75 ± 0.05 a | Moderate | 1231.00 ± 269.36 a,b,c | |
| Fraction 1 | 184.54 ± 36.43 c | 0.28 ± 0.05 a | Poor | 8222.58 ± 186.22 f | ||
| Fraction 2 | 68.71 ± 14.93 a,b,c | 0.64 ± 0.08 a | Moderate | 1504.58 ± 300.00 b,c | ||
| Fraction 3 | 48.97 ± 9.09 a,b | 1.02 ± 0.11 a,b | Strong | 1188.85 ± 229.66 a,b,c | ||
| P. angolensis | Barks | Crude extract | 7.11 ± 1.19 a | 6.21 ± 0.33 d | Very strong | 526.91 ± 101.74 a,b |
| Fraction 1 | 165.84 ± 7.09 b,c | 0.33 ± 0.08 a | Poor | 4396.88 ± 226.40 e | ||
| Fraction 2 | 74.39 ± 9.01 a,b,c | 0.63 ± 0.05 a | Moderate | 3021.61 ± 522.29 d | ||
| Fraction 3 | 11.30 ± 1.90 a | 4.46 ± 0.16 c | Very strong | 2162.85 ± 44.19 c,d | ||
| Leaves | Crude extract | 8.67 ± 1.22 a | 5.09 ± 0.32 c | Very strong | 578.55 ± 18.85 a,b | |
| Fraction 1 | 21.33 ± 3.19 a | 2.00 ± 0.43 b | Strong | 1480.34 ± 252.16 a,b,c,f | ||
| Fraction 2 | 10.46 ± 2.38 a | 4.29 ± 0.02 c | Very strong | 757.36 ± 68.61 a,b | ||
| Fraction 3 | 10.45 ± 1.74 a | 4.72 ± 0.11 c | Very strong | 583.24 ± 130.35 a,b | ||
| Plant Species | Plant Part | Samples | Anti-Inflammatory Activity—IC50 (mg/L) 1 | Cytotoxicity | ||
|---|---|---|---|---|---|---|
| Concentration (mg/L) | Cell Viability (%) 2 | p-Value 3 | ||||
| Negative Control a | 100 (100–100) | - | ||||
| J. paniculata | Barks | Crude extract | 784.24 ± 73.25 a | 250 b | 74.90 (71.95–91.91) | 0.037 a,b * |
| 500 c | 103.62 (70.34–112.75) | 0.487 a,c | ||||
| 750 d | 74.09 (70.87–94.23) | 0.037 ad * | ||||
| Fraction 3 | 274.86 ± 30.12 b | 250 e | 76.24 (64.97–87.52) | 0.053 a,e | ||
| 500 f | 82.69 (68.99–91.54) | 0.037 a,f * | ||||
| 750 g | 77.05 (70.87–83.22) | 0.053 a,g | ||||
| P. angolensis | Leaves | Crude extract | N.D. | 250 h | 59.60 (50.20–64.70) | 0.037 a,h * |
| 500 i | 54.50 (48.05–75.44) | 0.037 a,i * | ||||
| 750 j | 53.69 (51.01–63.90) | 0.037 a,j * | ||||
| Fraction 3 | N.D. | 250 k | 63.89 (52.89–81.88) | 0.037 a,k * | ||
| 500 l | 58.79 (56.11–66.56) | 0.037 a,l * | ||||
| 750 m | 91.01 (84.30–99.33) | 0.037 a,m * | ||||
| Plant Species | Plant Part | Samples | Concentration (mg/L) | Mean Difference (Negative Control—Sample) | 95% C.I. | p-Value |
|---|---|---|---|---|---|---|
| J. paniculata | Barks | Crude extract | 250 | 0.544 | 0.297–0.792 | <0.001 * |
| 500 | 0.422 | 0.175–0.670 | <0.001 * | |||
| 750 | 0.333 | 0.086–0.581 | 0.001 * | |||
| Fraction 3 | 250 | 0.644 | 0.397–0.892 | <0.001 * | ||
| 500 | 0.533 | 0.286–0.781 | <0.001 * | |||
| 750 | 0.656 | 0.408–0.903 | <0.001 * | |||
| P. angolensis | Leaves | Crude extract | 250 | 0.344 | 0.097–0.592 | 0.001 * |
| 500 | 0.300 | 0.053–0.547 | 0.006 * | |||
| 750 | 0.389 | 0.142–0.636 | <0.001 * | |||
| Fraction 3 | 250 | 0.622 | 0.375–0.870 | <0.001 * | ||
| 500 | 1.189 | 0.942–1.436 | <0.001 * | |||
| 750 | 0.656 | 0.408–0.903 | <0.001 * |
| Representative Image of the Cells at the Initial Moment (0 h) | Samples | 2 h | 24 h |
|---|---|---|---|
 | Negative control | 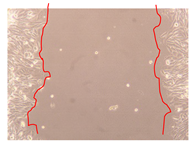 |  |
| J. paniculata barks crude extract (250 mg/L) | 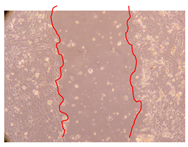 | 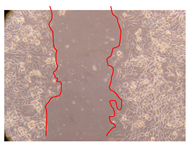 | |
| J. paniculata barks crude extract (500 mg/L) |  |  | |
| J. paniculata barks crude extract (750 mg/L) |  |  | |
| J. paniculata barks Fraction 3 (250 mg/L) | 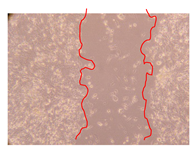 |  | |
| J. paniculata barks Fraction 3 (500 mg/L) |  | 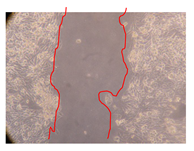 | |
| J. paniculata barks Fraction 3 (750 mg/L) |  | 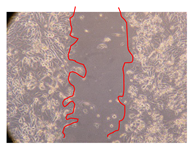 |
| Samples | 2 h | 24 h |
|---|---|---|
| P. angolensis leaves crude extract (250 mg/L) | 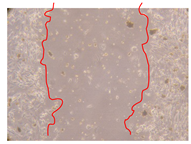 |  |
| P. angolensis leaves crude extract (500 mg/L) | 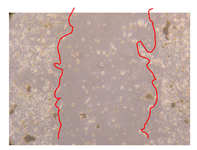 | 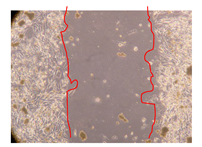 |
| P. angolensis leaves crude extract (750 mg/L) |  |  |
| P. angolensis leaves Fraction 3 (250 mg/L) |  |  |
| P. angolensis leaves Fraction 3 (500 mg/L) | 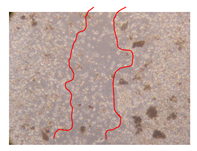 |  |
| P. angolensis leaves Fraction 3 (750 mg/L) | 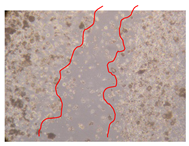 | 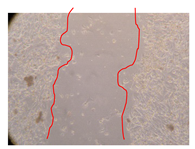 |
| Phenolic Compounds | Retention Time (min) | Wavelength (nm) |
|---|---|---|
| Gallic acid | 3.826 | 280 |
| Chlorogenic acid | 10.710 | 322 |
| Caffeic acid | 13.540 | 322 |
| Vanillic acid | 13.597 | 263 |
| Syringic acid | 16.171 | 280 |
| p-Coumaric acid | 20.774 | 291 |
| Taxifolin | 24.973 | 291 |
| Rutin | 25.569 | 255 |
| Ferulic acid | 25.865 | 322 |
| Ellagic acid | 27.266 | 255 |
| Rosmarinic acid | 32.852 | 322 |
| Quercetin | 41.964 | 360 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia paniculata and Pterocarpus angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation. Molecules 2020, 25, 1828. https://doi.org/10.3390/molecules25081828
Santos ES, Luís Â, Gonçalves J, Rosado T, Pereira L, Gallardo E, Duarte AP. Julbernardia paniculata and Pterocarpus angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation. Molecules. 2020; 25(8):1828. https://doi.org/10.3390/molecules25081828
Chicago/Turabian StyleSantos, Eugénia Solange, Ângelo Luís, Joana Gonçalves, Tiago Rosado, Luísa Pereira, Eugenia Gallardo, and Ana Paula Duarte. 2020. "Julbernardia paniculata and Pterocarpus angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation" Molecules 25, no. 8: 1828. https://doi.org/10.3390/molecules25081828
APA StyleSantos, E. S., Luís, Â., Gonçalves, J., Rosado, T., Pereira, L., Gallardo, E., & Duarte, A. P. (2020). Julbernardia paniculata and Pterocarpus angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation. Molecules, 25(8), 1828. https://doi.org/10.3390/molecules25081828









