Development and Study of Semi-Solid Preparations Containing the Model Substance Corticotropin (ACTH): Convenience Application in Neurodegenerative Diseases
Abstract
1. Introduction
2. Results and Discussion
2.1. Ointment pH Measurements by the Potentiometric Method
2.2. Spreadability of the Ointments
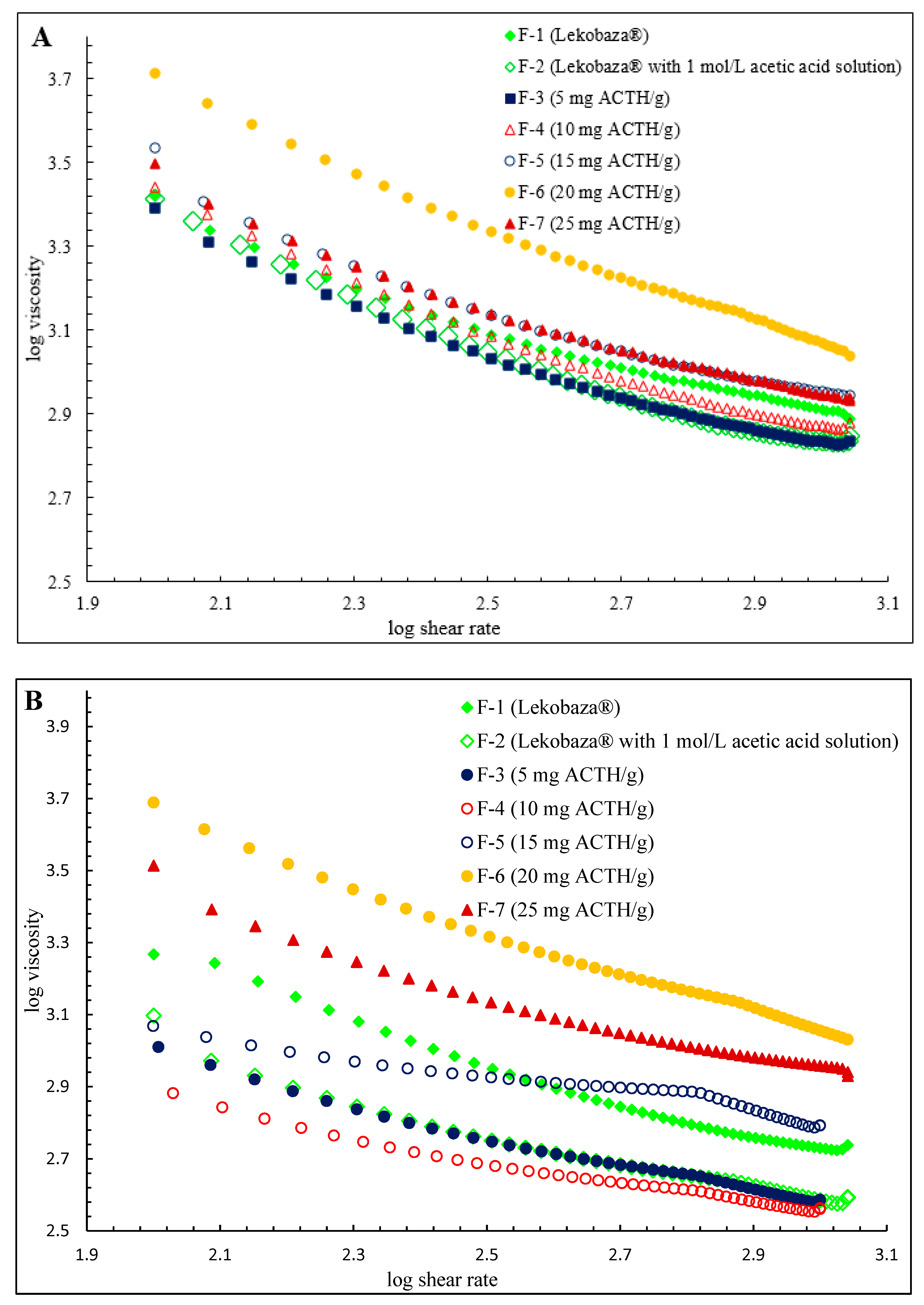
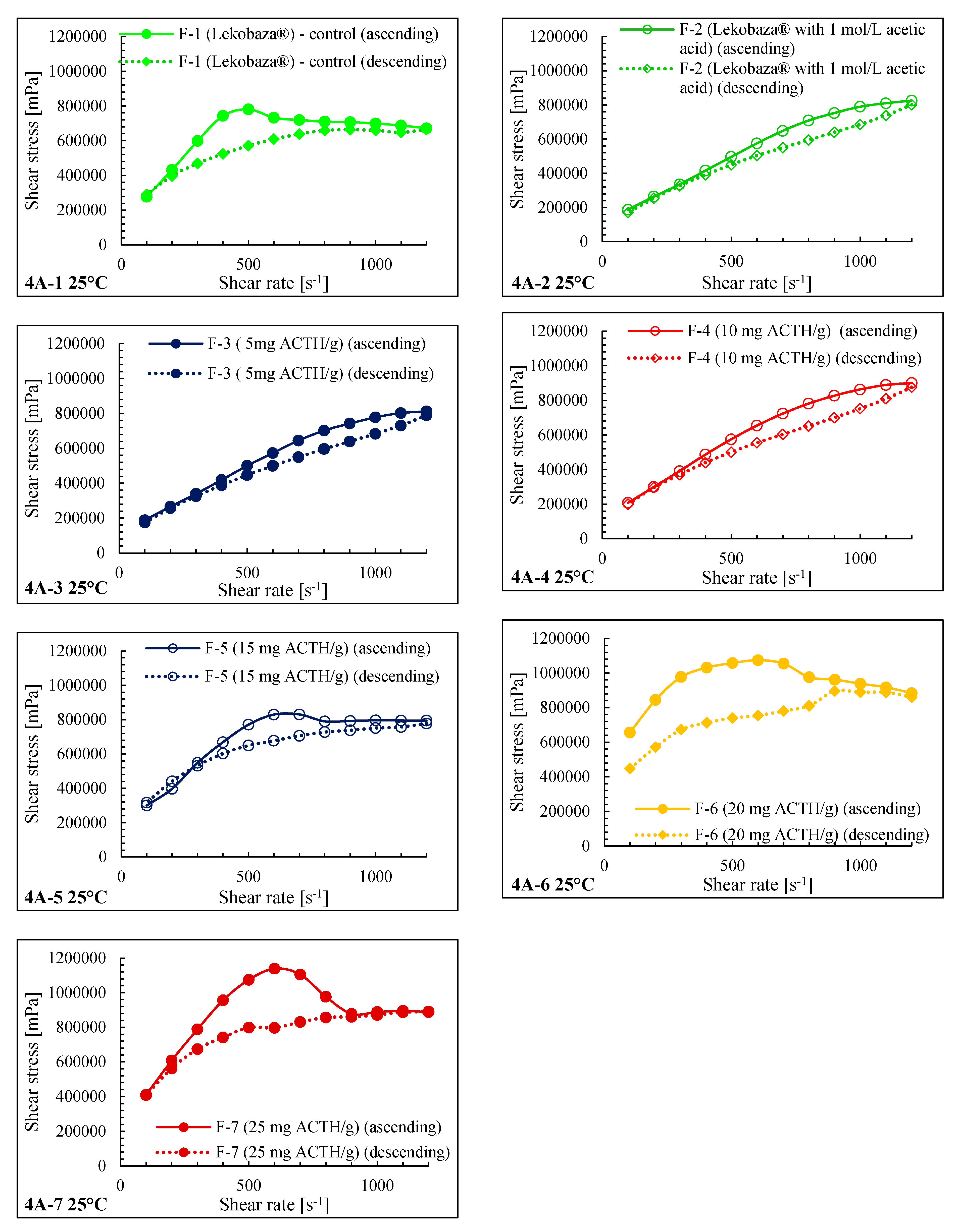
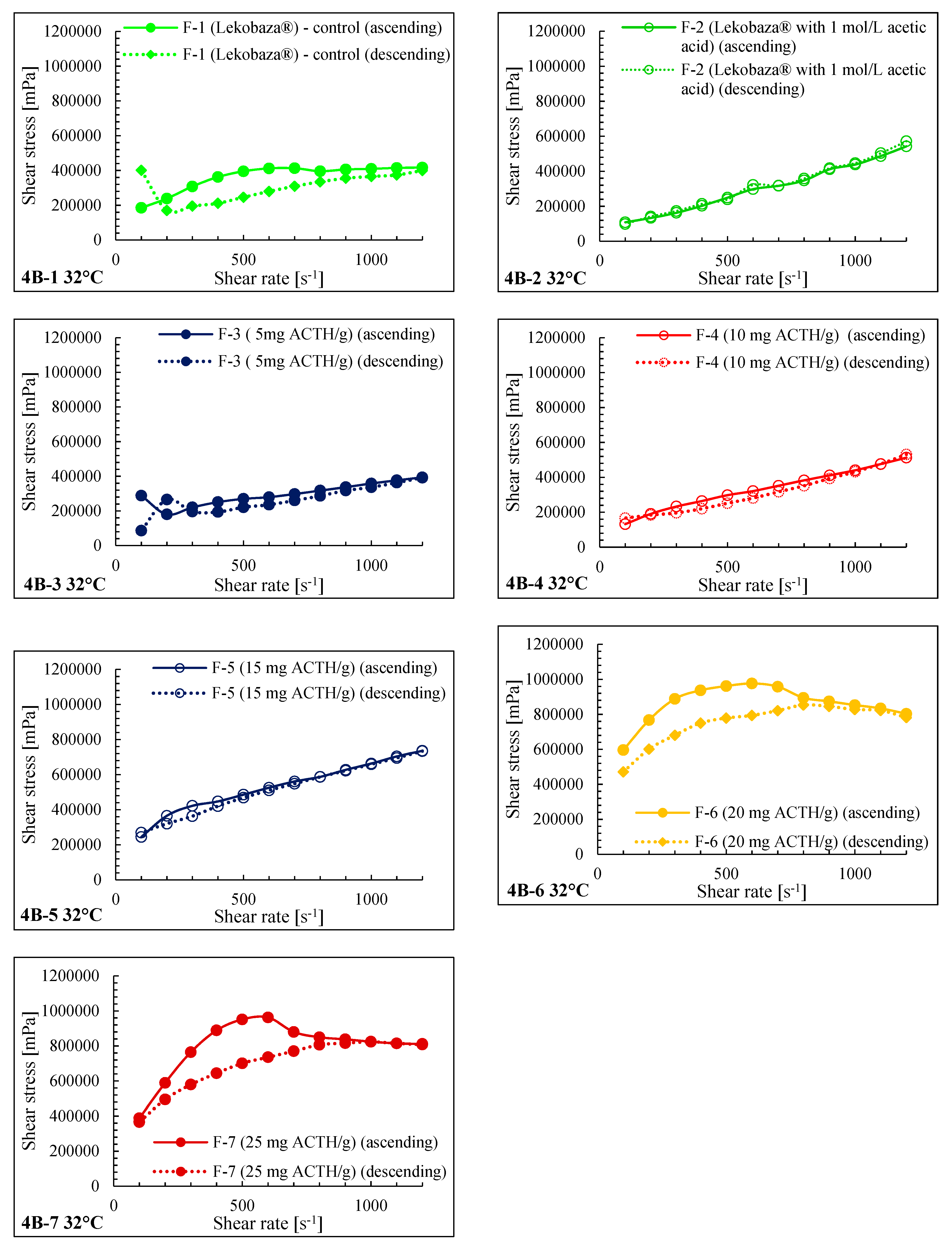
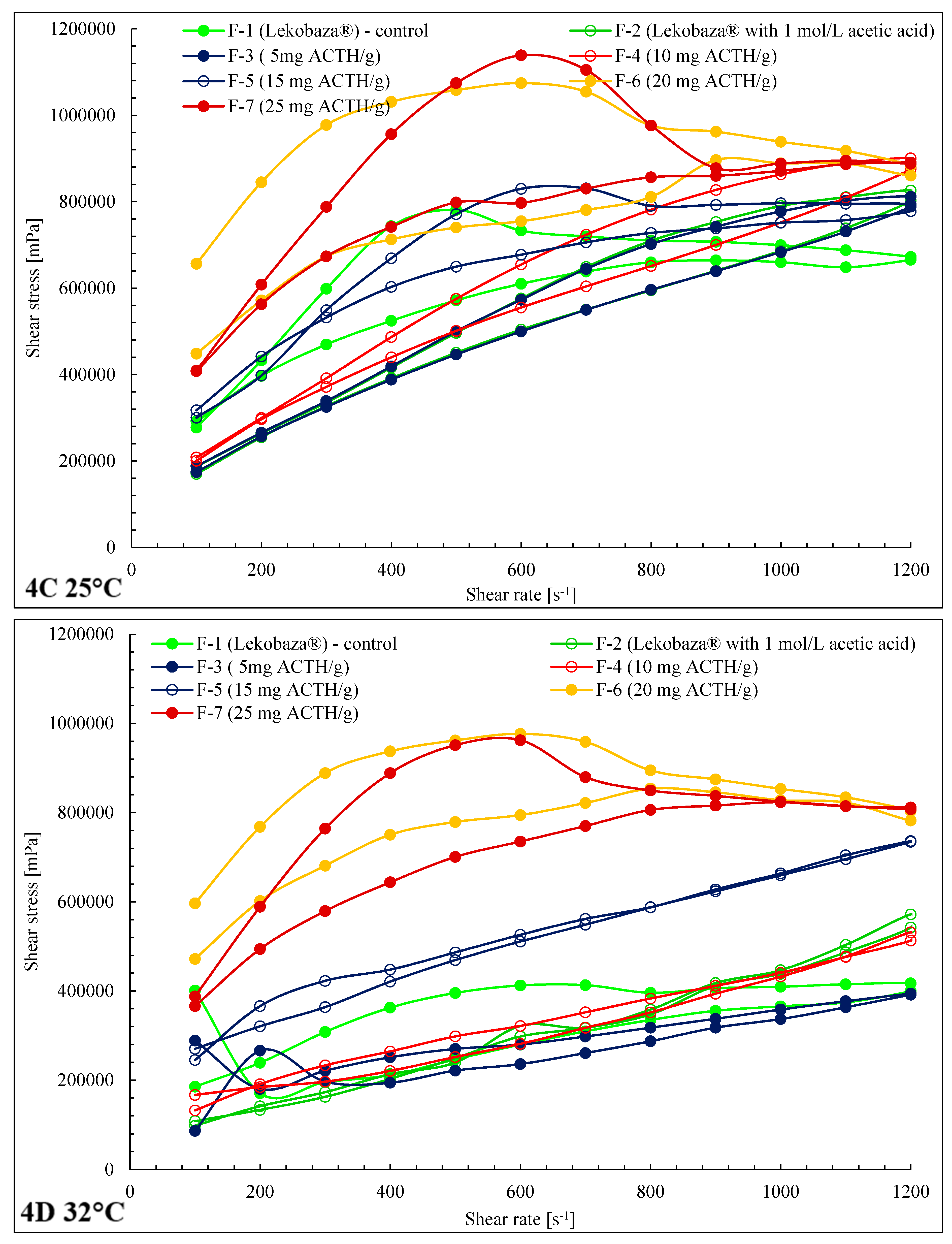
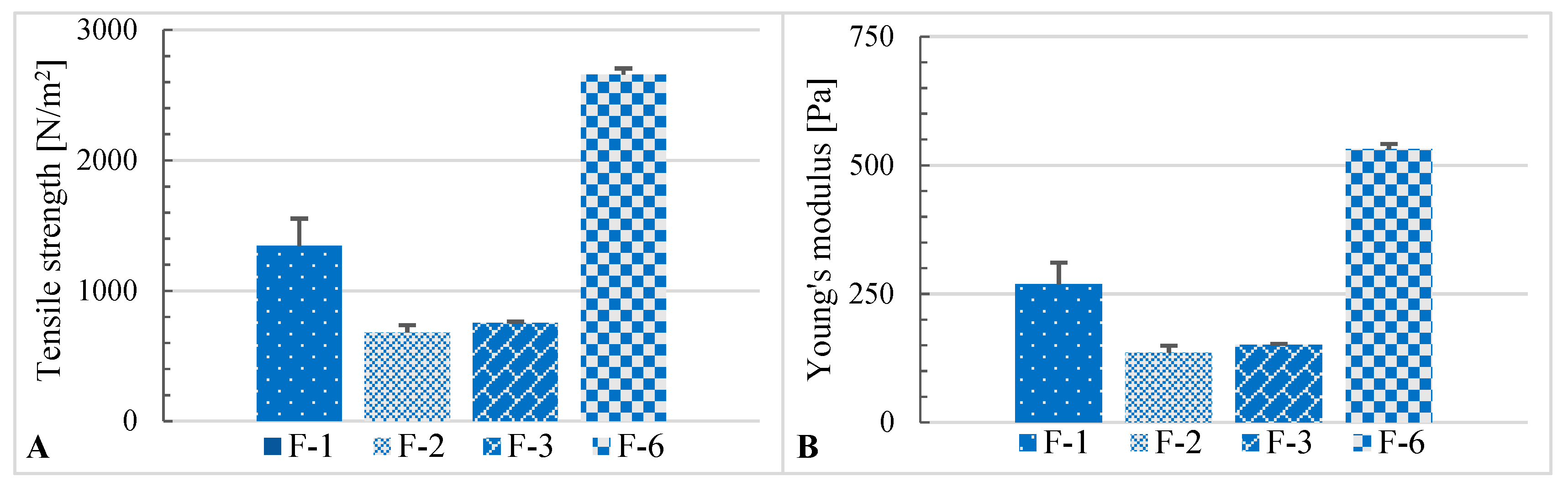
2.3. Rheological Properties
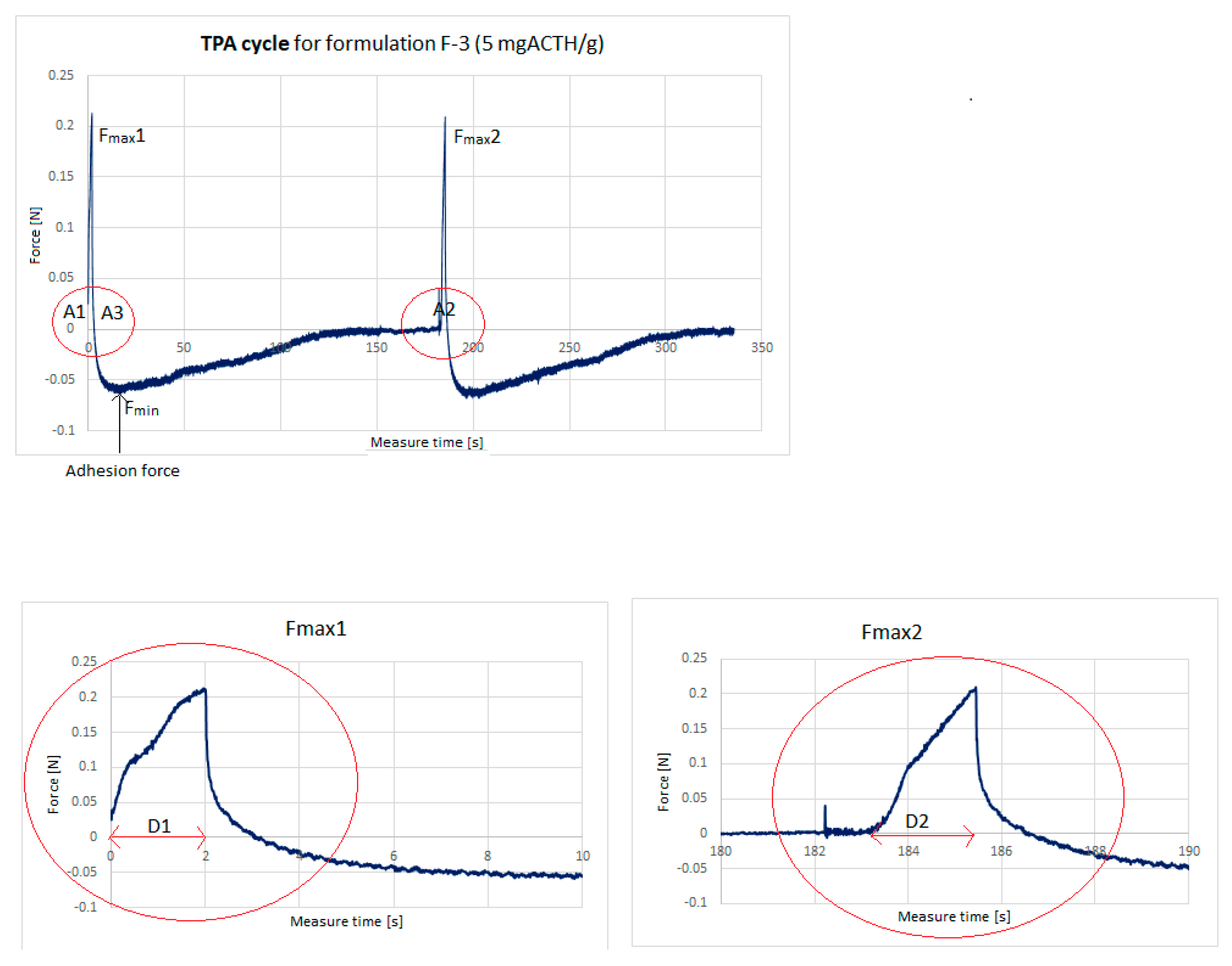
2.4. Texture Analysis
- Hardness (the force required to attain a given deformation) was found from maximum force Fmax1.
- Cohesiveness (the ratio of the area under the force–time curve produced on the second compression cycle to that on the first compression cycle, where successive compressions are separated by a defined recovery period).
- Adhesiveness (the work required to overcome the attractive forces between the surface of the sample and the surface of the probe).
- Elasticity: The D2/D1 ratio between two compression phase distances.
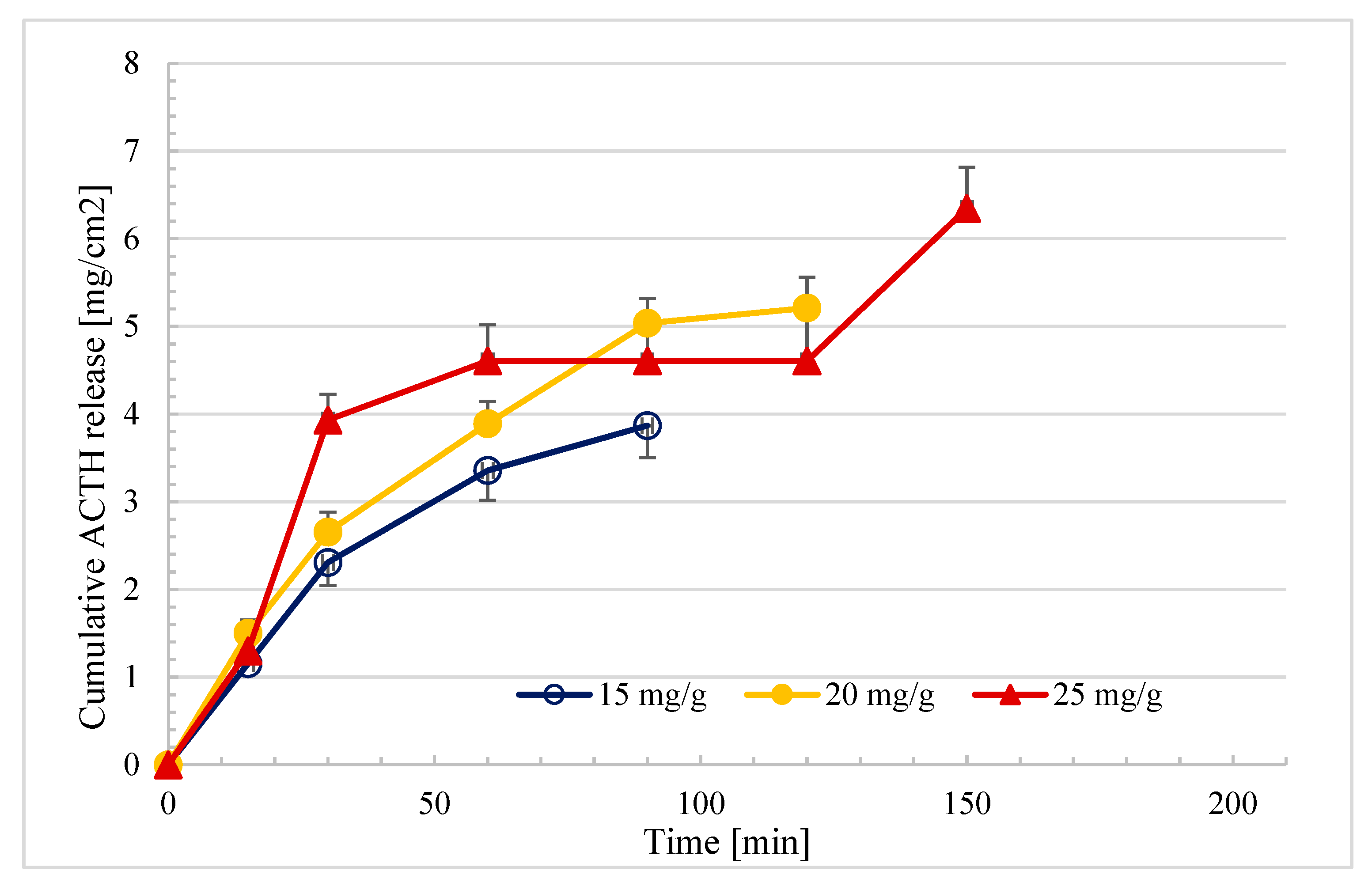
2.5. In Vitro Drug Release Profiles of the Ointments
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ointment with Corticotropin
3.3. pH Measurement
3.4. Spreadability
3.5. Rheological Experiments
3.6. Texture Analysis
3.7. In Vitro Drug Release from the Ointments
3.7.1. Methodology of ACTH Determination
3.7.2. ACTH Release from Ointment Formulations in Vitro
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- N’Da, D.D. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.; Woolfson, A.D. Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Wiley: Hoboken, NJ, USA, 2012; p. 86. [Google Scholar]
- Kretsos, K.; Kasting, G.B. A Geometrical Model of Dermal Capillary Clearance. Math. Biosci. 2007, 208, 430–453. [Google Scholar] [CrossRef] [PubMed]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin Permeabilization for Transdermal Drug Delivery: Recent Advances and Future Prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- Han, T.; Das, D.B. Potential of Combined Ultrasound and Microneedles for Enhanced Transdermal Drug Permeation: A Review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef]
- Brambilla, D.; Luciani, P.; Leroux, J. Break through Discoveries in Drug Delivery Technologies: The Next 30 years. J. Control. Release 2014, 190, 9–14. [Google Scholar] [CrossRef]
- Schuetz, Y.B.; Naik, A.; Guy, R.H.; Kalia, Y.N. Emerging Strategies for the Transdermal Delivery of Peptide and Protein Drugs. Expert Opin. Drug Deliv. 2005, 2, 533–548. [Google Scholar] [CrossRef]
- Shahzad, Y.; Louw, R.; Gerber, M.; du Plessis, J. Breaching the Skin Barrier through Temperature Modulations. J. Control. Release 2015, 202, 1–13. [Google Scholar] [CrossRef]
- Subramony, J.A. Needle Free Parenteral Drug Delivery: Leveraging active transdermal technologies for pediatric use. Int. J. Pharm. 2013, 455, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Guy, R.H. Transdermal Drug Delivery: 30 Years of War and Still Fighting. J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Jasti, B.R. Theory and Practice of Contemporary Pharmaceutics; CRC press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Li, N.; Larin, E.M.; Kerman, K. A Miniaturized Impedimetric Immunosensor for the Competitive Detection of Adrenocorticotropic Hormone. Sensors 2017, 17, 2836. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Payet, N. 60 Years of POMC: Adrenal and extra-adrenal functions of ACTH. J. Mol. Endocrinol. 2016, 56, 135–156. [Google Scholar] [CrossRef]
- Clark, A.J.; Forfar, R.; Hussain, M.; Jerman, J.; McIver, E.; Taylor, D.; Chan, L. ACTH Antagonists. Front. Endocrinol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Fleseriu, M.; Findling, J.W.; Koch, C.A.; Schlaffer, S.M.; Buchfelder, M.; Gross, C. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing’s disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J. Clin. Endocrinol. Metab. 2014, 99, 3718–3727. [Google Scholar] [CrossRef]
- Montero-Melendez, T. ACTH: The forgotten therapy. Semin. Immunol. 2015, 27, 216–226. [Google Scholar] [CrossRef]
- Philbin, M.; Niewoehner, J.; Wan, G.J. Clinical and Economic Evaluation of Repository Corticotropin Injection: A Narrative Literature Review of Treatment Efficacy and Healthcare Resource Utilization for Seven Key Indications. Adv. Ther. 2017, 34, 1775–1790. [Google Scholar] [CrossRef]
- Lorusso, P.; Bottai, A.; Mangione, E.; Innocenti, M.; Cupisti, A.; Egidi, M.F. Low-dose synthetic adrenocorticotropic hormone-analog therapy for nephrotic patients: Results from a single-center pilot study. Int. J. Nephrol. Renovasc. Dis. 2015, 8, 7–12. [Google Scholar] [CrossRef][Green Version]
- O’Neil, M.J. The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals; MerckandCo Inc.: Whitehouse Station, NJ, USA, 2006; p. 24. [Google Scholar]
- Acthar gel. Available online: https://www.acthar.com/pdf/Acthar-PI.pdf (accessed on 10 April 2019).
- Cork, M.J.; Danby, S. Skin barrier breakdown: A renaissance in emollient therapy. Br. J. Nurs. 2009, 18, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, J. Formulating at pH 4-5: How lower pH benefits the skin and formulations. Cosmet. Toilet. 2008, 123, 61–70. [Google Scholar]
- Panther, D.; Jacob, S. The importance of acidification in atopic eczema: An underexplored avenue for treatment. J. Clin. Med. 2015, 4, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. Influence of aqueous cream BP on corneocyte size, maturity, skin protease activity, protein content and transepidermal water loss. Br. J. Dermtol. 2011, 164, 1304–1310. [Google Scholar] [CrossRef]
- Antonijević, M.D.; Owusu-Ware, S.; Sanchon-Lopez, B. Emollient product design: Objective measurements of formulation structure, texture and performance, and subjective assessments of user acceptability. Clin. Exp. Dermtol. 2018, 43, 423–429. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Dolińska, B.; Kołodziejska, J.; Ryszka, F. Influence of plasma on the physical properties of ointments with quercetin. Acta Pharm. 2017, 67, 569–578. [Google Scholar] [CrossRef]
- Kolpakova, O.A.; Kucherenko, N.V.; Kukhtenko, H.P. Research of rheological properties of ointment with water soluble protein-polysaccharide complex of oyster mushroom. J. Pharm. Sci. Res. 2019, 11, 1880–1883. [Google Scholar]
- Hegdahl, T.; Gjerdet, N.R. Flowing of light-bodied elastic impression materials. Acta Odontol. Scand. 1981, 39, 33–38. [Google Scholar] [CrossRef]
- Vennat, B.; Gross, D.; Pourrat, A. Procyanidin gels based on cellulose and carrageenan derivatives. Drug Dev. Ind. Pharm. 1992, 18, 1535–1548. [Google Scholar] [CrossRef]
- Tal-Figiel, B.; Kwiecień, M.; Figiel, W. The influence of production process conditions on the rheological properties of pastes. Tech. Trans. Mech. 2012, 109, 243–248. [Google Scholar]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, Z.; Li, D.; Wu, M. Effects of Ball Milling Processes on the Microstructure and Rheological Properties of Microcrystalline Cellulose as a Sustainable Polymer Additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Agume, A.; Njintang, N.; Mbofung, C. Effect of Soaking and Roasting on the Physicochemical and Pasting Properties of Soybean Flour. Foods 2017, 6, 12. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Jadach, B.; Ancukiewicz, K.; Gadziński, P.; Wagner, D.; Białas, W. Badaniareologiczne I analiza tekstury termowrażliwych hydrożeli dopochwowych z chlorowodorkiem benzydaminy. Farm. Współczesna 2018, 11, 72–82. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F.; O’Neill, M.J. Mucoadhesive, syringeable drug delivery system for controlled application of metronidazole to the periodontal pocket: In vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J. Control. Rel. 1997, 49, 71–79. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Mishra, M.K.; Pathak, S.; Kesharwani, A.; Kesharwani, A. Semi solid dosage forms manufacturing: Tools, critical process parameters, strategies, optimization and recent advances. Indo Am. J. Pharm. Res. 2017, 7, 882–893. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Texture analysis and flow rheometry of novel, bioadhesive, antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef]
- Wróblewska, M.; Słyż, J.; Winnicka, K. Rheological and textural properties of hydrogels, containing sulfur as a model drug, made using different polymers types. Polimery 2019, 64, 208–215. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of Semisolid Formulations an Update. Pharm. Technol. 2002, 26, 84–105. [Google Scholar]
- Dash, S.; Murthy, P.M.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complexrate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Shah, V.P.; Elkins, J.; Lam, S.Y.; Skelly, J.P. Determination of in vitro drug release from hydrocortisone creams. Int. J. Pharm. 1989, 53, 53–59. [Google Scholar] [CrossRef]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, A.; Turakka, L.; Raty, T.; Lehmussaari, K. Release of hydrocortisone from topical semi solid preparations using three different in vitro methods. Acta Pharm. Fenn. 1989, 98, 93–99. [Google Scholar]
- Klein, R.R.; Bechtel, J.L.; Burchett, K.; Thakker, K.D. Technical Note: Hydrocortisone as a Performance Verification Test Reference Standard for In Vitro Release Testing. Dissolution Technol. 2010, 11, 37–38. [Google Scholar] [CrossRef]
- Choi, J.; Choi, M.K.; Chong, S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Effect of fatty acids on the transdermal delivery of donepezil: In vitro and in vivo evaluation. Int. J. Pharm. 2012, 422, 83–90. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Malinowska, M.; Sikora, E.; Ogonowski, J. Transport przeznaskórkowy aktywnych składników kosmetycznych. Wiadomości Chem. 2013, 67, 1–24. [Google Scholar]
- Office for Registration of Medicinal Products, Medical Devices & Biocidal Products. Polish Pharmacopoeia XI; Polskie Towarzystwo Farmaceutyczne: Warszawa, Poland, 2017; ISBN 978-837-887-509-3. [Google Scholar]
Sample Availability: Samples of the compounds of prepared ointments are available from the authors. |



| Formulation | F-1 | F-2 | F-3 | F-4 | F-5 | F-6 | F-7 |
|---|---|---|---|---|---|---|---|
| pH Value | 6.30 ± 0.02 | 3.43 ± 0.02 | 3.61 ± 0.07 | 3.79 ± 0.04 | 3.86 ± 0.02 | 3.92 ± 0.02 | 4.00 ± 0.02 |
| Formulation | R2 | Surface Area (cm2) | i(S) | |
|---|---|---|---|---|
| F-1 | 20.899ln(x) − 92.743 | 0.943 | 19,281.4 | - |
| F-2 | 21.851ln(x) − 93.754 | 0.933 | 21,786.7 | 1.130 |
| F-3 | 19.898ln(x) − 82.552 | 0.931 | 21,246.4 | 1.102 |
| F-4 | 16.791ln(x) − 67.144 | 0.942 | 19,111.5 | 0.991 |
| F-5 | 20.814ln(x) − 89.592 | 0.957 | 20,523.5 | 1.064 |
| F-6 | 20.474ln(x) − 86.238 | 0.937 | 21,215.6 | 1.100 |
| F-7 | 17.752ln(x) − 80.112 | 0.966 | 15,660.8 | 0.812 |
| Formu-Lation | Shear Rate | |||||
|---|---|---|---|---|---|---|
| 300 s−1 | 700 s−1 | 1100 s−1 | ||||
| Viscosity (mPa·s) at 25 °C | Viscosity (mPa·s) at 32 °C | Viscosity (mPa·s) at 25 °C | Viscosity (mPa·s) at 32 °C | Viscosity (mPa·s) at 25 °C | Viscosity (mPa·s) at 32 °C | |
| F-1 | 1907 ± 43 | 754 ± 13 | 1257 ± 26 | 528 ± 42 | 741 ± 21 | 471 ± 8 |
| F-2 | 1019 ± 74 * | 597 ± 7 * | 747 ± 25 * | 435 ± 2 * | 611 ± 16 * | 407 ± 12 * |
| F-3 | 1102 ± 28 * | 584 ± 10 * | 738 ± 22 * | 423 ± 45 * | 596 ± 12 * | 397 ± 12 * |
| F-4 | 1408 ± 57 * | 606 ± 31 * | 809 ± 16 * | 439 ± 7 * | 651 ± 10 * | 401 ± 2 * |
| F-5 | 1622 ± 13 * | 1423 ± 14 * | 1096 ± 56 * | 984 ± 25 * | 877 ± 19 * | 644 ± 29 * |
| F-6 | 2749 ± 63 * | 2272 ± 51 * | 1295 ± 137* | 1148 ± 10 * | 914 ± 44 * | 858 ± 13 * |
| F-7 | 2071 ± 89 * | 1853 ± 32 * | 1364 ± 88* | 1236 ± 25 * | 798 ± 26 * | 782 ± 5 * |
| Formulation | Time (s) | Hardness (N) | Cohesiveness | Adhesiveness (mJ) | Elasticity | Adhesion Force Fmin (N) |
|---|---|---|---|---|---|---|
| F-1 | 336 | 0.419 ± 0.070 | 1.107 ± 0.050 | 0.567 ± 0.058 | 1.130 ± 0.026 | −0.130 ± 0.003 |
| F-2 | 335 | 0.205 ± 0.018 | 0.885 ± 0.043 | 0.325 ± 0.050 | 0.950 ± 0.021 | −0.053 ± 0.002 |
| F-3 | 335 | 0.227 ± 0.004 | 0.869 ± 0.036 | 0.400 ± 0.000 | 0.947 ± 0.042 | −0.059 ± 0.005 |
| F-6 | 334 | 0.801 ± 0.015 | 0.360 ± 0.048 | 1.683 ± 0.075 | 0.787 ± 0.166 | −0.260 ± 0.012 |
| Formulation | Zero Order | First Order | Higuchi Model | Korsmeyer-Peppas Model | Weibull Method |
|---|---|---|---|---|---|
| Regression Coefficient R2 | |||||
| F-5 (15 mg/g) | 0.92 | 0.96 | 0.98 | 0.96 | 0.97 |
| F-6 (20 mg/g) | 0.91 | 0.96 | 0.98 | 0.98 | 0.98 |
| F-7 (25 mg/g) | 0.78 | 0.90 | 0.92 | 0.81 | 0.81 |
| Formulation | Average Release Rate (mg/cm2/min1/2) ± SD | R2 |
|---|---|---|
| F-5 (15 mg/g) | 0.41 ± 0.02 | 0.98 |
| F-6 (20 mg/g) | 0.48 ± 0.05 | 0.98 |
| F-7 (25 mg/g) | 0.52 ± 0.03 * | 0.92 |
| Ingredient | ACTH (mg) | 1 mol/L Acetic Acid Solution (mL) | Lekobaza® (g) | |
|---|---|---|---|---|
| Formulation | ||||
| F-1 | - | - | added to 10.0 | |
| F-2 | - | 1.0 | added to 10.0 | |
| F-3 | 50.0 | 1.0 | added to 10.0 | |
| F-4 | 100.0 | 1.0 | added to 10.0 | |
| F-5 | 150.0 | 1.0 | added to 10.0 | |
| F-6 | 200.0 | 1.0 | added to 10.0 | |
| F-7 | 250.0 | 1.0 | added to 10.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemiradzka, W.; Dolińska, B.; Ryszka, F. Development and Study of Semi-Solid Preparations Containing the Model Substance Corticotropin (ACTH): Convenience Application in Neurodegenerative Diseases. Molecules 2020, 25, 1824. https://doi.org/10.3390/molecules25081824
Siemiradzka W, Dolińska B, Ryszka F. Development and Study of Semi-Solid Preparations Containing the Model Substance Corticotropin (ACTH): Convenience Application in Neurodegenerative Diseases. Molecules. 2020; 25(8):1824. https://doi.org/10.3390/molecules25081824
Chicago/Turabian StyleSiemiradzka, Wioletta, Barbara Dolińska, and Florian Ryszka. 2020. "Development and Study of Semi-Solid Preparations Containing the Model Substance Corticotropin (ACTH): Convenience Application in Neurodegenerative Diseases" Molecules 25, no. 8: 1824. https://doi.org/10.3390/molecules25081824
APA StyleSiemiradzka, W., Dolińska, B., & Ryszka, F. (2020). Development and Study of Semi-Solid Preparations Containing the Model Substance Corticotropin (ACTH): Convenience Application in Neurodegenerative Diseases. Molecules, 25(8), 1824. https://doi.org/10.3390/molecules25081824






