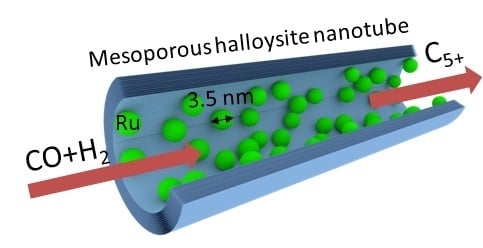
Ruthenium-Loaded Halloysite Nanotubes as Mesocatalysts for Fischer–Tropsch Synthesis
Abstract
1. Introduction
2. Results and Discussion
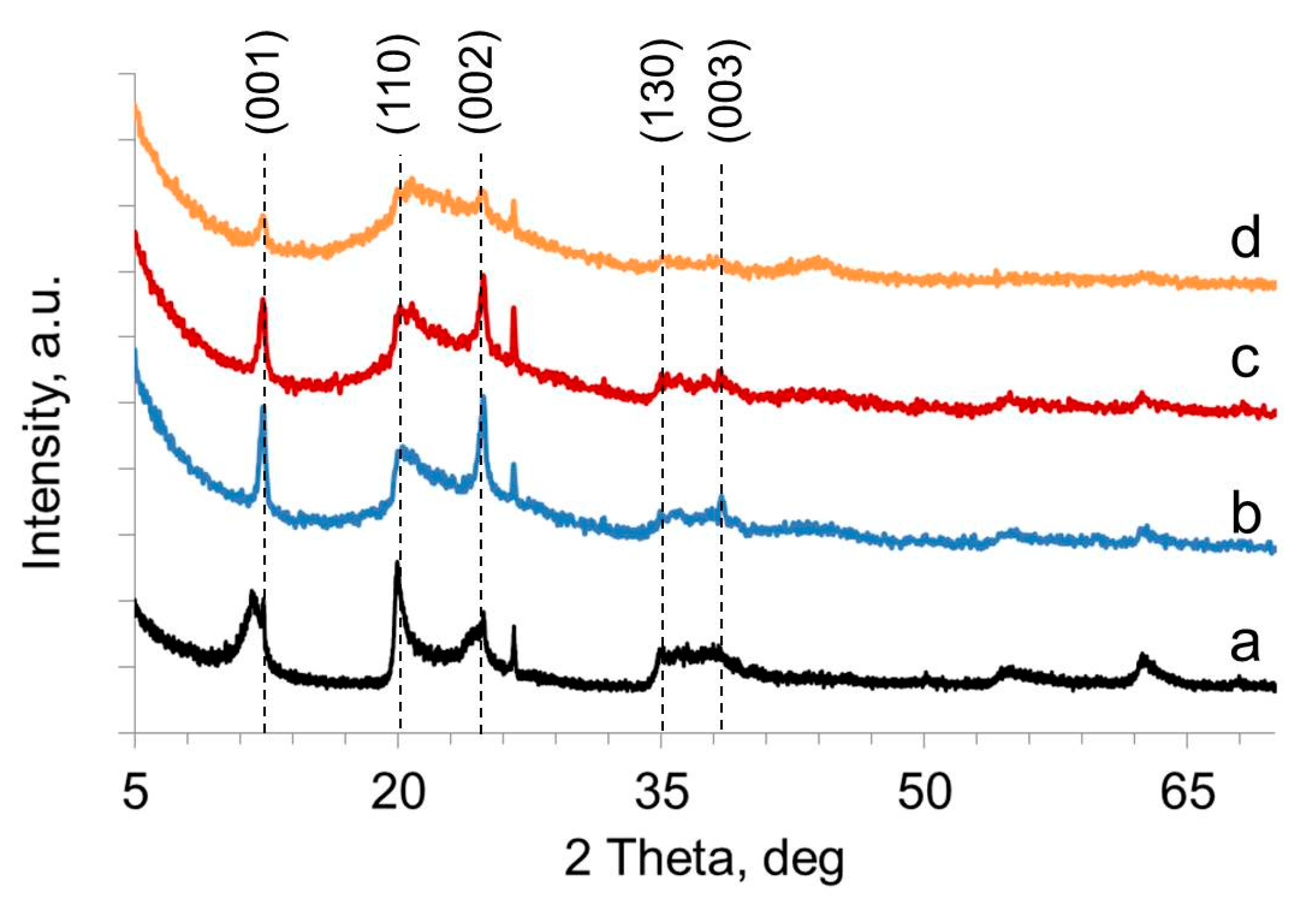
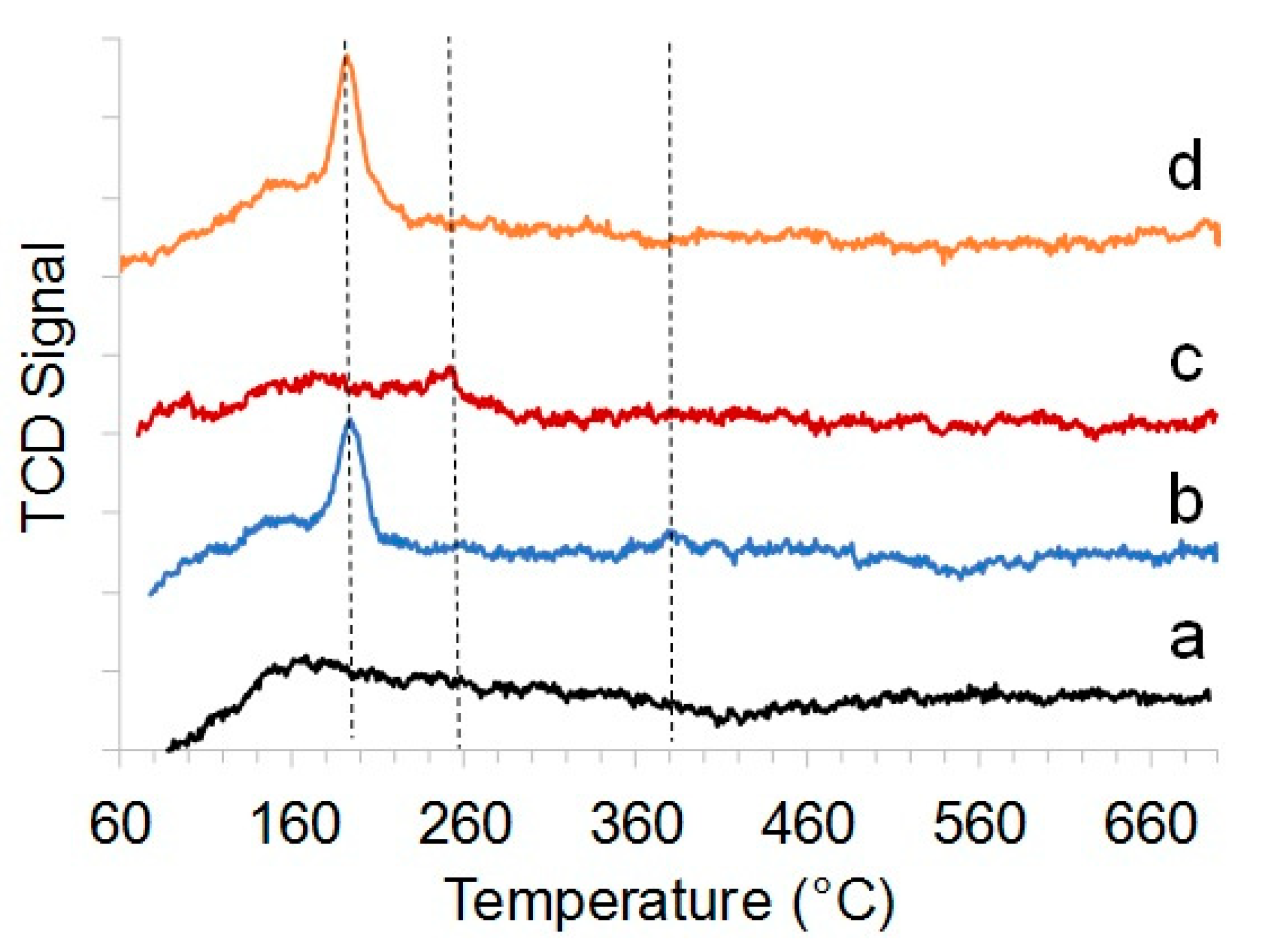
2.1. Structure, Morphology, and Composition of Ru-Loaded Halloysite Catalysts
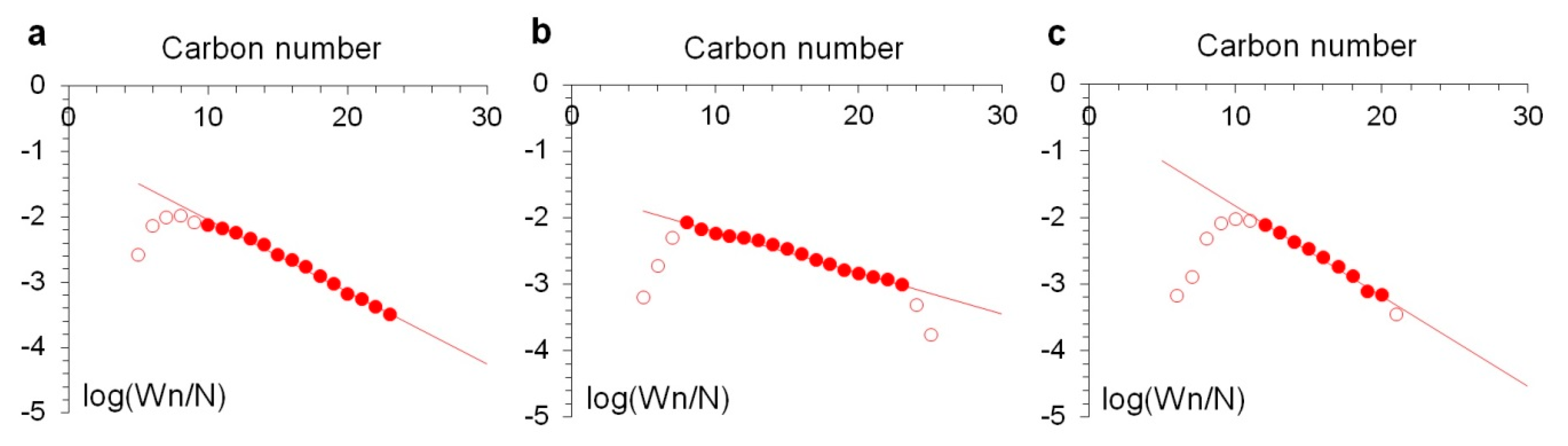
2.2. Catalytic Efficiency of Ru-Loaded Halloysite Catalysts in Fischer–Tropsch Synthesis
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of Biomass and Upgrading of Bio-Oil: A Review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Komaya, T.; Bell, A.T.; Wengsieh, Z.; Gronsky, R.; Engelke, F.; King, T.S.; Pruski, M. Effects of Dispersion and Metal-Metal Oxide Interactions on Fischer-Tropsch Synthesis over Ru/TiO2 and TiO2-Promoted Ru/SiO2. J. Catal. 1994, 150, 400–406. [Google Scholar] [CrossRef]
- Xiao, C.X.; Cai, Z.P.; Wang, T.; Kou, Y.; Yan, N. Aqueous-Phase Fischer–Tropsch Synthesis with a Ruthenium Nanocluster Catalyst. Angew. Chem. Int. Ed. 2008, 47, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, E.; Soled, S.L.; Fiato, R.A. Fischer-Tropsch synthesis on cobalt and ruthenium. Metal dispersion and support effects on reaction rate and selectivity. J. Catal. 1992, 137, 212–224. [Google Scholar] [CrossRef]
- Khadzhiev, S.N. Nanoheterogeneous catalysis: Definition, state, and research prospects. Petrol. Chem. 2016, 56, 465–479. [Google Scholar] [CrossRef]
- Buller, S.; Strunk, J. Nanostructure in energy conversion. J. Energy Chem. 2016, 25, 171–190. [Google Scholar] [CrossRef]
- Zaera, F. Shape-controlled nanostructures in heterogeneous catalysis. ChemSuschem 2013, 6, 1797–1820. [Google Scholar] [CrossRef]
- Dragutan, I.; Dragutan, V.; Demonceau, A. Special Issue on Ruthenium Complexes. Molecules 2017, 22, 255. [Google Scholar] [CrossRef]
- Cattaneo, S.; Naslhajian, H.; Somodi, F.; Evangelisti, C.; Villa, A.; Prati, L. Ruthenium on Carbonaceous Materials for the Selective Hydrogenation of HMF. Molecules 2018, 23, 2007. [Google Scholar] [CrossRef]
- Koh, T.; Koo, H.M.; Yu, T.; Lim, B.; Bae, J.W. Roles of Ruthenium–Support Interactions of Size-Controlled Ruthenium Nanoparticles for the Product Distribution of Fischer–Tropsch Synthesis. ACS Catal. 2014, 4, 1054–1060. [Google Scholar] [CrossRef]
- González-Carballo, J.M.; Finocchio, E.; García, S.; Rojas, S.; Ojeda, M.; Busca, G.; Fierro, J.L.G. Support effects on the structure and performance of ruthenium catalysts for the Fischer–Tropsch synthesis. Catal. Sci. Technol. 2011, 1, 1013–1023. [Google Scholar] [CrossRef]
- Phaahlamohlaka, T.N.; Kumia, D.O.; Dlamini, M.W.; Jewell, L.L.; Covillea, N.J. Ruthenium nanoparticles encapsulated inside porous hollow carbonspheres: A novel catalyst for Fischer–Tropsch synthesis. Catal. Today. 2016, 275, 76–83. [Google Scholar] [CrossRef]
- Tingjun, F.; Zhenhua, L. Review of recent development in Co-based catalysts supported on carbon materials for Fischer–Tropsch synthesis. Chem. Eng. Sci. 2015, 135, 3–20. [Google Scholar]
- Miners, S.A.; Rance, G.A.; Khlobystov, A.N. Chemical reactions confined within carbon nano-tubes. Chem. Soc. Rev. 2016, 45, 4727–4746. [Google Scholar] [CrossRef]
- Lvov, Y.; Abhishek, P.; Fu, Y.; Fakhrullin, R.; Kryuchkova, M.; Batasheva, S.; Stavitskaya, A.; Glotov, A.; Vinokurov, V. Interfacial Self-Assembly in Halloysite Nanotube Composites. Langmuir 2019, 35, 8646–8657. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Glotov, A.P.; Ivanov, E.V.; Gushchin, P.A.; Lvov, Y.M.; Maximov, A.L.; Muradov, A.V.; Karakhanov, E.A. Core-shell nanoarchitecture: Schiff-base assisted synthesis of ruthenium in clay nanotubes. Pure Appl. Chem. 2018, 90, 825. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Glotov, A.P.; Novikov, A.A.; Zolotukhina, A.V.; Kotelev, M.S.; Gushchin, P.A.; Ivanov, E.V.; Darrat, Y.; Lvov, Y.M. Nanoparticles Formed onto/into Halloysite Clay Tubules: Architectural Synthesis and Applications. Chem. Rec. 2018, 18, 858–867. [Google Scholar] [CrossRef]
- Glotov, A.P.; Stavitskaya, A.V.; Chudakov, Y.A.; Artemova, M.I.; Smirnova, E.M.; Demikhova, N.R.; Shabalina, T.N.; Gureev, A.A.; Vinokurov, V.A. Nanostructured Ruthenium Catalysts in Hydrogenation of Aromatic Compounds. Petrol. Chem. 2018, 58, 1221–1226. [Google Scholar] [CrossRef]
- Vinokurov, V.; Glotov, A.; Chudakov, Y.; Stavitskaya, A.; Ivanov, E.; Gushchin, P.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Core/Shell Ruthenium–Halloysite Nanocatalysts for Hydrogenation of Phenol. Ind. Eng. Chem. Res. 2017, 56, 14043–14052. [Google Scholar] [CrossRef]
- Sufang, C.; Jinlin, L.; Yuhua, Z.; Daohong, Z.; Junjiang, Z. Effect of preparation method on halloysite supported cobalt catalysts for Fischer-Tropsch synthesis. J. Nat. Gas Chem. 2012, 21, 426–430. [Google Scholar]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Nassir, M.H.; Al-Amiery, A.A. Impact of Sulfuric Acid Treatment of Halloysite on Physico-Chemical Property Modification. Materials 2016, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: Ftir, xrf, and xrd studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Abdullayev, E.; Joshi, A.; Wei, W.B.; Zhao, Y.F.; Lvov, Y. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F.; Yan, W.; Liu, D.; He, H. Changes in Structure, Morphology, Porosity, and Surface Activity of Mesoporous Halloysite Nanotubes Under Heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- Mammadova, T.A.; Hasankhanova, N.V.; Teyubov, K.S.; Askerova, E.N.; Latifova, T.S.; Abbasov, V.M. Use of natural nanotubes of halloysite clay for thermochemical conversion of cottonseed oil. Catal. Sustain. Energy 2015, 2, 28–32. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M.; Hassina, Z.-B.; Zerroual, L. Trichlorophenol removal from aqueous solutions by modified halloysite: Kinetic and equilibrium studies. Desalin. Water Treat. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Ouyang, J.; Guo, B.; Fu, L.; Yang, H.; Hu, Y.; Tang, A.; Long, H.; Jin, Y.; Chen, J.; Jiang, J. Radical guided selective loading of silver nanoparticles at interior lumen and out surface of halloysite nanotubes. Mater. Des. 2016, 110, 169–178. [Google Scholar] [CrossRef]
- Barrientos-Ramírez, S.; Ramos-Fernández, E.V.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Pastor-Blas, M.M.; González-Montiel, A. Use of nanotubes of natural halloysite as catalyst support in the atom transfer radical polymerization of methyl methacrylate. Micropor. Mesopor. Mater. 2009, 120, 132–140. [Google Scholar] [CrossRef]
- Eliseev, O.L.; Savost’yanov, A.P.; Sulima, S.I.; Lapidus, A.L. Recent development in heavy paraffins synthesis from CO and H2. Mendeleev Commun. 2018, 28, 345–351. [Google Scholar] [CrossRef]
- Novak, S.; Madon, R.J.; Suhl, H. Models of hydrocarbon product distributions in Fischer–Tropsch synthesis. J. Chem. Phys. 1981, 74, 6083–6091. [Google Scholar] [CrossRef]
- Novak, S.; Madon, R.J.; Suhl, H. Secondary effects in the Fischer-Tropsch synthesis. J. Catal. 1982, 77, 141–151. [Google Scholar] [CrossRef]
- Friedel, R.A.; Anderson, R.B. Composition of synthetic liquid fuels. I. Product distribution and analysis of C5-C8 paraffin isomers from cobalt catalyst. J. Am. Chem. Soc. 1950, 72, 1212–1215. [Google Scholar] [CrossRef]
- Erley, W.; McBreen, P.; Ibach, H. Evidence for CHx surface species after the hydrogenation of CO over an Fe(110) single crystal surface. J. Catal. 1983, 84, 229–234. [Google Scholar] [CrossRef]
- Wang, C.J.; Ekerdt, J.G. Evidence for alkyl intermediates during Fischer-Tropsch synthesis and their relation to hydrocarbon products. J. Catal. 1984, 86, 239–244. [Google Scholar] [CrossRef]
- Kaminsky, M.; Winograd, N.; Geoffroy, G.; Vannice, M.A. Direct SIMS observation of methylidyne, methylene, and methyl intermediates on a nickel (III) methanation catalyst. J. Am. Chem. Soc. 1986, 108, 1315–1316. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
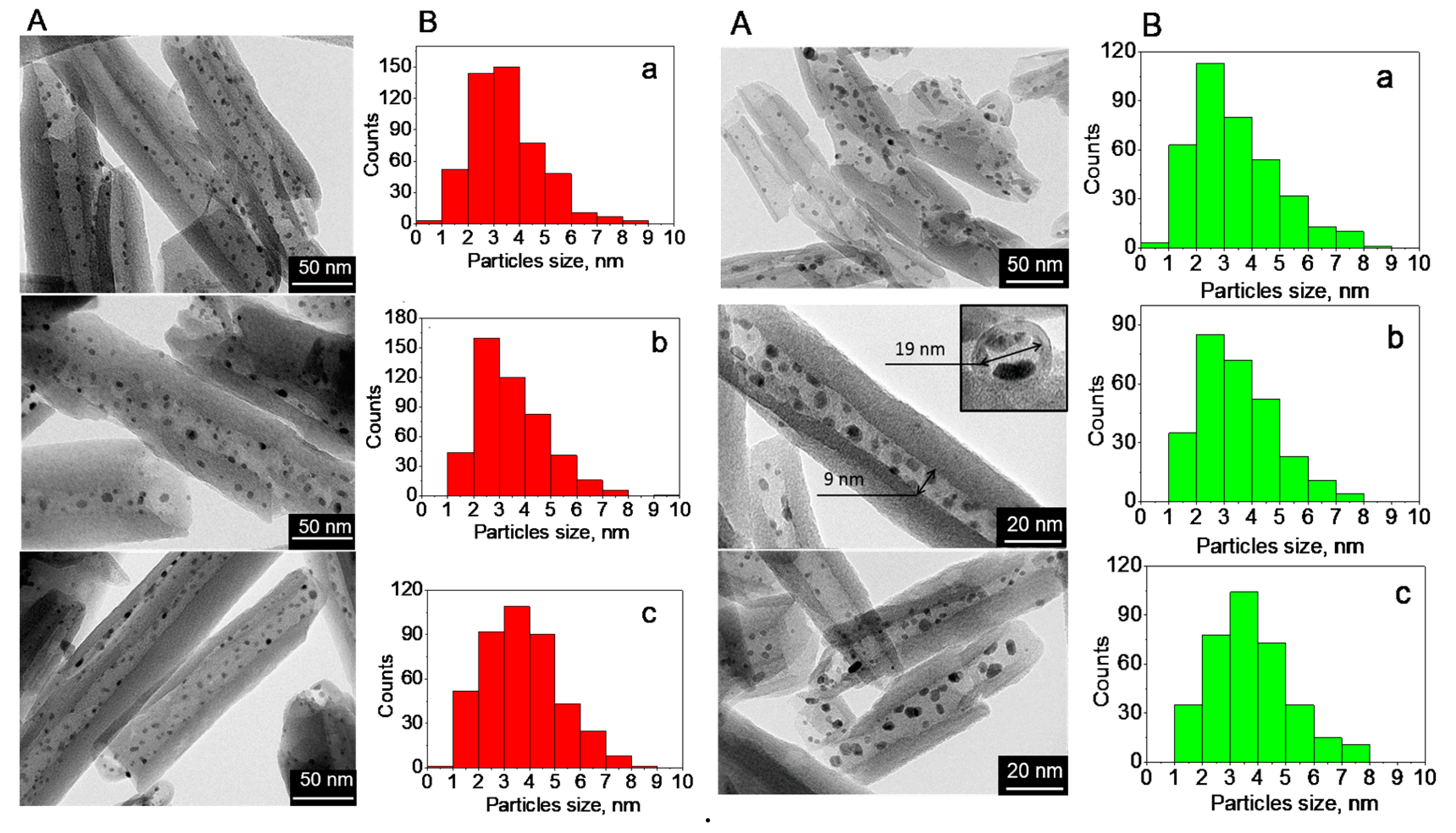
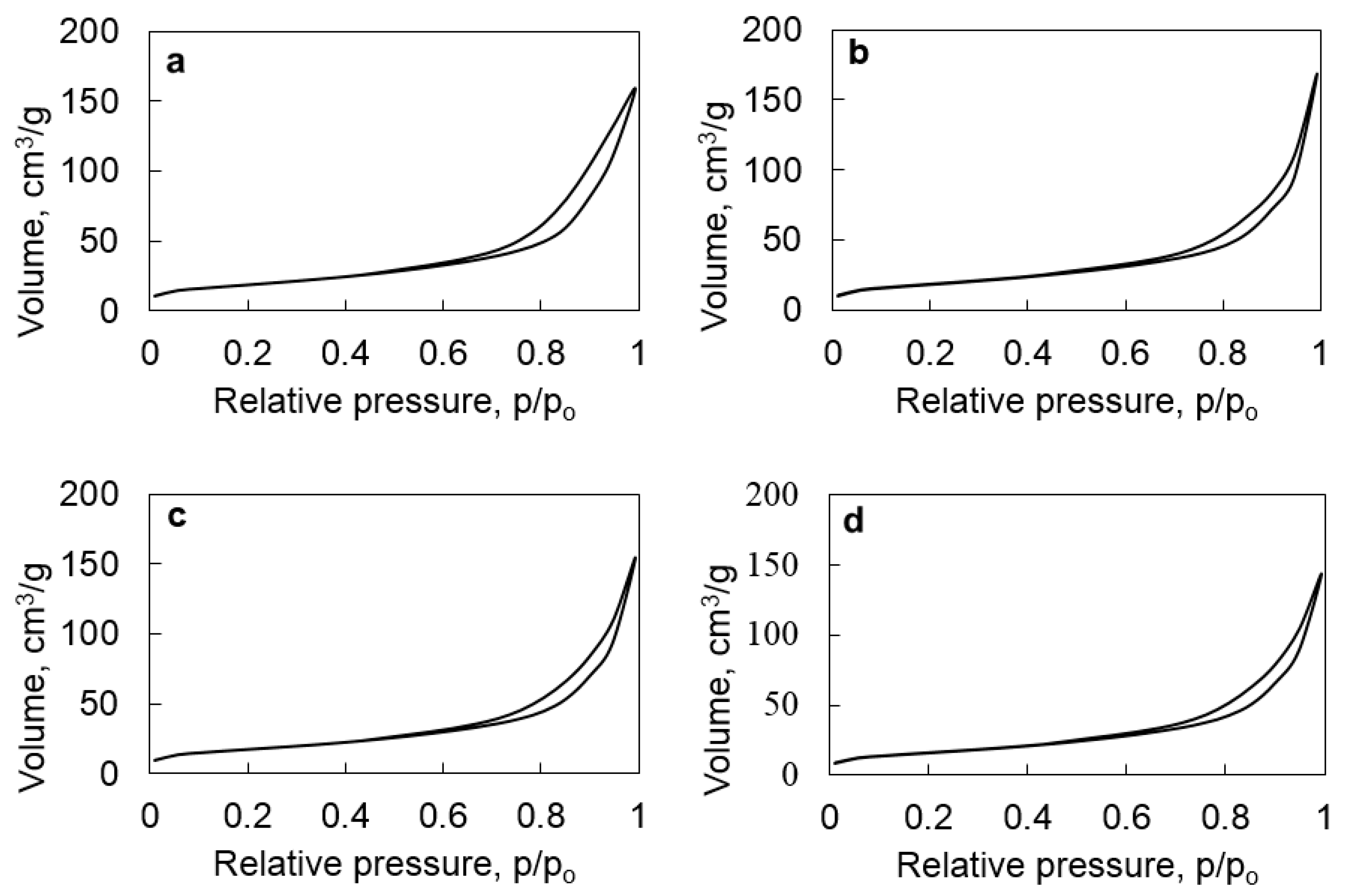
| Catalyst | Elemental Composition, wt. % | Surface Area, BET, m2/g | Average Particles Size (TEM), nm | Total Acidity, µmol/g | ||
|---|---|---|---|---|---|---|
| Si | Al | Ru | ||||
| HNT | 23.6 | 25.3 | - | 66 | None | 178 |
| HNT@Ru-1 | 23.5 | 23.8 | 2.0 | 63 | 3.5 | 315 |
| HNT@Ru-2 | 23.0 | 24.0 | 2.0 | 60 | 3.5 | 129 |
| HNT@Ru-3 | 22.5 | 24.3 | 2.2 | 58 | 3.5 | 250 |
| Parameter | HNT@Ru-1 | HNT@Ru-2 | HNT@Ru-3 |
|---|---|---|---|
| CO conversion, % | 15.6 | 17.8 | 18.8 |
| Ru–time yield × 103, molCO/(molRu s) | 29.3 | 33.4 | 32.1 |
| CH4 selectivity, % | 52.8 | 19.9 | 28.8 |
| C2–C4 selectivity, % | 20.3 | 1.6 | 3.2 |
| C5+ selectivity, % | 26.7 | 78.0 | 67.7 |
| CO2 selectivity, % | 0.2 | 0.5 | 0.3 |
| % olefins in C5+ | 19.2 | 12.9 | 26.5 |
| ASF α | 0.78 | 0.87 | 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavitskaya, A.; Mazurova, K.; Kotelev, M.; Eliseev, O.; Gushchin, P.; Glotov, A.; Kazantsev, R.; Vinokurov, V.; Lvov, Y. Ruthenium-Loaded Halloysite Nanotubes as Mesocatalysts for Fischer–Tropsch Synthesis. Molecules 2020, 25, 1764. https://doi.org/10.3390/molecules25081764
Stavitskaya A, Mazurova K, Kotelev M, Eliseev O, Gushchin P, Glotov A, Kazantsev R, Vinokurov V, Lvov Y. Ruthenium-Loaded Halloysite Nanotubes as Mesocatalysts for Fischer–Tropsch Synthesis. Molecules. 2020; 25(8):1764. https://doi.org/10.3390/molecules25081764
Chicago/Turabian StyleStavitskaya, Anna, Kristina Mazurova, Mikhail Kotelev, Oleg Eliseev, Pavel Gushchin, Aleksandr Glotov, Ruslan Kazantsev, Vladimir Vinokurov, and Yuri Lvov. 2020. "Ruthenium-Loaded Halloysite Nanotubes as Mesocatalysts for Fischer–Tropsch Synthesis" Molecules 25, no. 8: 1764. https://doi.org/10.3390/molecules25081764
APA StyleStavitskaya, A., Mazurova, K., Kotelev, M., Eliseev, O., Gushchin, P., Glotov, A., Kazantsev, R., Vinokurov, V., & Lvov, Y. (2020). Ruthenium-Loaded Halloysite Nanotubes as Mesocatalysts for Fischer–Tropsch Synthesis. Molecules, 25(8), 1764. https://doi.org/10.3390/molecules25081764