Recent Advances in the Application of Antibacterial Complexes Using Essential Oils
Abstract
1. Introduction
2. EOs as Antimicrobial Agents
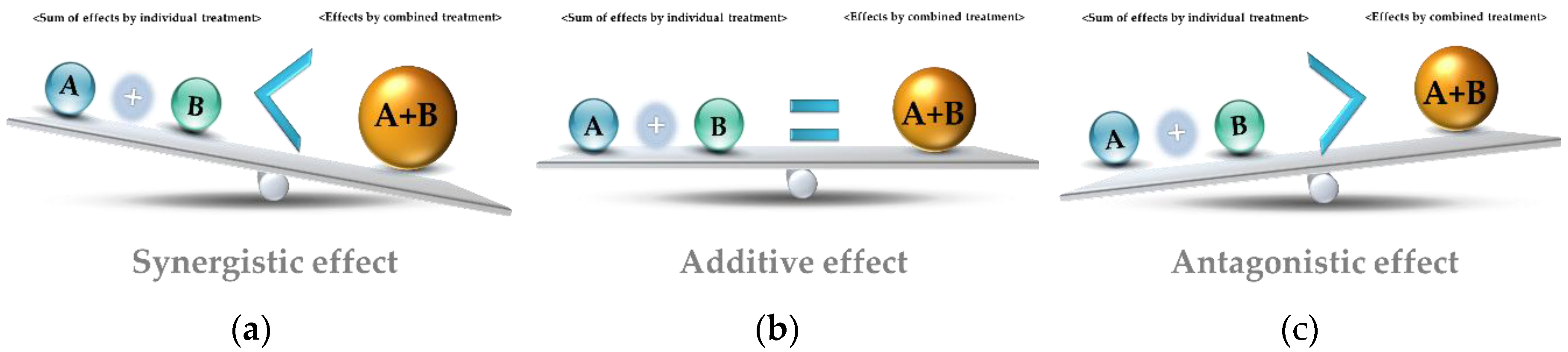
3. Antibacterial Complex Using EOs
4. Antimicrobial Mechanisms of EO Complex against Pathogens
5. Practical Application of EO-Based Antibacterial Complex
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalemba, D.A.A.K.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed. Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and Anti-Inflammatory Effects of Essential Oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial Properties of Plant Essential Oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Mourey, A.; Canillac, N. Anti-Listeria monocytogenes Activity of Essential Oils Components of Conifers. Food Control. 2002, 13, 289–292. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial Activity of Selected Plant Essential Oils Against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Božović, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagnoli, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball-New Approaches. Molecules 2017, 22, 203. [Google Scholar] [CrossRef]
- Bae, Y.S.; Rhee, M.S. Short-Term Antifungal Treatments of Caprylic Acid with Carvacrol or Thymol Induce Synergistic 6-Log Reduction of Pathogenic Candida albicans by Cell Membrane Disruption and Efflux Pump Inhibition. Cell. Physiol. Biochem. 2019, 53, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D. Antiviral Activity of the Essential Oil of Melaleuca aternifolia (Maiden amp; Betche) Cheel (Tea Tree) Against Tobacco Mosaic Virus. J. Essent. Oil Res. 1995, 7, 641–644. [Google Scholar] [CrossRef]
- Azzouz, M.A.; Bullerman, L.B. Comparative Antimycotic Effects of Selected Herbs, Spices, Plant Components and Commercial Antifungal Agents. J. Food Prot. 1982, 45, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.-P.; Fyfe, L.; Smith, H. Plant Active Components–A Resource for Antiparasitic Agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Oregano Essential Oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Marked Synergistic Bactericidal Effects and Mode of Action of Medium-Chain Fatty Acids in Combination with Organic Acids Against Escherichia coli O157:H7. Appl. Environ. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, M.R.; Park, J.W.; Park, K.H.; Chung, M.S.; Ryu, S.; Kang, D.H. Use of Organic Acids to Inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on Organic Fresh Apples and Lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Essential Oils in Foods: Extraction, Stabilization, and Toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A Review on Recent Research Results (2008–2010) on Essential Oils as Antimicrobials and Antifungals. A Review. Flavour Frag. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Kim, S.S.; Kang, D.H. Synergistic Effect of Carvacrol and Ohmic Heating for Inactivation of E. coli O157:H7, S. Typhimurium, L. monocytogenes, and MS-2 Bacteriophage in Salsa. Food Control. 2017, 73, 300–305. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A Review on the Use of Essential Oils for Postharvest Decay Control and Maintenance of Fruit Quality During Storage. Crop. Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, P.S.; Lazar, V.; Papp, B.; Arnoldini, M.; zur Wiesch, P.A.; dBusa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Banhoeffer, S. Antagonism between Bacteriostatic and Bactericidal Antibiotics is Prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Highly Enhanced Bactericidal Effects of Medium Chain Fatty Acids (Caprylic, Capric, and Lauric Acid) Combined with Edible Plant Essential Oils (Carvacrol, Eugenol, β-Resorcylic Acid, Trans-Cinnamaldehyde, Thymol, and Vanillin) Against Escherichia coli O157:H7. Food Control. 2016, 60, 447–454. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Aliberti, L.; Amato, M.; de Feo, V.; Camele, I. Chemical Composition and Antimicrobial Activity of Chia (Salvia hispanica L.) Essential Oil. Eur. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Grul’ová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; de Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy Between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Moon, H.; Rhee, M.S. Synergism Between Carvacrol or Thymol Increases the Antimicrobial Efficacy of Soy Sauce with No Sensory Impact. Int. J. Food Microbiol. 2016, 217, 35–41. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of Action of Spanish Oregano, Chinese Cinnamon, and Savory Essential Oils Against Cell Membranes and Walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C.-I. Antibacterial Activity of Some Essential Oil Components Against Five Foodborne Pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of Bactericidal Action of Cinnamaldehyde Against Listeria monocytogenes and of Eugenol Against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of Antimicrobial Action of Vanillin Against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of Combined Antibacterial Effects of Eugenol, Cinnamaldehyde, Thymol, and Carvacrol against E. coli with an Improved Method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Zhou, F.; Ji, B.; Zhang, H.; Jiang, H.; Yang, Z.; Li, J.; Li, J.; Yan, W. The Antibacterial Effect of Cinnamaldehyde, Thymol, Carvacrol and Their Combinations Against the Foodborne Pathogen Salmonella Typhimurium. J. Food Saf. 2007, 27, 124–133. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol is Essential for Action Against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Kim, H.O.; Park, S.W.; Park, H.D. Inactivation of Escherichia coli O157:H7 by Cinnamic Aldehyde Purified from Cinnamomum cassia Shoot. Food Microbiol. 2004, 21, 105–110. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.l.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro Antimicrobial Activity and Chemical Composition of Sardinian Thymus Essential Oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents Against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, H.W.; Moon, H.; Rhee, M.S. Sodium Chloride Significantly Enhances the Bactericidal Actions of Carvacrol and Thymol Against the Halotolerant Species Escherichia coli O157:H7, Listeria monocytogenes, and Staphylococcus aureus. Lwt-Food Sci. Technol. 2020, 109015. [Google Scholar] [CrossRef]
- Chung, D.; Cho, T.J.; Rhee, M.S. Citrus Fruit Extracts with Carvacrol and Thymol Eliminated 7-log Acid-adapted Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes: A Potential of Effective Natural Antibacterial Agents. Food Res. Int. 2018, 107, 578–588. [Google Scholar] [CrossRef]
- Abadias, M.; Alegre, I.; Usall, J.; Torres, R.; Viñas, I. Evaluation of Alternative Sanitizers to Chlorine Disinfection for Reducing Foodborne Pathogens in Fresh-cut Apple. Postharvest Biol. Technol. 2011, 59, 289–297. [Google Scholar] [CrossRef]
- Lin, C.M.; Sheu, S.R.; Hsu, S.C.; Tsai, Y.H. Determination of Bactericidal Efficacy of Essential Oil Extracted from Orange Peel on the Food Contact Surfaces. Food Control. 2010, 21, 1710–1715. [Google Scholar] [CrossRef]
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial Activity of Leaf and Bark Cinnamon Essential Oils Against Listeria monocytogenes and Salmonella typhimurium in Broth System and on Celery. J. Food Process. Preserv. 2019, 43, e13888. [Google Scholar] [CrossRef]
- de Sousa Guedes, J.P.; da Costa Medeiros, J.A.; e Silva, R.S.d.S.; de Sousa, J.M.B.; da Conceicao, M.L.; de Souza, E.L. The Efficacy of Mentha arvensis L. and M. piperita L. Essential Oils in Reducing Pathogenic Bacteria and Maintaining Quality Characteristics in Cashew, Guava, Mango, and Pineapple Juices. Int. J. Food Microbiol. 2016, 238, 183–192. [Google Scholar] [CrossRef]
- Bor, T.; Gyawali, R.; Ibrahim, S.A. Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157: H7 in Laboratory Medium. Foods 2016, 5, 14. [Google Scholar] [CrossRef]
- Piskernik, S.; Klančnik, A.; Riedel, C.T.; Brøndsted, L.; Možina, S.S. Reduction of Campylobacter jejuni by Natural Antimicrobials in Chicken Meat-Related Conditions. Food Control. 2011, 22, 718–724. [Google Scholar] [CrossRef]
- Rabiey, S.; Hosseini, H.; Rezaei, M. The Hurdle Effect of Bunium persicum Essential Oil, Smoke and NaCl for Controlling the Listeria monocytogenes Growth in Fish Model Systems. J. Food Saf. 2013, 33, 137–144. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial Properties of Microemulsions Formulated with Essential Oils, Soybean Oil, and Tween 80. Int. J. Food Microbiol. 2016, 226, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.A.N.; Afaisen, S.J.; Gadi, R.A.M.A. Antimicrobial Activity of Noni Fruit Essential Oil on Escherichia coli O157:H7 and Salmonella Enteritidis. Micronesica 2016, 5, 1–10. [Google Scholar]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial Efficacies of Essential oils/Nanoparticles Incorporated Polylactide Films Against L. monocytogenes and S. typhimurium on Contaminated Cheese. Int. J. Food Prop. 2017, 20, 53–67. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and Structural Characterization of Chitosan Flms Containing Eucalyptus globulus Essential Oil: Potential as an Antimicrobial Carrier for Packaging of Sliced Sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Antimicrobial Activity of Nanodispersed Thymol in Tryptic Soy Broth. J. Food Prot. 2013, 76, 440–447. [Google Scholar] [CrossRef]
- Ribes, S.; Ruiz-Rico, M.; Pérez-Esteve, É; Fuentes, A.; Barat, J.M. Enhancing the Antimicrobial Activity of Eugenol, Carvacrol and Vanillin Immobilised on Silica Supports Against Escherichia coli or Zygosaccharomyces rouxii in Fruit Juices by Their Binary Combinations. Lwt-Food Sci. Technol. 2019, 113, 108326. [Google Scholar] [CrossRef]
- Campion, A.; Morrissey, R.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. Use of Enhanced Nisin Derivatives in Combination with Food-grade Oils or Citric Acid to Control Cronobacter sakazakii and Escherichia coli O157:H7. Food Microbiol. 2017, 65, 254–263. [Google Scholar] [CrossRef]
- Smid, E.J.; Gorris, L.G.M. Handbook of Food Preservation; Rahman, M.S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 285–308. [Google Scholar]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of Antilisterial Action of Cilantro Oil on Vacuum Packed Ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, S.A.; Lee, N.Y.; Rhee, M.S. New Decontamination Method Based on Caprylic Acid in Combination with Citric Acid or Vanillin for Eliminating Cronobacter sakazakii and Salmonella enterica serovar Typhimurium in Reconstituted Infant Formula. Int. J. Food Microbiol. 2013, 166, 499–507. [Google Scholar] [CrossRef]
- Roller, S.; Seedhar, P. Carvacrol and Cinnamic Acid Inhibit Microbial Growth in Fresh-Cut Melon and Kiwifruit at 4 °C and 8 °C. Lett. Appl. Microbiol. 2002, 35, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Thippareddi, H.; Friedman, M. Control of Clostridium perfringens in Cooked Ground Beef by Carvacrol, Cinnamaldehyde, Thymol, or Oregano Oil During Chilling. J. Food Prot. 2006, 69, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Weissinger, W.R.; McWatters, K.H.; Beuchat, L.R. Evaluation of Volatile Chemical Treatments for Lethality to Salmonella on Alfalfa Seeds and Sprouts. J. Food Prot. 2001, 64, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kang, J.H.; Song, K.B. Antibacterial Activities of a Cinnamon Essential Oil with Cetylpyridinium Chloride Emulsion Against Escherichia coli O157:H7 and Salmonella Typhimurium in Basil Leaves. Food Sci. Biotechnol. 2018, 27, 47–55. [Google Scholar] [CrossRef]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles Against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef]
- Rhoades, J.; Kargiotou, C.; Katsanidis, E.; Koutsoumanis, K.P. Use of Marination for Controlling Salmonella enterica and Listeria monocytogenes in Raw Beef. Food Microbiol. 2013, 36, 248–253. [Google Scholar] [CrossRef]
- Desai, M.A.; Soni, K.A.; Nannapaneni, R.; Schilling, M.; Silva, J.L. Reduction of Listeria monocytogenes in Raw Catfish Fillets by Essential Oils and Phenolic Constituent Carvacrol. J. Food Sci. 2012, 77, M516–M522. [Google Scholar] [CrossRef]
- Tassou, C.C.; Drosinos, E.H.; Nychas, G.J.E. Effects of Essential Oil from Mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in Model Food Systems at 4 °C and 10 °C. J. Appl. Bacteriol. 1995, 78, 593. [Google Scholar] [CrossRef]
- Menon, K.V.; Garg, S.R. Inhibitory Effect of Clove Oil on Listeria monocytogenes in Meat and Cheese. Food Microbiol. 2001, 18, 647–650. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.K.; Bhunia, A.K.; Stroshine, R.L. Efficacy of Chlorine Dioxide, Ozone, and Thyme Essential Oil or a Sequential Washing in Killing Escherichia coli O157:H7 on Lettuce and Baby Carrots. Lwt-Food Sci. Technol. 2002, 35, 720–729. [Google Scholar] [CrossRef]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial Activity of Carvacrol Toward Bacillus cereus on Rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Iten, F.; Saller, R.; Abel, G.; Reichling, J. Additive Antmicrobial Effects of the Active Components of the Essential Oil of Thymus vulgaris–Chemotype Carvacrol. Planta Med. 2009, 75, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Djouahri, A.; Saka, B.; Boudarene, L.; Benseradj, F.; Aberrane, S.; Aitmoussa, S.; Chelghoum, C.; Lamari, L.; Sabaou, N.; Baaliouamer, A. In vitro Synergistic/Antagonistic Antibacterial and Anti-Inflammatory Effect of Various Extracts/Essential Oil from Cones of Tetraclinis articulata (Vahl) Masters with Antibiotic and Anti-Inflammatory Agents. Ind. Crop. Prod. 2014, 56, 60–66. [Google Scholar] [CrossRef]
- Wendakoon, C.N.; Sakaguchi, M. Combined Effect of Sodium Chloride and Clove on Growth and Biogenic Amine Formation of Enterobacter aerogenes in Mackerel Muscle Extract. J. Food Prot. 1993, 56, 410–413. [Google Scholar] [CrossRef]
- Hulankova, R.; Borilova, G.; Steinhauserova, I. Combined Antimicrobial Effect of Oregano Essential Oil and Caprylic Acid in Minced Beef. Meat Sci. 2013, 95, 190–194. [Google Scholar] [CrossRef]
- Pol, I.E.; Smid, E.J. Combined Action of Nisin and Carvacrol on Bacillus cereus and Listeria monocytogenes. Lett. Appl. Microbiol. 1999, 29, 166–170. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The Antimicrobial Effect of Thyme Essential Oil, Nisin, and Their Combination Against Listeria monocytogenes in Minced Beef During Refrigerated Storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The Antimicrobial Effect of Thyme Essential Oil, Nisin and Their Combination Against Escherichia coli O157:H7 in Minced Beef During Refrigerated Storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P.S. The Antimicrobial Effect of Oregano Essential Oil, Nisin and Their Combination Against Salmonella Enteritidis in Minced Sheep Meat During Refrigerated Storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef]
- Moon, H.; Kim, N.H.; Kim, S.H.; Kim, Y.; Ryu, J.H.; Rhee, M.S. Teriyaki Sauce with Carvacrol or Thymol Effectively Controls Escherichia coli O157:H7, Listeria monocytogenes, Salmonella Typhimurium, and Indigenous Flora in Marinated Beef and Marinade. Meat Sci. 2017, 129, 147–152. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial Properties of Lauric Arginate Alone or in Combination with Essential Oils in Tryptic Soy Broth and 2% Reduced Fat Milk. Int. J. Food Microbiol. 2013, 166, 77–84. [Google Scholar] [CrossRef]
- Char, C.D.; Guerrero, S.N.; Alzamora, S.M. Mild Thermal Process Combined with Vanillin Plus Citral to Help Shorten the Inactivation Time for Listeria innocua in Orange Juice. Food Bioprocess. Technol. 2010, 3, 752–761. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol Against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of Action of Carvacrol on the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in Membrane Fatty Acids Composition of Microbial Cells Induced by Addiction of Thymol, Carvacrol, Limonene, Cinnamaldehyde, and Eugenol in the Growing Media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils Against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Hamoud, R.; Sporer, F.; Reichling, J.; Wink, M. Antimicrobial Activity of a Traditionally Used Complex Essential Oil Distillate (Olbas® Tropfen) in Comparison to Its Individual Essential Oil Ingredients. Phytomedicine 2012, 19, 969–976. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of Essential Oils: A Review on Their Interaction with Food Components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-Based Packaging Films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Growth Inhibition of Escherichia coli O157:H7 and Listeria monocytogenes by Carvacrol and Eugenol Encapsulated in Surfactant Micelles. J. Food Prot. 2005, 68, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Influence of Organic Matter, Cations and Surfactants on the Antimicrobial Activity of Melaleuca alternifolia (Tea Tree) Oil In Vitro. J. Appl. Microbiol. 1999, 86, 446–452. [Google Scholar] [CrossRef]
- Dashipour, A.; Razavilar, V.; Hosseini, H.; Shojaee-Aliabadi, S.; German, J.B.; Ghanati, K.; Khakpour, M.; Khaksar, R. Antioxidant and Antimicrobial Carboxymethyl Cellulose Films Containing Zataria multiflora Essential Oil. Int. J. Biol. Macromol. 2015, 72, 606–613. [Google Scholar] [CrossRef]
- Yorgancioglu, A.; Bayramoglu, E.E. Production of Cosmetic Purpose Collagen Containing Antimicrobial Emulsion with Certain Essential Oils. Ind. Crop. Prod. 2013, 44, 378–382. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of Essential Oil and Surfactant on the Physical and Antimicrobial Properties of Corn and Wheat Starch Films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-Natural Composite Wound Dressing Films of Essential Oils Encapsulated in Sodium Alginate with Antimicrobial Properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical Composition and Antimicrobial Activity of Garlic Essential Oils Evaluated in Organic Solvent, Emulsifying, and Self-Microemulsifying Water Based Delivery Systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential Oils and Herbal Extracts as Antimicrobial Agents in Cosmetic Emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, Antioxidant and Antimicrobial Properties of Chitosan Films Containing Eucalyptus globulus Essential Oil. Lwt-Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical Characterization and Antimicrobial Activity of Food-Grade Emulsions and Nanoemulsions Incorporating Essential Oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and Lavender Essential Oils as Antioxidant and Antimicrobial additives of Biogenic Gelatin Films. Ind. Crop. Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Wu, J.E.; Lin, J.; Zhong, Q. Physical and Antimicrobial Characteristics of Thyme Oil Emulsified with Soluble Soybean Polysaccharide. Food Hydrocoll. 2014, 39, 144–150. [Google Scholar] [CrossRef]
- Zivanovic, S.; Chi, S.; Draughon, A.F. Antimicrobial Activity of Chitosan Films Enriched with Essential Oils. J. Food Sci. 2005, 70, M45–M51. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and Antioxidant Efficiency of Nanoemulsion-Based Edible Coating Containing Ginger (Zingiber officinale) Essential Oil and Its Effect on Safety and Quality Attributes of Chicken Breast Fillets. Food Control. 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Fuselli, S.R.; Gende, L.B.; de la Rosa, S.B.G.; Eguaras, M.J.; Fritz, R. Inhibition of Paenibacillus larvae subsp. larvae by the Essential Oils of Two Wild Plants and Their Emulsifying Agents. Span. J. Agric. Res. 2005, 3, 220–224. [Google Scholar] [CrossRef]
- Orafidiya, L.O.; Oyedele, A.O.; Shittu, A.O.; Elujoba, A.A. The Formulation of an Effective Topical Antibacterial Product Containing Ocimum gratissimum Leaf Essential Oil. Int. J. Pharm. 2001, 224, 177–183. [Google Scholar] [CrossRef]
- Patrone, V.; Campana, R.; Vittoria, E.; Baffone, W. In vitro Synergistic Activities of Essential Oils and Surfactants in Combination with Cosmetic Preservatives Against Pseudomonas aeruginosa and Staphylococcus aureus. Curr. Microbiol. 2010, 60, 237–241. [Google Scholar] [CrossRef]
- Haba, E.; Bouhdid, S.; Torrego-Solana, N.; Marqués, A.; Espuny, M.J.; García-Celma, M.J.; Manresa, A. Rhamnolipids as Emulsifying Agents for Essential Oil Formulations: Antimicrobial Effect Against Candida albicans and Methicillin-Resistant Staphylococcus aureus. Int. J. Pharm. 2014, 476, 134–141. [Google Scholar] [CrossRef]


| Essential Oils | Target Microorganisms | Antimicrobial Effects (Minimum Inhibitory Concentration; MIC; μL/mL) | References |
|---|---|---|---|
| Carvacrol | Escherichia coli | 0.225–0.4 | [32,36,37] |
| Escherichia coli O157:H7 | 3 | ||
| Salmonella Typhimurium | 0.225–0.25 | ||
| Listeria monocytogenes | 0.375–5 | [33] | |
| Staphylococcus aureus | 0.175–0.45 | [41] | |
| Bacillus cereus | 0.1875–0.9 | [38] | |
| Thymol | Escherichia coli | 0.225–0.4 | [32,36] |
| Salmonella Typhimurium | 0.056–0.25 | ||
| Listeria monocytogenes | 0.45 | [33] | |
| Staphylococcus aureus | 0.14–0.225 | [41] | |
| Bacillus cereus | 0.45 | [40] | |
| Eugenol | Escherichia coli | 1.0–1.6 | [18,33,36] |
| Escherichia coli O157:H7 | 1.7 | ||
| Salmonella Typhimurium | 0.5 | ||
| Listeria monocytogenes | >1.0 | [34] | |
| Trans-cinnamaldehyde | Escherichia coli | 0.382–1 | [32,36,37,39] |
| Escherichia coli O157:H7 | 0.52 | ||
| Salmonella Typhimurium | 0.382–1 | ||
| Listeria monocytogenes | 3.82 | [34] | |
| Vanillin | Escherichia coli | 2.183 | [35] |
| Listeria innocua | 5.093 |
| Medium | Treatment Conditions | Target Microorganisms | Singular Treatment 1 | Antibacterial Effects (Log Reduction; Log CFU/g or mL) | References |
|---|---|---|---|---|---|
| 0.85% saline | 37 °C, 10 min | Escherichia coli O157:H7 | CAR/EUG/RA/TC/TM/VNL 1 mM | negligible (ca. <1) | [25] |
| 22 °C, 5 min | Escherichia coli Staphylococcus aureus | CAR/TM 2 mM | negligible (ca. <1) | [45] | |
| Deionized water | 22 °C, 10 min | Escherichia coli Listeria monocytogenes Staphylococcus aureus | TM 2 mM | negligible (ca. <1) | [44] |
| 22 °C, 10 min | Escherichia coli Listeria monocytogenes Staphylococcus aureus | CAR 2 mM | 1–2 | ||
| Deionized water (with 10 μg/μL Tween 80) | 1 min 2 | Escherichia coli O157:H7 Listeria innocua | CAR 0.875 μg/mL | >4 | [46] |
| 0.1% peptone water | 37 °C, 30 min | Escherichia coli O157:H7 | cinnamon bark oil 0.0625% | >3 | [66] |
| cinnamon leaf oil 0.0625% | >3 | ||||
| Salmonella Typhimurium | cinnamon bark oil 0.0625% | 4 | |||
| cinnamon leaf oil 0.0625% | >3 | ||||
| Brain heart infusion broth | 4 °C, 8 h | Escherichia coli | Mentha arvensis L. oil 0.625 μL/mL | >5 | [49] |
| Salmonella Enteritidis | Mentha piperita oil 5 μL/mL | >5 | |||
| 37 °C, 8 h | Escherichia coli | armoise oil 0.10% | >8.0 | [50] | |
| clove oil 0.10% | >7.5 | ||||
| Butterfield’s phosphate buffer | 2 min 2 | Escherichia coli Salmonella Typhimurium Staphylococcus aureus | orange oil 10% | 7 | [47] |
| Fish peptone broth | 4 °C, 12 d | Listeria monocytogenes | Bunium persicum (Black zira) oil 0.20% | 3.2 | [52] |
| Luria-Bertani broth | 22 °C, 3 h | Escherichia coli O157:H7 Cronobacter sakazakii | TM 150 μg/mL | 1 | [59] |
| CAR 300 μg/mL | 1 | ||||
| TC 350 μg/mL | 1 | ||||
| Mueller-Hinton broth | 4 °C, 24 h | Campylobacter jejuni | rosemary extract 310 μg/mL | 7 | [51] |
| 37 °C, 0.17 h | Escherichia coli Staphylococcus aureus | oregano oil 0.596 μg/mL | 5 | [67] | |
| Phosphate-buffered saline | 37 °C, 72 h | Salmonella Typhimurium | bark cinnamon oil 0.5% | >9 | [48] |
| 37 °C, 8 h | Listeria monocytogenes | leaf cinnamon oil 0.5% | >9 | ||
| 2 min 2 | Vibrio parahaemolyticus | orange oil 10% | 7 | [47] | |
| Tryptic soy broth | 32 °C, 24 h | Listeria monocytogenes | bark cinnamon oil 313 ppm | 2.0 | [49] |
| TM 625 ppm | 5.3 | ||||
| 37 °C, 16 h | Escherichia coli O157:H7 | noni oil 4 μL/mL | >8 | [54] | |
| Salmonella enterica | noni oil 4 μL/mL | >8 | |||
| 32 °C, 24 h | Escherichia coli O157:H7 | clove oil 600 μg/mL | >5 | [55] | |
| Salmonella Typhimurium | garlic/cinnamon oil 600 μg/mL | 3 | |||
| Listeria monocytogenes | garlic/clove oil 400 μg/mL | >5 | |||
| 37 °C, 24 h | Escherichia coli | Eucalyptus globulus oil 5 μL/mL | 8.7 | [56] | |
| Salmonella Enteritidis | Eucalyptus globulus oil 7.5 μL/mL | 8.1 | |||
| Bacillus cereus | Eucalyptus globulus oil 5 μL/mL | 9.0 | |||
| Staphylococcus aureus | |||||
| 35 °C, 3 h | Escherichia coli Staphylococcus aureus | TM 300 ppm | 4–5 | [57] | |
| Listeria monocytogenes | TM 500 ppm | 4–5 | |||
| Salmonella Typhimurium | |||||
| 37 °C, 24 h | Escherichia coli | EUG/VNL 125 μg/mL | 7 | [58] |
| Matrix | Treatment Condition | Target Microorganisms | Singular Treatment 1 | Antibacterial Effects (Log Reduction; Log CFU/g or mL) | Reference |
|---|---|---|---|---|---|
| Soy sauce | 22 °C, 10 min | Escherichia coli O157:H7 Salmonella Typhimurium Listeria monocytogenes | CAR/TM 1 mM | negligible (ca. <1) | [30] |
| Infant formula (reconstituted) | 45 °C, 30 min | Cronobacter sakazakii Salmonella Typhimurium | VNL < 30 mM | negligible (ca. <1) | [62] |
| Ground beef | Heat (60 °C, 1 h), vacuum package | Clostridium perfringens | CAR/TM/CA/oregano oil 0.1–2.0% | 3.2–5.0 | [64] |
| Ground beef | Marination with wine, storage (5 °C, 10 d) | Salmonella enterica Listeria monocytogenes | oregano oil 0.5% | 1.0–3.1 | [68] |
| Catfish fillet | Storage (4 °C, 14 d) | Listeria monocytogenes | CAR/thyme oil/oregano oil 1–5% | <4 | [69] |
| Taramosalata | Storage (4, 10 °C, 9 d) | Salmonella Enteritidis Listeria monocytogenes | mint oil 0.5–2.0% | 1.1–1.9 | [70] |
| Mozzarella cheese | Listeria monocytogenes | clove oil 0.5–1% | 1–3 | [71] | |
| Alfalfa seed | 60 °C (1, 3, 7 h) | Salmonella spp. | TM/CA 200–600 μg/mL of air | >3 | [65] |
| Honeydew | Storage (4 °C, 21 d) | Natural flora | CA 5–15 mM | <5.1 | [63] |
| Lettuce/baby carrot | 1–15 min | Escherichia coli O157:H7 | thyme oil 0.1–10.0 μg/mL | 1.5–2.0 | [72] |
| Boiled rice | Bacillus cereus | CAR 0.15–0.75 μg/mg | 1.0–3.8 | [73] |
| Components of the EO-Based Antibacterial Complex | Treatment Conditions | Target Microorganisms | Combined Treatment 1 | Antibacterial Effects (Log Reduction; Log CFU/g or mL) [combined effect] | Reference |
|---|---|---|---|---|---|
| Combination of EOs | 37 °C, 24 h | Escherichia coli | CA 100 mg/L + TM 100 mg/L | 2.2 [Synergism] | [36,37] |
| CA 100 mg/L + CAR 100 mg/L | 2.1 [Synergism] | ||||
| TM 100 mg/L + CAR 100 mg/L | 2.4 [Synergism] | ||||
| 37 °C, 24 h | Salmonella Typhimurium | CA 50 mg/L + TM 100 mg/L | 0.44 [Synergism] | [37] | |
| CA 50 mg/L + CAR 100 mg/L | 0.42 [Synergism] | ||||
| TM 100 mg/L + CAR 100 mg/L | 0.27 [Synergism] | ||||
| Medium chain fatty acid | 37 °C, 24 h | Escherichia coli O157:H7 | capric acid 0.4 mM + RA/CAR/EUG/TM/TC 0.4 mM | >7 [Synergism] | [25] |
| caprylic acid 1.0 mM + RA/CAR/EUG/TM/TC 1.0 mM | |||||
| lauric acid 0.5 mM + RA/CAR/TM 1.0 mM | |||||
| 40 °C, 10 min | Cronobacter sakazakii | caprylic acid 20 mM + VNL 30 mM | >7 [Synergism] | [62] | |
| 40 °C, 5 min | Salmonella Typhimurium | caprylic acid 20 mM + VNL 30 mM | |||
| Organic acid | 37 °C, 24 h | Salmonella Typhimurium | lactic acid 0.10% + CAR 200 µL/L | 0.37 [Synergism] | [37] |
| acetic acid 0.05% + TM 100 mg/L | 0.57 [Synergism] | ||||
| acetic acid 0.05% + CAR 100 µL/L | 0.15 [Synergism] | ||||
| Caprylic acid + citric acid | 3 °C, 10 d | Listeria monocytogenes | 0.5% caprylic acid + 0.1% citric acid + 0.2% oregano oil | <5 [Synergism] | [78] |
| Citrus fruit extracts | 22 °C, 5 min | Escherichia coli O157:H7 (Acid-adapted) | calamansi 10% + CAR/TM 2.0 mM | >6.9 [Synergism] | [45] |
| Salmonella Typhimurium (Acid-adapted) | calamansi/lemon 10% + CAR/TM 2.0 mM | ||||
| Listeria monocytogenes (Acid-adapted) | calamansi/lemon/lime 10% + CAR/TM 2.0 mM | ||||
| Lauric arginate (LAE) | 21 °C, 48 h | Listeria monocytogenes | LAE 375 ppm + cinnamon leaf oil/EUG/TM 3,000 ppm | >4 [Synergism] | [84] |
| Escherichia coli O157:H7 | LAE 375 ppm + cinnamon leaf oil/EUG 2,500 ppm | >2 log growth 2 [Antagonism] | |||
| LAE 375 ppm + TM 2,000 ppm | >2 log growth 2 [Antagonism] | ||||
| Salmonella Enteritidis | LAE 375 ppm + cinnamon leaf oil/EUG 2,500 ppm | >1 log growth 2 [Antagonism] | |||
| LAE 375 ppm + TM 2,000 ppm | >2 log growth 2 [Antagonism] | ||||
| Nisin | 8 °C, 20 min | Listeria monocytogenes | nisin 5.3 µg/mL + CAR 1.3 mmol/L | ca. 3 log reduction [Synergism] | [79] |
| 8 °C, 30 min | Bacillus cereus | nisin 5.3 µg/mL + CAR 0.7 mmol/L | ca. 3 log reduction [Synergism] | ||
| 4 °C, 12 d | Listeria monocytogenes | nisin 1000 IU/g + thyme essential oil 0.6% | 4.0 log reduction [Synergism] | [80] | |
| 4 °C, 12 d | Salmonella Enteritidis | nisin 500 IU/g + oregano essential oil 0.9% | ca. 4 log reduction [Synergism] | [82] | |
| 37 °C, 32 h | Escherichia coli O157:H7 | nisin 500 IU/g + thyme essential oil 0.6% | ca. 1 log reduction [Synergism] | [81] | |
| EDTA | 37 °C, 24 h | Salmonella Typhimurium | EDTA 75 mg/L + TM 100 mg/L | 0.7 log reduction [Synergism] | [37] |
| Sodium chloride | 22 °C, 1 min | Escherichia coli O157:H7 | sodium chloride 5% + CAR 2.0 mM | 7 log reduction [Synergism] | [44] |
| Listeria monocytogenes | |||||
| Staphylococcus aureus | sodium chloride 10% + CAR 2.0 mM | ||||
| 22 °C, 1 min | Escherichia coli O157:H7 | sodium chloride 3% + TM 2.0 mM | 7 log reduction [Synergism] | ||
| Listeria monocytogenes | sodium chloride 10% + TM 1.0 mM | ||||
| Staphylococcus aureus | sodium chloride 15% + TM 1.0 mM | ||||
| Soy sauce | 4 °C, 5 min | Escherichia coli O157:H7 | soy sauce + TM 0.5 mM | 7 log reduction [Synergism] | [30] |
| 4 °C, 5 min | Listeria monocytogenes | soy sauce + TM 0.5 mM | |||
| 4 °C, 10 min | Salmonella Typhimurium | soy sauce + TM 0.5 mM | |||
| Teriyaki sauce | 4 °C, 7 d | Escherichia coli O157:H7 | teriyaki sauce + TM/CAR 0.5% | 3.0–3.4 log reduction [Synergism] | [83] |
| Listeria monocytogenes | |||||
| Salmonella Typhimurium | |||||
| Biological silver nano particles (bio-AgNPs) | 37 °C, 24 h | Methicillin resistant Staphylococcus aureus | bio-AgNP 125 µM + Origanum vulgare oil 0.298 mg/mL | >5 log reduction [Synergism] | [67] |
| Escherichia coli | bio-AgNP 31.25 µM + Origanum vulgare oil 0.075 mg/mL | >5 log reduction [Synergism] |
| Species | Product Type | No. of Components Other than EO 1 | EO with Antibacterial Activity 2 | Test Method | Reference |
|---|---|---|---|---|---|
| Acinetobacter baumanii | EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] |
| Aeromonas sobria | EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] |
| Bacillus cereus | Carboxymethyl cellulose film | 2 | Zataria multiflora Boiss oil | Agar diffusion | [96] |
| Escherichia coli | Emulsion | 8 | Thymus vulgaris, Origanum onites | Agar diffusion | [97] |
| Corn and wheat starch film | 7 | cinnamon, lavender, lemongrass, lemon oil, peppermint, tea tree | Agar diffusion | [98] | |
| Wound dressing films | 3 | lemon oil | Agar diffusion | [99] | |
| Carboxymethyl cellulose film | 2 | Zataria multiflora Boiss oil | Agar diffusion | [96] | |
| Water-based emulsion | 2 | garlic oil | Agar diffusion | [100] | |
| Cream formulation | 7 | Lavandulla officinallis, Melaleuca alternifolia, Cinnamomum zeylanicum oils | Time-kill assay | [101] | |
| Chitosan film | 3 | Eucalyptus globulus oil | Agar diffusion | [102] | |
| Emulsion | 2 | lemongrass, majoram, clove, palmarosa, tea tree, rosewood, thyme, sage, geranium, mint | Time-kill assay | [103] | |
| Gelatin film | 2 | oregano, lavender oil | Agar diffusion | [104] | |
| Cellulose film | 2 | CA, EUG | Vapor diffusion | [93] | |
| Escherichia coli O157:H7 | Surfactant micelles | 1 | CAR, EUG | Broth dilution | [94] |
| Emulsion | 1 | thyme oil | Broth dilution | [105] | |
| Chitosan film | 3 | oregano oil | Agar diffusion | [106] | |
| Enterococcus faecalis | Emulsion | 8 | Origanum onites | Agar diffusion | [97] |
| EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] | |
| Klebsiella pneumoniae | EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] |
| Listeria monocytogenes | Surfactant micelles | 1 | CAR, EUG | Broth dilution | [94] |
| Water-based emulsion | 2 | garlic oil | Agar diffusion | [100] | |
| Edible coating | 2 | ginger oil | Agar diffusion | [107] | |
| Emulsion | 1 | thyme oil | Broth dilution | [105] | |
| Cellulose film | 2 | CA, EUG | Vapor diffusion | [93] | |
| Chitosan film | 3 | oregano oil | Agar diffusion | [106] | |
| Paenibacillus larvae | EO + Emulsifier | 1 | wild chamomile, Andean thyme oil | Broth dilution | [108] |
| Pseudomonas aeruginosa | Carboxymethyl cellulose film | 2 | Zataria multiflora Boiss oil | Agar diffusion | [96] |
| Cream formulation | 7 | Lavandulla officinallis, Melaleuca alternifolia, Cinnamomum zeylanicum oils | Time-kill assay | [101] | |
| Chitosan film | 3 | Eucalyptus globulus oil | Agar diffusion | [102] | |
| EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] | |
| Proteus spp. | Topical formulation | 1-4 | Ocimum gratissimum leaf oil | Agar diffusion | [109] |
| Staphylococcus aureus | Emulsion | 8 | Thymus vulgaris, Origanum onites | Agar diffusion | [97] |
| Topical formulation | 1-4 | Ocimum gratissimum leaf oil | Agar diffusion | [109] | |
| Wound dressing films | 3 | lemon oil | Agar diffusion | [99] | |
| Topical formulation | 1-4 | Ocimum gratissimum leaf oil | Agar diffusion | [109] | |
| EO + Preservative | 1 | mint, oregano, rosemary, sage | Broth dilution | [110] | |
| Carboxymethyl cellulose film | 2 | Zataria multiflora Boiss oil | Agar diffusion | [96] | |
| Water-based emulsion | 2 | garlic oil | Agar diffusion | [100] | |
| EO + Emulsifier | 1 | oregano oil, cinnamon oil, tea tree oil, lavender oil | Agar diffusion | [111] | |
| Chitosan film | 3 | Eucalyptus globulus oil | Agar diffusion | [102] | |
| EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] | |
| Gelatin film | 2 | oregano, lavender oil | Agar diffusion | [104] | |
| Cellulose film | 2 | CA, EUG | Vapor diffusion | [93] | |
| Serratia marcescens | EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] |
| Salmonella Typhimurium | Carboxymethyl cellulose film | 2 | Zataria multiflora Boiss oil | Agar diffusion | [99] |
| Water-based emulsion | 2 | garlic oil | Agar diffusion | [100] | |
| EO + Interfering substance | 1 | tea tree oil | Agar diffusion, broth dilution | [95] | |
| Edible coating | 2 | ginger oil | Agar diffusion | [107] | |
| Salmonella Enteritidis | Emulsion | 1 | thyme oil | Broth dilution | [105] |
| Cellulose film | 2 | CA, EUG | Vapor diffusion | [93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. https://doi.org/10.3390/molecules25071752
Cho TJ, Park SM, Yu H, Seo GH, Kim HW, Kim SA, Rhee MS. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules. 2020; 25(7):1752. https://doi.org/10.3390/molecules25071752
Chicago/Turabian StyleCho, Tae Jin, Sun Min Park, Hary Yu, Go Hun Seo, Hye Won Kim, Sun Ae Kim, and Min Suk Rhee. 2020. "Recent Advances in the Application of Antibacterial Complexes Using Essential Oils" Molecules 25, no. 7: 1752. https://doi.org/10.3390/molecules25071752
APA StyleCho, T. J., Park, S. M., Yu, H., Seo, G. H., Kim, H. W., Kim, S. A., & Rhee, M. S. (2020). Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules, 25(7), 1752. https://doi.org/10.3390/molecules25071752







