Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts
Abstract
1. Introduction
2. Results and Discussion
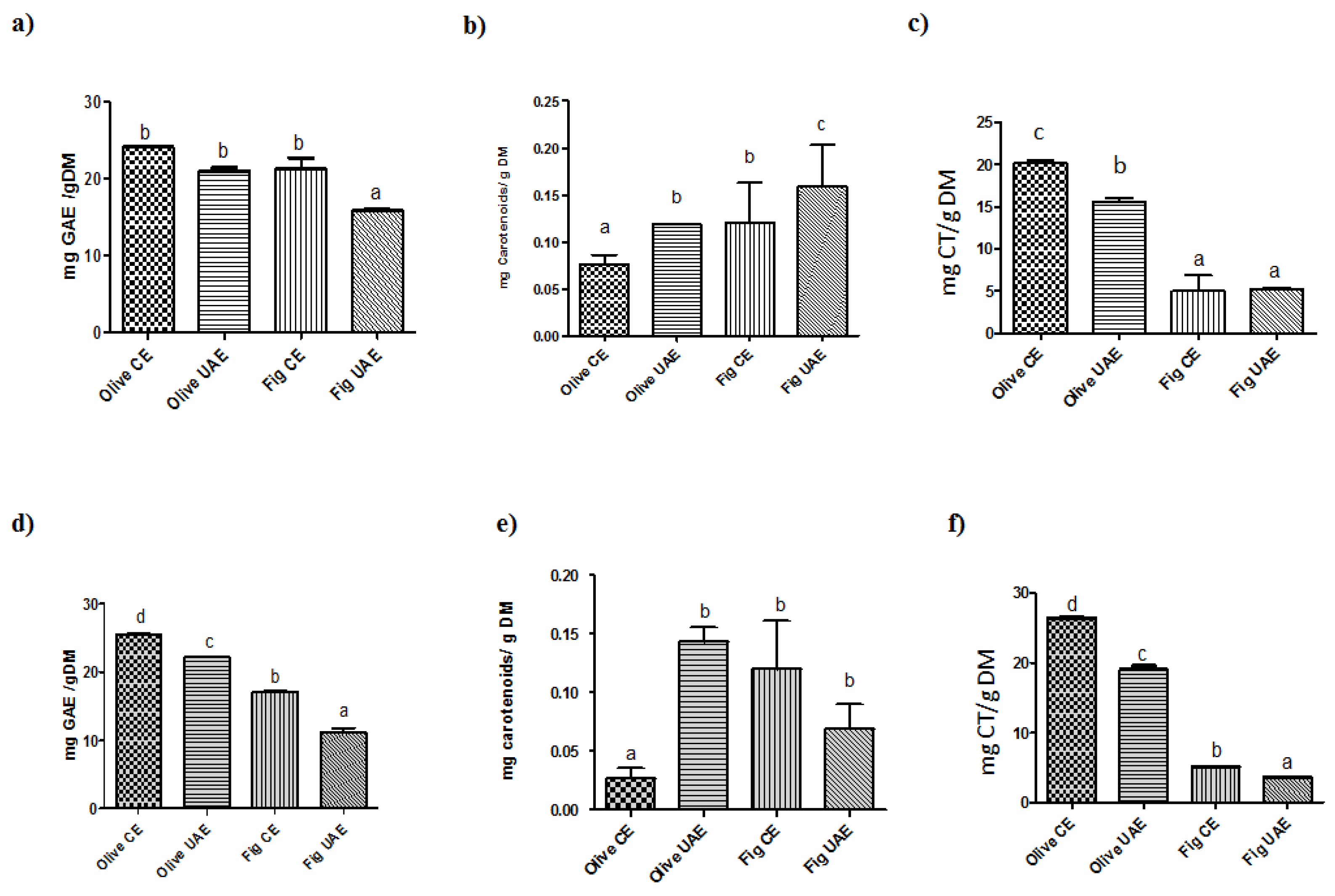
2.1. Contents of Total Phenolics, Flavonoids, and Carotenoids
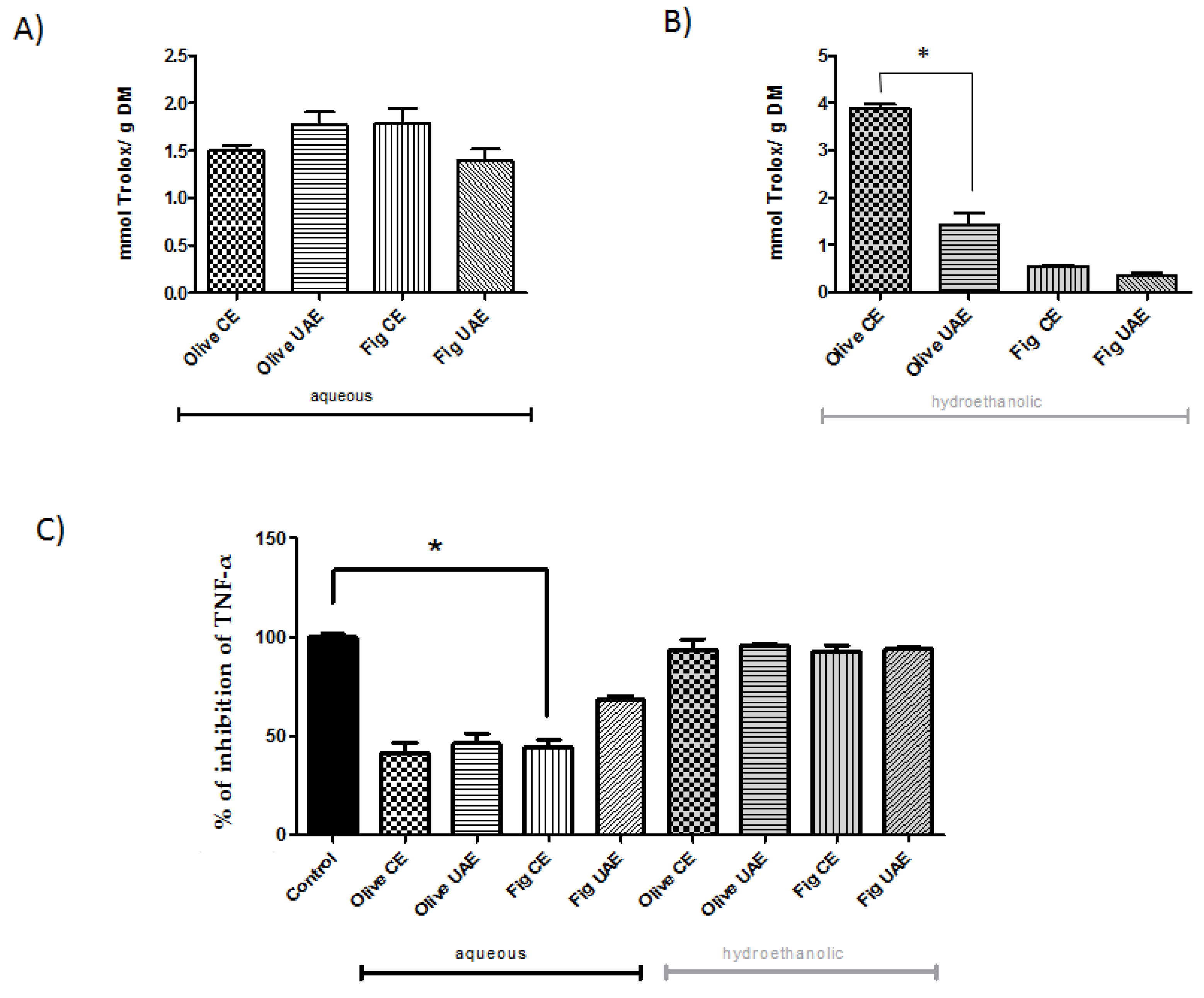
2.2. Individual Phenolic Composition, Antioxidant Capacity and Anti-Inflammatory Effects
2.3. Effect of Extracts on Bacterial Growth
3. Materials and Methods
3.1. Plant Materials
3.2. Chemical Reagents
3.3. Solvents and Extraction Methodology
3.4. Total Phenolic, Flavonoid and Carotenoids Contents
3.5. Antioxidant Capacity and Anti-Inflammatory Effects
3.6. Effect of Extracts on Bacterial Growth
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.J.; Jambrak, A.R.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Kapiszewska, M.; Sołtys, E.; Visioli, F.; Cierniak, A.; Zajac, G. The protective ability of the Mediterranean plant extract against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J. Physiol. Pharm. 2005, 56, 183–197. [Google Scholar]
- Şahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-Free Microwave-Assisted Extraction of Polyphenols from Olive Tree Leaves: Antioxidant and Antimicrobial Properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of drying method on oleuropein, total phenolic content, flavonoid content, and antioxidant activity of olive (Olea europaea) leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar] [CrossRef]
- Ghazi, F.; Rahmat, A.; Yassin, Z.; Shazini Ramli, N.; Amira Buslima, N. Determination of total polyphenols and nutritional composition of two different types of Ficus carica leaves cultivated in Saudi Arabia. Pak. J. Nutr. 2012, 11, 1061–1065. [Google Scholar]
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; & Kovačević, D.B. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 106, 19–28. [Google Scholar] [CrossRef]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Bubonja-Sonje, M.; Giacometti, J.; Abram, M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011, 127, 1821–1827. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuelsbioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Catalán, E.B.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochemistry 2017, 34, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Grimi, N.; Vorobiev, E. Evaluating the potential of cell disruption technologies for green selective extraction of antioxidant compounds from Stevia rebaudiana Bertoni leaves. J. Food Eng. 2015, 149, 222–228. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Ílbay, Z.; S.¸ahin, S.; Büyükkabasakal, K. A novel approach for olive leaf extraction through ultrasound technology: Response surface methodology versus artificial neural networks. Korean J. Chem. Eng. 2014, 31, 1661–1667. [Google Scholar]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Odeh, I.; Bisher, A.; Abbadi, J.; Qabbajeh, M. Effect of geographical region and harvesting date on antioxidant activity, phenolic and flavonoid content of olive leaves. J. Food Nutr. Res. 2014, 2, 925–930. [Google Scholar] [CrossRef]
- Mopuri, R.; Ganjayi, M.; Meriga, B.; Koorbanally, N.A.; Islam, M.S. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J. Food Drug Anal. 2018, 26, 201–210. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Samaras, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, M.; Do Carmo, M.A.V.; Cruz, T.M.; Azevedo, L.; Myoda, T.; Furtado, M.M.; Marques, M.B.; Sant’ana, A.S.; Genovese, M.I.; Oh, W.Y.; et al. Camu-camu seed (Myrciaria dubia)-from side stream to an antioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient. Food Chem. 2020, 310, 125909. [Google Scholar] [CrossRef] [PubMed]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Medina, E.; Romero-Gil, V.; Garrido-Fernández, A.; Arroyo-López, F.N. Survival of foodborne pathogens in natural cracked olive brines. Food Microbiol. 2016, 59, 104–111. [Google Scholar] [CrossRef]
- Refaat, J.; Yehia, S.Y.; Ramadan, M.A.; Kamel, M.S. Rhoifolin: A review of sources and biological activities. Int. J. Pharm. 2015, 2, 102–109. [Google Scholar]
- Alvarez, M.D.L.A.; Debattista, N.B.; Pappano, N.B. Synergism of flavonoids with bacteriostatic action against Staphylococcus aureus ATCC 25 923 and Escherichia coli ATCC 25 922. Biocell 2006, 30, 39–42. [Google Scholar]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71, S110–S116. [Google Scholar] [CrossRef]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Rodríguez Vaquero, M.J.; Alberto, M.R.; de Nadra, M.C. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharm. Off. J. Pol. Physiol. Soc. 2012, 63, 497–503. [Google Scholar]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Carreres, J.E.; Micol, V.; García-Pérez, J.V. Influence of olive leaf processing on the bioaccessibility of bioactive polyphenols. J. Agric. Food Chem. 2014, 62, 6190–6198. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S.; Cárcel, J.A.; García-Pérez, J.V. Ultrasound-assisted air-drying of apple (Malus domestica L.) and its effects on the vitamin of the dried product. Food Bioprocess. Technol. 2015, 8, 1503–1511. [Google Scholar] [CrossRef]
- Abdelkebir, R.; Alcántara, C.; Falcó, I.; Sánchez, G.; Garcia-Perez, J.V.; Neffati, M.; Collado, M.C. Effect of ultrasound technology combined with binary mixtures of ethanol and water on antibacterial and antiviral activities of Erodium glaucophyllum extracts. Innov. Food Sci. Emerg. Technol. 2019, 52, 189–196. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Lee, H.; Castle, W.S. Seasonal changes of carotenoid pigments and color in hamlin, earlygold, and budd blood orange juices. J. Agric. Food Chem. 2000, 49, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Env.. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of statistical methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Compound Name | Formula | Expected m/z | CE | UAE |
|---|---|---|---|---|
| Oleoside 11-methylester | C17H24O11 | 403.1246 | 18537 ± 151 | 18128 ± 290 |
| Rhoifolin | C27H30O14 | 577.1563 | 6932 ± 574 | 6123 ± 429 |
| Demethyloleuropein | C24H30O13 | 525.1614 | 5676 ± 317 | 4692 ± 143 |
| Querc-3-O-gal-7-O-rhamnoside | C27H30O16 | 609.1461 | 3555 ± 319 | 2651 ± 137 |
| Phloretin xylosyl-galactoside | C26H32O14 | 567.1719 | 4164 ± 222 | 3369 ± 124 |
| 3-Hydroxyphloretin 2-O-xylosyl-gluc | C26H32O15 | 583.1668 | ND | 956 ± 57 |
| Kaempferol 3-rutinoside | C27H30O15 | 593.1512 | ND | 2682 ± 213 |
| Kaempferol 3-O-sophoroside | C27H30O16 | 609.1461 | 3555 ± 319 | 2651 ± 137 |
| Verbascoside | C29H36O15 | 623.1981 | 2692 ± 21 | 2250 ± 122 |
| Apigenin 6-C-glucoside | C21H20O10 | 431.0984 | 1956 ± 61 | 1593 ± 100 |
| 1-Sinapoyl-2-feruloylgentiobiose | C33H40O18 | 723.2142 | 1622 ± 124 | 1601 ± 153 |
| Isorhamnetin 7-O-rhamnoside | C22H22O11 | 461.1089 | 1457 ± 125 | 1083 ± 101 |
| Hydroxytyrosol | C8H10O3 | 153.0557 | 766 ± 26 | 646 ± 13 |
| Diosmin | C28H32O15 | 607.1668 | 649 ± 21 | 548 ± 36 |
| Kaempferol 3-O-rhamnosyl-rhamnosyl-gluc | C33H40O19 | 739.2091 | 639 ± 32 | 616 ± 19 |
| Hydroxytyrosol 1-O-glucoside | C14H20O9 | 331.1035 | 667 ± 81 | 671 ± 23 |
| Protocatechuic acid 4-O-glucoside | C13H16O9 | 315.0722 | 595 ± 17 | 488 ± 17 |
| Sinapoyl glucose | C17H22O10 | 385.114 | 488 ± 24 | ND |
| Matairesinol | C20H22O6 | 357.1344 | 455 ± 27 | 506 ± 18 |
| Kaempferol | C15H10O6 | 285.0405 | 474 ± 65 | 216 ± 50 |
| p-HPEA-EA | C19H22O7 | 361.1293 | 350 ± 61 | ND |
| 3,4-DHPEA-EA | C19H22O8 | 377.1242 | ND | 929 ± 139 |
| 3-Methylcatechol | C7H8O2 | 123.0452 | 339 ± 58 | ND |
| Quercetin 3-O-glucoside | C21H20O12 | 463.0882 | 294 ± 8 | 171 ± 28 |
| Oleoside dimethylester | C18H26O11 | 417.1402 | 285 ± 9 | 201 ± 22 |
| 4-Hydroxybenzoic acid 4-O-gluc | C13H16O8 | 299.0772 | 185 ± 5 | 156 ± 11 |
| p-Coumaric acid | C9H8O3 | 163.0401 | 170 ± 1 | 126 ± 23 |
| Dihydroquercetin 3-O-glucoside | C21H22O12 | 465.1039 | 163 ± 16 | 117 ± 12 |
| 3-Sinapoylquinic acid | C18H22O10 | 397.114 | 111 ± 13 | ND |
| Quercetin | C15H10O7 | 301.0354 | 46 ± 5 | ND |
| Rosmadial | C20H24O5 | 343.1551 | ND | 79 ± 45 |
| Compound Name | Formula | Expected m/z | CE | UAE |
|---|---|---|---|---|
| Apigenin 6-C-glucoside 8-C-arabinoside | C26H28O14 | 563.1406 | 16475 ± 2471 | 11765.26 ± 1038 |
| Apigenin 6-C-glucoside | C21H20O10 | 431.0984 | 886 ± 30 | 509 ± 112 |
| Quercetin 3- rutinoside | C27H30O16 | 609.1461 | 5008 ± 504 | 3539 ± 114 |
| Rhoifolin | C27H30O14 | 577.1563 | 860 ± 83 | 578 ± 29 |
| 3-Feruloylquinic acid | C17H20O9 | 367.1035 | 2115 ± 126 | 417 ± 63 |
| 4-Hydroxycoumarin | C9H6O3 | 161.0244 | 1280 ± 180 | 719 ± 157 |
| Ferulic acid | C10H10O4 | 193.0506 | 515 ± 97 | 292 ± 47 |
| Kaempferol 3-O-xylosyl-glucoside | C26H28O15 | 579.1355 | 864 ± 71 | 602 ± 86 |
| Kaempferol 3-O-xylosyl-rutinoside | C32H38O19 | 725.1935 | ND | 238 ± 27 |
| 3-Sinapoylquinic acid | C18H22O10 | 397.114 | 1294 ± 73 | 395 ± 73 |
| Sinapoyl glucose | C17H22O10 | 385.114 | 751 ± 162 | 485 ± 99 |
| Kaempferol 3-O-rhamnoside | C21H20O10 | 431.0984 | 886 ± 30 | 509 ± 112 |
| Kaempferol 3-O-rutinoside | C27H30O15 | 593.1512 | 570 ± 16 | 368 ± 31 |
| Isorhamnetin 7-O-rhamnoside | C22H22O11 | 461.1089 | 511 ± 26 | 285 ± 37 |
| p-Coumaroyl malic acid | C13H12O7 | 279.051 | 184 ± 25 | ND |
| Resveratrol | C14H12O3 | 227.0714 | 177 ± 37 | 98 ± 37 |
| Didymin | C28H34O14 | 593.1876 | 145 ± 18 | ND |
| Chrysoeriol | C16H12O6 | 299.0561 | 128 ± 46 | ND |
| Oleoside 11-methylester | C17H24O11 | 403.1246 | 128 ± 15 | 70 ± 24 |
| 4-Hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 299.0772 | 104 ± 22 | ND |
| Rosmadial | C20H24O5 | 343.1551 | 60 ± 26 | 101 ± 80 |
| Protocatechuic acid 4-O-glucoside | C13H16O9 | 315.0722 | 187 ± 5 | 120 ± 14 |
| Cyanidin 3-O-(6-succinyl-glucoside) | C25H25O14 | 548.1172 | 86 ± 13 | 39 ± 7 |
| Dihydrocaffeic acid | C9H10O4 | 181.0506 | 29 ± 10 | 13 ± 5 |
| Quercetin 3-O-glucosyl-rhamnosyl-glucoside | C33H40O21 | 771.1989 | 155 ± 138 | 154 ± 22 |
| Condition | Composition | Method * | Specific Growth Rate (h−1) | ‡MOD |
|---|---|---|---|---|
| Salmonella enterica | ||||
| Bacteria | water | - | 0.432 ± 0.006 a | 1.407 ± 0.033 c |
| Bacteria | EtOH | - | 0.484 ± 0.047 bcd | 1.418 ± 0.037 c |
| Olive leaves | water | CE | 0.334 ± 0.060 a | 1.406 ± 0.064 c |
| UAE | 0.398 ± 0.017 ab | 1.362 ± 0.006 bc | ||
| EtOH | CE | 0.322 ± 0.039 a | 1.236 ± 0.002 ab | |
| UAE | 0.345 ± 0.005 ab | 1.187 ± 0.074 a | ||
| Fig leaves | water | CE | 0.515 ± 0.043 cd | 1.471 ± 0.008 c |
| UAE | 0.559 ± 0.051 d | 1.457 ± 0.011 c | ||
| EtOH | CE | 0.571 ± 0.014 d | 1.435 ± 0.024 d | |
| UAE | 0.550 ± 0.15 d | 1.369 ± 0.006 bc | ||
| Listeria innocua | ||||
| Bacteria | water | - | 0.252 ± 0.22 a | 1.269 ± 0.017 abc |
| Bacteria | EtOH | - | 0.248 ± 0.042 a | 1.385 ± 0.003 bcd |
| Olive leaves | water | CE | 0.316 ± 0.034 a | 1.262 ± 0.037 abc |
| UAE | 0.274 ± 0.079 a | 1.208 ± 0.004 a | ||
| EtOH | CE | 0.358 ± 0.058 a | 1.189 ± 0.013 bcd | |
| UAE | 0.348 ± 0.063 a | 1.254 ± 0.090 d | ||
| Fig leaves | water | CE | 0.184 ± 0.009 a | 1.383 ± 0.008 a |
| UAE | 0.233 ± 0.034 a | 1.423 ± 0.058 ab | ||
| EtOH | CE | 0.235 ± 0.040 a | 1.407 ± 0.033 cd | |
| UAE | 0.300 ± 0.056 a | 1.391 ± 0.013 bcd | ||
| Staphylococcus aureus | ||||
| Bacteria | water | - | 0.556 ± 0.002 a | 1.851 ± 0.064 a |
| Bacteria | EtOH | - | 0.561 ± 0.075 a | 1.882 ± 0.001 a |
| Olive leaves | water | CE | 0.580 ± 0.024 a | 1.939 ± 0.033 a |
| UAE | 0.636 ± 0.016 a | 1.899 ± 0.013 a | ||
| EtOH | CE | 0.755 ± 0.144 a | 1.970 ± 0.028 a | |
| UAE | 0.657 ± 0.031 a | 1.989± 0.069 a | ||
| Fig leaves | water | CE | 0.638 ± 0.074 a | 1.901 ± 0.031 a |
| UAE | 0.587 ± 0.003 a | 1.857 ± 0.028 a | ||
| EtOH | CE | 0.601 ± 0.010 a | 1.851 ± 0.012 a | |
| UAE | 0.585 ± 0.006 a | 1870 ± 0.045 a | ||
| Condition | Composition | Method | Specific Growth Rate (h−1) | ‡MOD |
|---|---|---|---|---|
| Lactobacillus casei | ||||
| Bacteria | water | - | 0.392 ± 0.001 a | 2.797 ± 0.027 a |
| Bacteria | EtOH | - | 0.383 ± 0.003 a | 2.837 ± 0.018 ab |
| Olive leaves | water | CE | 0.398 ± 0.016 a | 2.857 ± 0.058 ab |
| UAE | 0.388 ± 0.005 a | 2.884 ± 0.033 ab | ||
| EtOH | CE | 0.383 ± 0.008 a | 2.904 ± 0.071 abc | |
| UAE | 0.398 ± 0.007 a | 2.852 ± 0.083 ab | ||
| Fig leaves | water | CE | 0.437 ± 0.002b | 3.077 ± 0.023 c |
| UAE | 0.441 ± 0.003b | 3.024 ± 0.041 bc | ||
| EtOH | CE | 0.390 ± 0.009 a | 2.873 ± 0.062 ab | |
| UAE | 0.389 ± 0.007 a | 2.885 ± 0.006 abc | ||
| Bifidobacterium lactis | ||||
| Bacteria | water | -- | 0.234 ± 0.021 a | 2.451 ± 0.052 a |
| Bacteria | EtOH | 0.236 ± 0.016 a | 2.463 ± 0.095 a | |
| Olive leaves | water | CE | 0.255 ± 0.008 a | 2.634 ± 0.081 a |
| UAE | 0.223 ± 0.005 a | 2.461 ± 0.013 a | ||
| EtOH | CE | 0.233± 0.011 a | 2.492 ± 0.047 a | |
| UAE | 0.229 ± 0.017 a | 2.481 ± 0.008 a | ||
| Fig leaves | water | CE | 0.252 ± 0.003 a | 2.481 ± 0.013 a |
| UAE | 0.263 ± 0.007 a | 2.540 ± 0.036 a | ||
| EtOH | CE | 0.217 ± 0.006 a | 2.386 ± 0.050 a | |
| UAE | 0.234 ± 0.005 a | 2.476 ± 0.012 a | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. https://doi.org/10.3390/molecules25071718
Alcántara C, Žugčić T, Abdelkebir R, García-Pérez JV, Jambrak AR, Lorenzo JM, Collado MC, Granato D, Barba FJ. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules. 2020; 25(7):1718. https://doi.org/10.3390/molecules25071718
Chicago/Turabian StyleAlcántara, Cristina, Tihana Žugčić, Radhia Abdelkebir, Jose V. García-Pérez, Anet Režek Jambrak, José M. Lorenzo, María Carmen Collado, Daniel Granato, and Francisco J. Barba. 2020. "Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts" Molecules 25, no. 7: 1718. https://doi.org/10.3390/molecules25071718
APA StyleAlcántara, C., Žugčić, T., Abdelkebir, R., García-Pérez, J. V., Jambrak, A. R., Lorenzo, J. M., Collado, M. C., Granato, D., & Barba, F. J. (2020). Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules, 25(7), 1718. https://doi.org/10.3390/molecules25071718










