Lignans in Knotwood of Norway Spruce: Localisation with Soft X-ray Microscopy and Scanning Transmission Electron Microscopy with Energy Dispersive X-ray Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Knotwood Compounds
2.2. Extractives in the Knotwood
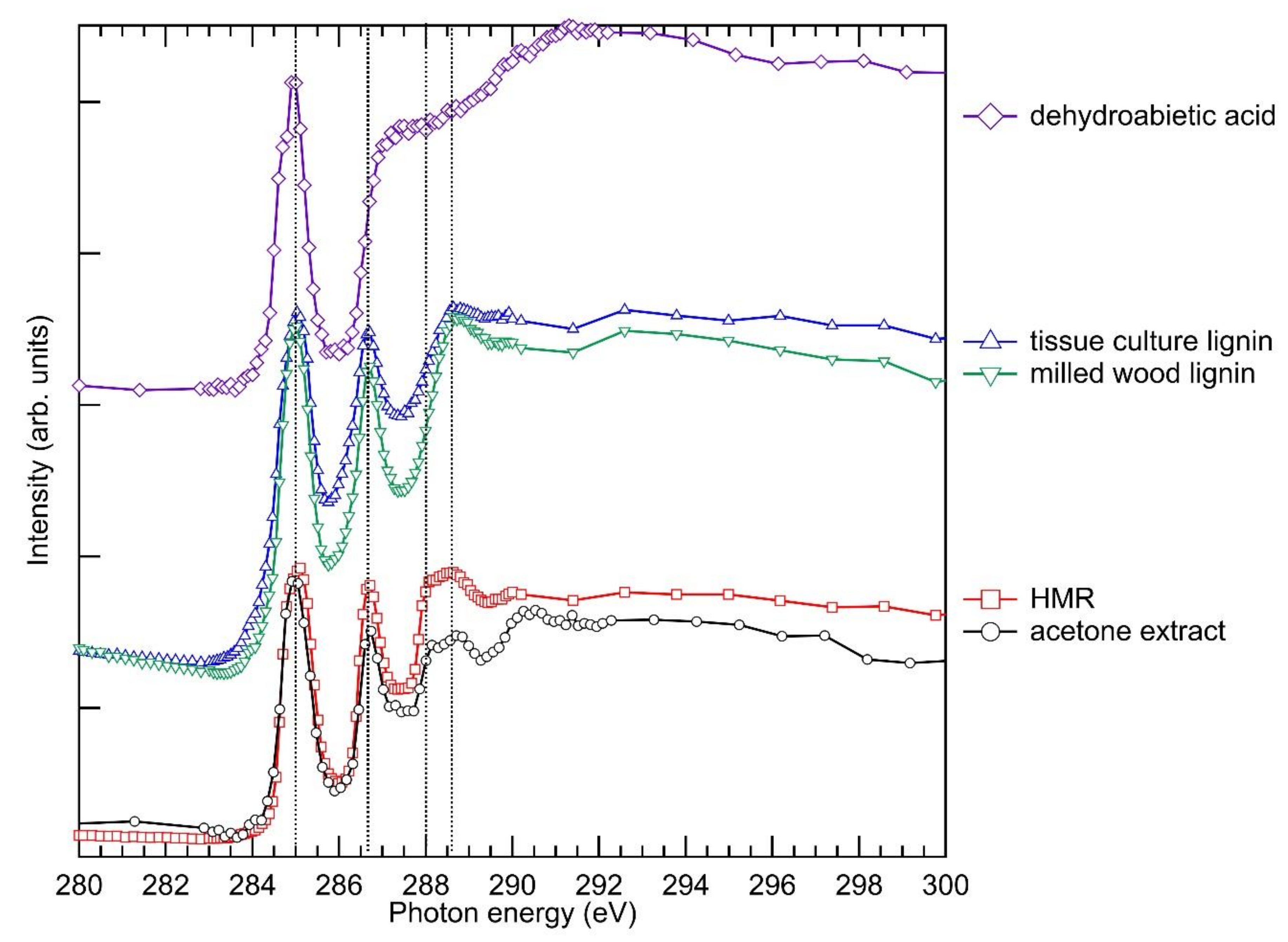
2.3. X-ray Absorption Spectra of the Acetone Extract and of Selected Reference Compounds
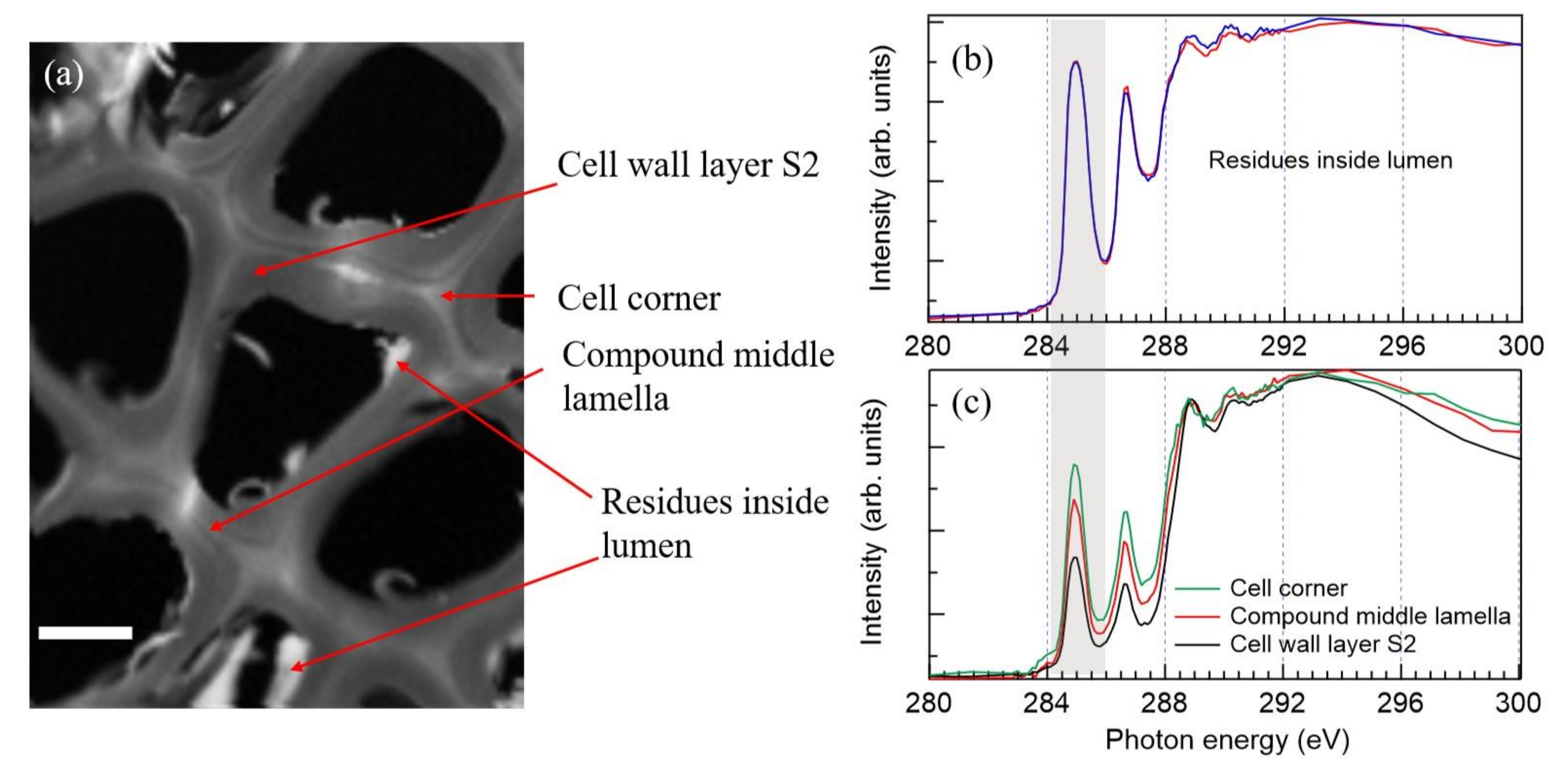
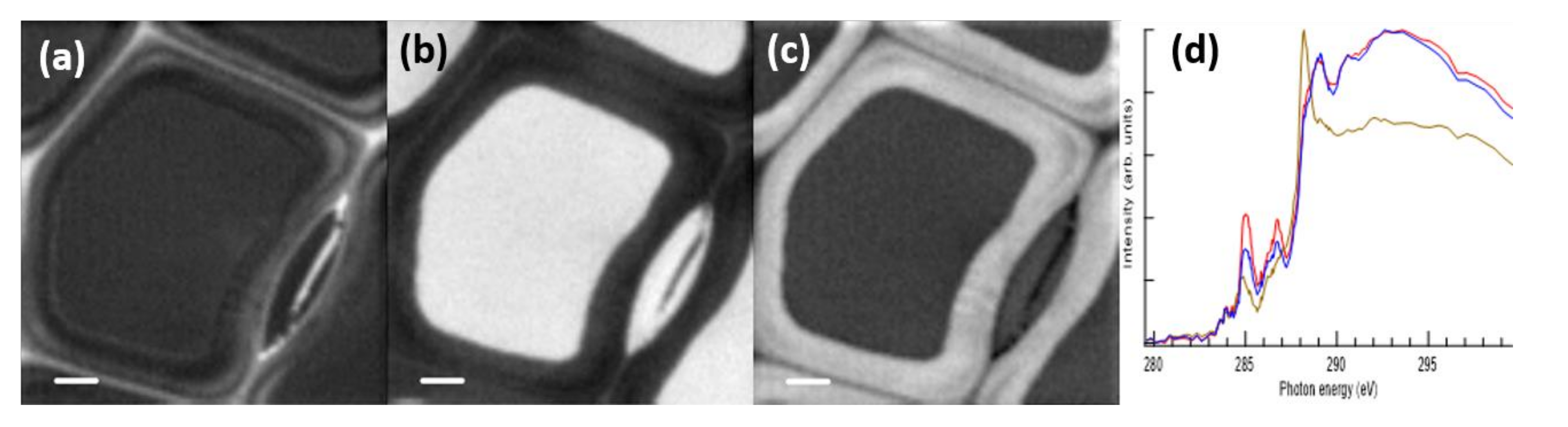
2.4. STXM Imaging of Cryosectioned Samples
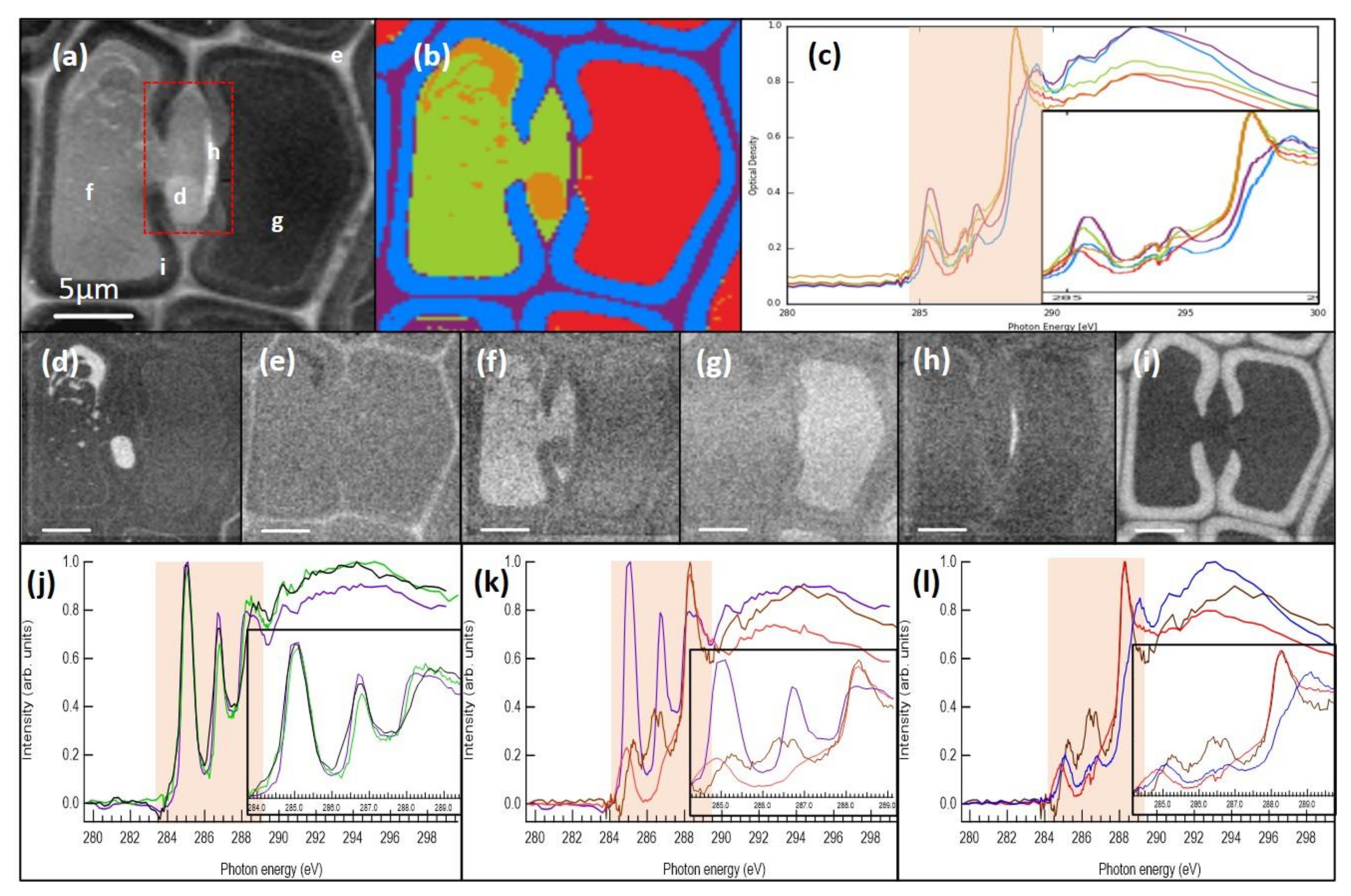
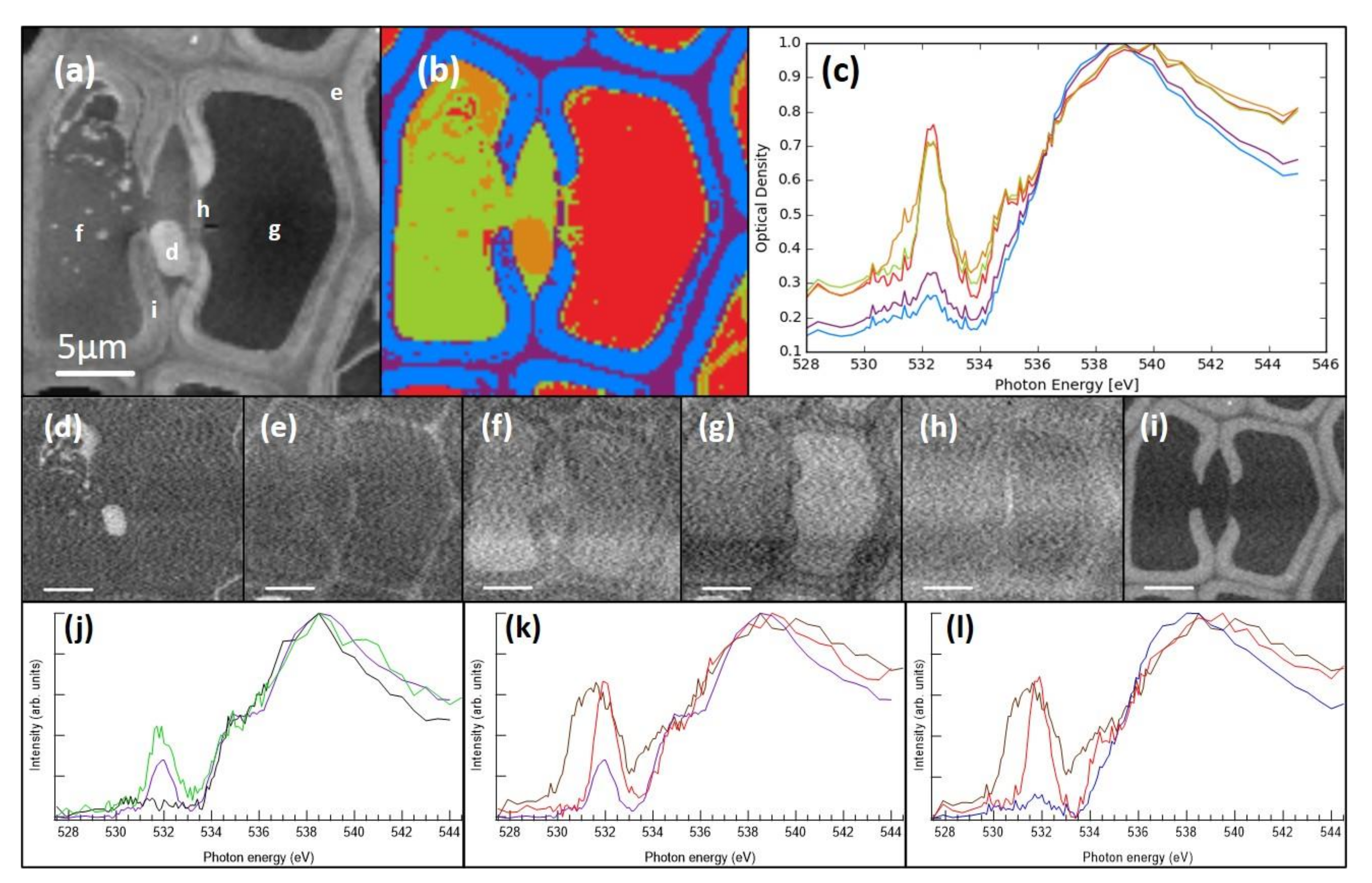
2.5. STXM Imaging of Resin-Embedded Samples
2.6. STEM-EDS Imaging of Resin-Embedded Samples
3. Materials and Methods
3.1. Chemical Analyses
3.1.1. GC-FID/GC-MS Analysis of Extractives
3.1.2. Hemicelluloses and Pectins
3.1.3. Cellulose
3.1.4. Lignin
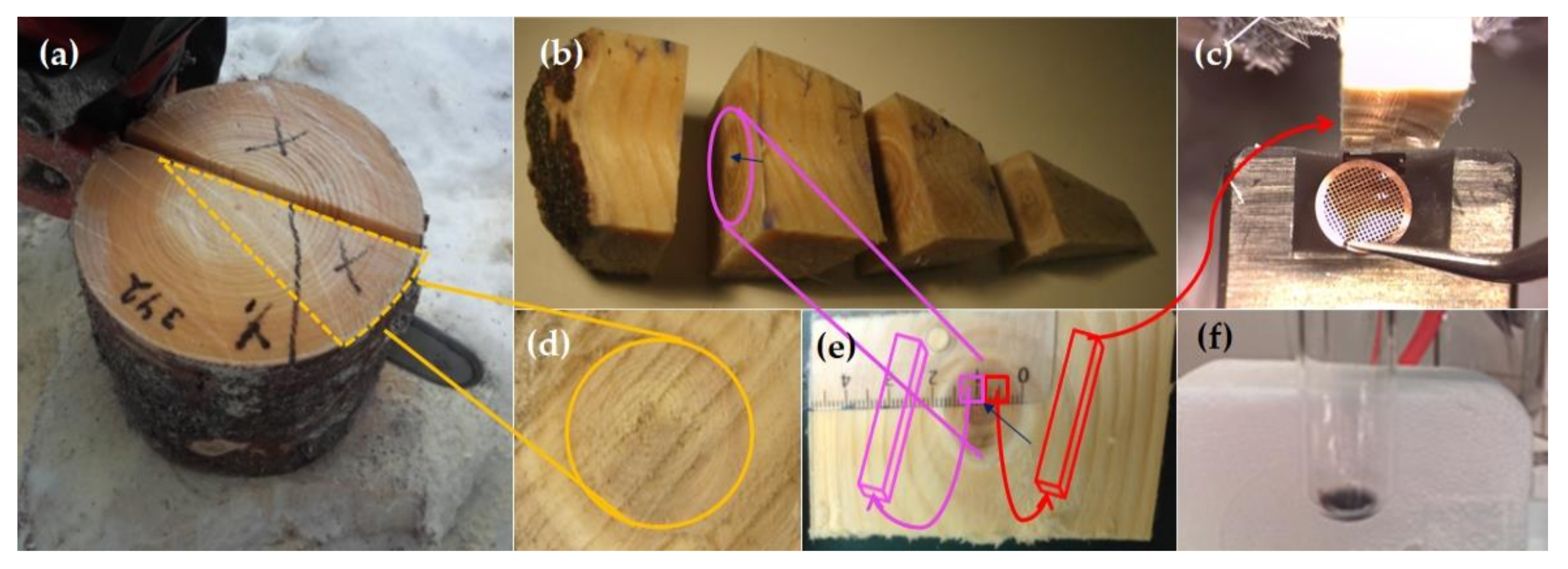
3.2. Resin-Embedded Samples
3.3. Cryosectioned Samples
3.4. Reference Samples
- Hydroxymatairesinol: a mixture of both isomers HMR2 and HMR1 (93:7, w/w), approximately 90% purity (obtained from Åbo Akademi University, Turku, Finland). The isolation was carried out according to Eklund and Raitanen (2019) [48].
- Tissue culture lignin: extracellular lignin was collected from a Norway spruce cell suspension culture (line A3/85) [49,50,51]. Lignin was pelleted by centrifugation from the culture medium and washed with water. Lignin-bound proteins [52] were extracted with buffered 1 M NaCl, after which the carbohydrates bound to lignin [53] were diminished by a treatment with glycosyl hydrolases according to Warinowski et al. (2016) [52]. After several washes with water, the lignin was lyophilised.
- Milled wood lignin (MWL) was prepared from Norway spruce sapwood according to the Björkman procedure (1956) [54].
- Microcrystalline cellulose, CAS Number: 9004-34-6 (Sigma-Aldrich Co., St. Louis, MO, USA).
- Norway spruce galactoglucomannan (GGM) with residual arabinoglucurunoxylan and pectin was extracted with pressurised hot water, concentrated and precipitated with ethanol [55].
- Dehydroabietic acid, CAS Number: 1740-19-8 (Sigma-Aldrich Co., St. Louis, MO, USA).
- Acetone extract of the Norway spruce knotwood: the knotwood extract was prepared according to the protocol presented in Section 3.1.1.
3.5. Scanning Transmission X-ray Microscopy
3.6. Scanning Transmission Electron Microscopy with Energy Dispersive Spectroscopy
3.7. OsO4 Binding Experiment
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Willför, S.; Hemming, J.; Reunanen, M.; Eckerman, C.; Holmbom, B. Lignans and Lipophilic Extractives in Norway Spruce Knots and Stemwood. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Holmbom, B.; Eckerman, C.; Eklund, P.C.; Hemming, J.; Nisula, L.; Reunanen, M.; Sjöholm, R.; Sundberg, A.; Sundberg, K.; Willför, S. Knots in trees—A new rich source of lignans. Phytochem. Rev. 2003, 2, 331–340. [Google Scholar] [CrossRef]
- Piispanen, R.; Willför, S.; Saranpaa, P.; Holmbom, B. Variation of lignans in Norway spruce (Picea abies [L.] Karst.) knotwood: Within-stem variation and the effect of fertilisation at two experimental sites in Finland. Trees 2007, 22, 317–328. [Google Scholar] [CrossRef]
- Eklund, P.C.; Willför, S.; Smeds, A.I.; Sundell, F.J.; Sjöholm, R.E.; Holmbom, B.R. A New Lariciresinol-Type Butyrolactone Lignan Derived from Hydroxymatairesinol and Its Identification in Spruce Wood. J. Nat. Prod. 2004, 67, 927–931. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Kangas, L.; Saarinen, N.; Mutanen, M.; Ahotupa, M.; Hirsinummi, R.; Unkila, M.; Perälä, M.; Soininen, P.; Laatikainen, R.; Korte, H.; et al. Antioxidant and antitumor effects of hydroxymatairesinol (HM-3000, HMR), a lignan isolated from the knots of spruce. Eur. J. Cancer Prev. 2002, 11, 48–57. [Google Scholar]
- Buchert, J.; Mustranta, A.; Tamminen, T.; Spetz, P.; Holmbom, B. Modification of Spruce Lignans with Trametes hirsuta Laccase. Holzforschung 2002, 56, 579–584. [Google Scholar] [CrossRef]
- Markus, H.; Plomp, A.J.; Mäki-Arvela, P.; Bitter, J.H.; Murzin, D.Y. The influence of acidity of carbon nanofibre-supported palladium catalysts in the hydrogenolysis of hydroxymatairesinol. Catal. Lett. 2007, 113, 141–146. [Google Scholar] [CrossRef]
- Bernas, H.; Plomp, A.J.; Bitter, J.H.; Murzin, D.Y. Influence of Reaction Parameters on the Hydrogenolysis of Hydroxymatairesinol Over Carbon Nanofibre Supported Palladium Catalysts. Catal. Lett. 2008, 125, 8–13. [Google Scholar] [CrossRef]
- Barone, G.; Manni, G.L.; Prestianni, A.; Duca, D.; Bernas, H.; Murzin, D.Y. Hydrogenolysis of hydroxymatairesinol on Y derived catalysts: A computational study. J. Mol. Catal. A Chem. 2010, 333, 136–144. [Google Scholar] [CrossRef]
- Piispanen, R.; Gierlinger, N.; Saranpää, P. Cell wall chemistry and knotwood structure of Norway spruce (Picea abies [L.] Karst.) studied by confocal Raman microscopy. In Proceedings of the Cell Wall Macromolecules and Reaction Wood COST E-50 Conference, Warsaw, Poland, 19–20 October 2006; p. 19. [Google Scholar]
- Huttula, M.; Patanen, M.; Piispanen, R.; Ohigashi, T.; Kosugi, N.; Swaraj, S.; Belkhou, R.; Pranovich, A.; Jyske, T.; Kilpeläinen, P.O.; et al. STXM Chemical Mapping of Norway Spruce Knotwood Lignans. Microsc. Microanal. 2018, 24, 482–483. [Google Scholar] [CrossRef][Green Version]
- Hitchcock, A.P.; Dynes, J.J.; Johansson, G.; Wang, J.; Botton, G. Comparison of NEXAFS microscopy and TEM-EELS for studies of soft matter. Micron 2008, 39, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Dünisch, O.; Bauch, J.; Muller, M.; Greis, O. Subcellular Quantitative Determination of K and Ca in Phloem, Cambium, and Xylem Cells of Spruce (Picea abies [L.] Karst.) During Earlywood and Latewood Formation. Holzforschung 1998, 52, 582–588. [Google Scholar] [CrossRef]
- Rapp, A.; Bestgen, H.; Adam, W.; Peek, R.-D. Electron Energy Loss Spectroscopy (EELS) for Quantification of Cell-Wall Penetration of a Melamine Resin. Holzforschung 1999, 53, 111–117. [Google Scholar] [CrossRef]
- Reza, M.; Kontturi, E.; Jääskeläinen, A.-S.; Vuorinen, T.; Ruokolainen, J. Transmission Electron Microscopy for Wood and Fiber Analysis—A Review. BioResources 2015, 10, 6230–6261. [Google Scholar] [CrossRef]
- Cody, G.D.; Brandes, J.; Jacobsen, C.; Wirick, S. Soft X-ray induced chemical modification of polysaccharides in vascular plant cell walls. J. Electron Spectrosc. Relat. Phenom. 2009, 170, 57–64. [Google Scholar] [CrossRef]
- Karunakaran, C.; Christensen, C.R.; Gaillard, C.; Lahlali, R.; Blair, L.M.; Perumal, V.; Miller, S.S.; Hitchcock, A.P. Introduction of Soft X-ray Spectromicroscopy as an Advanced Technique for Plant Biopolymers Research. PLoS ONE 2015, 10, e0122959. [Google Scholar] [CrossRef]
- Boyce, C.K.; Cody, G.D.; Feser, M.; Jacobsen, C.; Knoll, A.H.; Wirick, S. Organic chemical differentiation within fossil plant cell walls detected with X-ray spectromicroscopy. Geology 2002, 30, 1039–1042. [Google Scholar] [CrossRef]
- Boyce, C.K.; Zwieniecki, M.A.; Cody, G.D.; Jacobsen, C.; Wirick, S.; Knoll, A.H.; Holbrook, N.M. Evolution of xylem lignification and hydrogel transport regulation. Proc. Natl. Acad. Sci. USA 2004, 101, 17555–17558. [Google Scholar] [CrossRef]
- Jeremic, D.; Goacher, R.E.; Yan, R.; Karunakaran, C.; Master, E.R. Direct and up-close views of plant cell walls show a leading role for lignin-modifying enzymes on ensuing xylanases. Biotechnol. Biofuels 2014, 7, 496. [Google Scholar] [CrossRef]
- Feldman, D. Wood—chemistry, ultrastructure, reactions, by D. Fengel and G. Wegener, Walter de Gruyter, Berlin and New York, 1984, 613 pp. Price: 245 DM. J. Polym. Sci. Part C Polym. Lett. 1985, 23, 601–602. [Google Scholar] [CrossRef]
- Bertaud, F.; Holmbom, B. Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci. Technol. 2004, 38, 245–256. [Google Scholar] [CrossRef]
- Willför, S.; Sundberg, A.; Hemming, J.; Holmbom, B. Polysaccharides in some industrially important softwood species. Wood Sci. Technol. 2005, 39, 245–257. [Google Scholar] [CrossRef]
- Timell, T.E. Recent progress in the chemistry and topochemistry of compression wood. Wood Sci. Technol. 1982, 16, 83–122. [Google Scholar] [CrossRef]
- Pranovich, A.; Eckerman, C.; Holmbom, B. Determination of methanol released from wood and mechanical pulp by headspace solid-phase microextraction. J. Pulp Pap. Sci. 2002, 28, 199–203. [Google Scholar]
- Hosia, M.; Lindholm, C.-A.; Toivanen, P.; Nevalainen, K. Undersökningar rörande utnyttjandet av barrträdsgrenar som råmaterial för kemisk massa och hård fiberskiva 1. Investigations on Utilizing Softwood Branches as Pulp and Hardboard Raw Material. Papper och. Trä. 1971, 53, 49–66. (In Swedish) [Google Scholar]
- Anttonen, S.; Manninen, A.-M.; Saranpää, P.; Kainulainen, P.; Linder, S.; Vapaavuori, E. Effects of long-term nutrient optimisation on stem wood chemistry in Picea abies. Trees 2002, 16, 386–394. [Google Scholar] [CrossRef]
- Hägglund, E.; Larsson, S. Om grankvistens kemiska sammansättning och dess förhållande vid sulfitkokningsprocessen. Sven. Papp. 1937, 40, 356–360. (In Swedish) [Google Scholar]
- Ishii, I.; Hitchcook, A. The oscillator strengths for C1s and O1s excitation of some saturated and unsaturated organic alcohols, acids and esters. J. Electron Spectrosc. Relat. Phenom. 1988, 46, 55–84. [Google Scholar] [CrossRef]
- Kolczewski, C.; Püttner, R.; Martins, M.; Schlachter, A.S.; Snell, G.; Sant’Anna, M.M.; Hermann, K.; Kaindl, G. Spectroscopic analysis of small organic molecules: A comprehensive near-edge x-ray-absorption fine-structure study of C6-ring-containing molecules. J. Chem. Phys. 2006, 124, 034302. [Google Scholar] [CrossRef]
- Solomon, D.; Lehmann, J.; Kinyangi, J.; Liang, B.; Heymann, K.; Dathe, L.; Hanley, K.; Wirick, S.; Jacobsen, C. Carbon (1s) NEXAFS Spectroscopy of Biogeochemically Relevant Reference Organic Compounds. Soil Sci. Soc. Am. J. 2009, 73, 1817–1830. [Google Scholar] [CrossRef]
- aXis2000. Available online: http://unicorn.mcmaster.ca/aXis2000.html (accessed on 19 March 2020).
- Whiting, P.; Goring, D.A.I. Chemical characterization of tissue fractions from the middle lamella and secondary wall of black spruce tracheids. Wood Sci. Technol. 1982, 16, 261–267. [Google Scholar] [CrossRef]
- Donaldson, L. Lignification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Hänninen, T.; Tukiainen, P.; Svedström, K.; Serimaa, R.; Saranpaa, P.; Kontturi, E.; Hughes, M.; Vuorinen, T. Ultrastructural evaluation of compression wood-like properties of common juniper (Juniperus communis L.). Holzforschung 2012, 66, 389–395. [Google Scholar] [CrossRef][Green Version]
- Gierlinger, N. Revealing changes in molecular composition of plant cell walls on the micron-level by Raman mapping and vertex component analysis (VCA). Front. Plant Sci. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Urquhart, S.G.; Ade, H. Trends in the Carbonyl Core (C 1S, O 1S)→π*C=OTransition in the Near-Edge X-ray Absorption Fine Structure Spectra of Organic Molecules. J. Phys. Chem. B 2002, 106, 8531–8538. [Google Scholar] [CrossRef]
- Terashima, N.; Fukushima, K. Heterogeneity in formation of lignin? XI: An autoradiographic study of the heterogeneous formation and structure of pine lignin. Wood Sci. Technol. 1988, 22, 259–270. [Google Scholar] [CrossRef]
- Tokareva, E.N.; Pranovich, A.V.; Fardim, P.; Daniel, G.; Holmbom, B. Analysis of wood tissues by time-of-flight secondary ion mass spectrometry. Holzforschung 2007, 61, 647–655. [Google Scholar] [CrossRef]
- Sachs, I.B. Torus of the Bordered-pit Membrane in Conifers. Nature 1963, 198, 906–907. [Google Scholar] [CrossRef]
- Bland, D.E.; Foster, R.C.; Logan, A.F. The Mechanism of Permanganate and Osmium Tetroxide Fixation and the Distribution of Lignin in the Cell Wall ofPinus radiata. Holzforschung 1971, 25, 137–143. [Google Scholar] [CrossRef]
- Singh, A.P.; Daniel, G. The S2 Layer in the Tracheid Walls of Picea abies Wood: Inhomogeneity in Lignin Distribution and Cell Wall Microstructure. Holzforschung 2001, 55, 373–378. [Google Scholar] [CrossRef]
- Frey-Wyssling, A. The Plant Cell Wall. Part 3, No. 5. In Handbuch der Pflenzenanatomie, 3rd ed.; Gebrüder Borntraeger: Berlin, Germany, 1976; p. 294. ISBN 97834431400901976. [Google Scholar]
- Sundheq, A.; Sundherg, K.; Lillandt, C.; Holmhom, B.; Sundberg, A. Determination of hemicelluloses and pectins in wood and pulp fibres by acid methanolysis and gas chromatography. Nord. Pulp Pap. Res. J. 1996, 11, 216–219. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Klason Lignin: Modifications to Improve the Precision of the Standardized Determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- Pierson, J.; Fernández, J.J.; Bos, E.; Amini, S.; Gnaegi, H.; Vos, M.; Bel, B.; Adolfsen, F.; Carrascosa, J.L.; Peters, P.J. Improving the technique of vitreous cryo-sectioning for cryo-electron tomography: Electrostatic charging for section attachment and implementation of an anti-contamination glove box. J. Struct. Boil. 2010, 169, 219–225. [Google Scholar] [CrossRef]
- Eklund, P.C.; Raitanen, J.-E. 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules 2019, 24, 220. [Google Scholar] [CrossRef]
- Simola, L.K.; Lemmetyinen, J.; Santanen, A. Lignin release and photomixotrophism in suspension cultures of Picea abies. Physiol. Plant. 1992, 84, 374–379. [Google Scholar] [CrossRef]
- Kärkönen, A.; Koutaniemi, S.; Mustonen, M.; Syrjänen, K.; Brunow, G.; Kilpeläinen, I.; Teeri, T.H.; Simola, L.K. Lignification related enzymes in Picea abies suspension cultures. Physiol. Plant. 2002, 114, 343–353. [Google Scholar] [CrossRef]
- Laitinen, T.; Morreel, K.; Delhomme, N.; Gauthier, A.; Schiffthaler, B.; Nickolov, K.; Brader, G.; Lim, K.-J.; Teeri, T.H.; Street, N.R.; et al. A Key Role for Apoplastic H2O2 in Norway Spruce Phenolic Metabolism. Plant Physiol. 2017, 174, 1449–1475. [Google Scholar] [CrossRef]
- Warinowski, T.; Koutaniemi, S.; Kärkönen, A.; Sundberg, I.; Toikka, M.; Simola, L.K.; Kilpeläinen, I.; Teeri, T.H. Peroxidases Bound to the Growing Lignin Polymer Produce Natural Like Extracellular Lignin in a Cell Culture of Norway Spruce. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Giummarella, N.; Balakshin, M.; Koutaniemi, S.; Kärkönen, A.; Lawoko, M. Nativity of lignin carbohydrate bonds substantiated by biomimetic synthesis. J. Exp. Bot. 2019, 70, 5591–5601. [Google Scholar] [CrossRef]
- Björkman, A. Studies on Finely Divided Wood I. Svensk Papperstid. 1956, 59, 477–485. [Google Scholar]
- Song, T.; Pranovich, A.; Sumerskiy, I.; Holmbom, B. Extraction of galactoglucomannan from spruce wood with pressurised hot water. Holzforschung 2008, 62, 659–666. [Google Scholar] [CrossRef]
- Belkhou, R.; Stanescu, S.; Swaraj, S.; Besson, A.; LeDoux, M.; Hajlaoui, M.; Dalle, D. HERMES: A soft X-ray beamline dedicated to X-ray microscopy. J. Synchrotron Radiat. 2015, 22, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, T.; Arai, H.; Araki, T.; Kondo, N.; Shigemasa, E.; Ito, A.; Kosugi, N.; Katoh, M. Construction of the Scanning Transmission X-ray Microscope Beamline at UVSOR. J. Phys. Conf. Ser. 2013, 463, 012006. [Google Scholar] [CrossRef]
- MANTiS. Available online: http://spectromicroscopy.com/ (accessed on 19 March 2020).
- Lerotic, M.; Mak, R.; Wirick, S.; Meirer, F.; Jacobsen, C. MANTiS: A program for the analysis of X-ray spectromicroscopy data. J. Synchrotron Radiat. 2014, 21, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Kukk, E.; Snell, G.; Bozek, J.D.; Cheng, W.-T.; Berrah, N. Vibrational structure and partial rates of resonant Auger decay of the N1s?2? core excitations in nitric oxide. Phys. Rev. A 2001, 63, 062702. [Google Scholar] [CrossRef]
- Kukk, E.; Yabashi, M.; Hergenhahn, U.; Liu, X.-J.; Prümper, G.; Yoshida, H.; Tamenori, Y.; Makochekanwa, C.; Tanaka, T.; Kitajima, M.; et al. Violation of the Franck-Condon Principle due to Recoil Effects in High Energy Molecular Core-Level Photoionization. Phys. Rev. Lett. 2005, 95, 133001. [Google Scholar] [CrossRef]
Sample Availability



| Component | mg g−1 Knotwood |
|---|---|
| Cellulose | 369.6 |
| Non-cellulosic sugars | |
| Mannose | 83.7 |
| Glucose | 36.4 |
| Galactose | 37.1 |
| Xylose | 61.6 |
| Arabinose | 14.4 |
| Rhamnose | 1.8 |
| Glucuronic acid | 1.5 |
| Galacturonic acid | 11.7 |
| 4-O-Methylglucuronic acid | 6.7 |
| Total non-cellulosic carbohydrates | 255.0 |
| Total carbohydrates | 624.5 |
| Sample Knot ID | Extraction Solvent | Knotwood Extractives (mg g−1) |
|---|---|---|
| Eastern knot 1 | Acetone only | 208.8 |
| Eastern knot 1 | Hexane | 4.0 |
| Northern knot 2 | Acetone only | 181.9 |
| Northern knot 2 | Hexane | 4.5 |
| Eastern knot 1 | Acetone 3 | 194.43 |
| Northern knot 2 | Acetone 3 | 174.43 |
| Chemical Component | Eastern Knot 1 | Northern Knot 2 | Eastern Knot 1 | Northern Knot 2 |
|---|---|---|---|---|
| Acetone Extract (%) | Acetone Extract after Pre-extraction with Hexane (%) | |||
| Acid C15:0 | data | 0.00 | 0.00 | 0.00 |
| Acid C16:1 | 0.01 | 0.00 | 0.01 | 0.01 |
| Acid C16:0 | 0.00 | 0.08 | 0.00 | 0.01 |
| Acid C18:3 | 0.03 | 0.03 | 0.00 | 0.01 |
| Acid C18:2 | 0.04 | 0.08 | 0.01 | 0.03 |
| Acid C9-18:1 | 0.03 | 0.05 | 0.02 | 0.03 |
| Acid C11-18:1 | 0.01 | 0.03 | 0.00 | 0.00 |
| Dehydroabietic acid | 0.07 | 0.09 | 0.03 | 0.02 |
| Acid C20:3 | 0.04 | 0.00 | 0.00 | 0.00 |
| Acid C22:0 | 0.03 | 0.03 | 0.03 | 0.06 |
| Acid C23:0 | 0.03 | 0.00 | 0.00 | 0.03 |
| Acid C24:0 | 0.08 | 0.03 | 0.05 | 0.04 |
| Sum of fatty and resin acids | 0.37 | 0.43 | 0.17 | 0.24 |
| 7R-Todolactol | 1.23 | 1.24 | 1.04 | 1.39 |
| Secoisolariciresinol | 3.30 | 4.17 | 3.81 | 4.04 |
| 7S-Todolactol | 3.35 | 3.26 | 2.78 | 3.30 |
| 7R-Isoliovil | 0.69 | 0.66 | 0.63 | 0.55 |
| α-Conidendric acid | 2.13 | 1.79 | 2.23 | 1.69 |
| 7`-Hydroxymatairesinol | 3.95 | 4.03 | 3.84 | 3.67 |
| Hydroxymatairesinol HMR1 | 21.72 | 23.29 | 23.68 | 23.24 |
| Hydroxymatairesinol HMR2 | 37.26 | 36.42 | 37.23 | 36.57 |
| α-Conidendrin | 2.23 | 2.24 | 2.74 | 2.07 |
| 9´-Hydroxymatairesinol | 0.88 | 1.02 | 0.58 | 1.08 |
| 7`-Oxo-matairesinol | 0.36 | 0.28 | 0.40 | 0.35 |
| Lariciresinol | 0.94 | 0.97 | 0.78 | 0.96 |
| iso-Hydroxymatairesinol | 2.47 | 2.38 | 2.28 | 2.41 |
| 7`-Oxolariciresinol | 0.34 | 0.32 | 0.31 | 0.29 |
| epi-iso-Hydroxymatairesinol | 0.38 | 0.38 | 0.38 | 0.37 |
| 7R-Todolactol | 1.23 | 1.24 | 1.04 | 1.39 |
| Sum of lignans | 81.23 | 82.45 | 82.72 | 81.98 |
| Sesquilignans 3 | 9.74 | 9.40 | 9.07 | 10.14 |
| Dilignans 3 | 5.84 | 5.23 | 5.07 | 5.24 |
| Sum of sesqui/dilignans | 15.57 | 14.63 | 14.14 | 15.38 |
| Sum of non-identified | 2.83 | 2.50 | 2.97 | 2.39 |
| Total exractives | 100.00 | 100.00 | 100.00 | 100.00 |
| Atom–% | Mass–% | |||||
|---|---|---|---|---|---|---|
| C | O | Os | C | O | Os | |
| 1 | 92.55 | 7.42 | 0.03 | 89.94 ± 0.57 | 9.60 ± 0.22 | 0.45 ± 0.08 |
| 2 | 91.38 | 8.00 | 0.62 | 81.69 ± 0.79 | 9.53 ± 0.31 | 8.79 ± 0.46 |
| 3 | 95.39 | 4.59 | 0.02 | 93.73 ± 0.76 | 6.01 ± 0.22 | 0.26 ± 0.10 |
| Atom–% | Mass–% | |||||
|---|---|---|---|---|---|---|
| C | O | Os | C | O | Os | |
| 1 | 91.68 | 8.26 | 0.06 | 88.44 ± 0.75 | 10.62 ± 0.30 | 0.94 ± 0.16 |
| 2 | 92.73 | 7.25 | 0.02 | 90.30 ± 1.15 | 9.41 ± 0.43 | 0.29 ± 0.22 |
| 3 | 93.42 | 6.54 | 0.04 | 90.92 ± 0.61 | 8.48 ± 0.22 | 0.60 ± 0.10 |
| 4 | 94.61 | 5.33 | 0.06 | 92.11 ± 0.71 | 6.90 ± 0.22 | 0.98 ± 0.06 |
| 5 | 95.54 | 5.38 | 0.08 | 91.83 ± 0.50 | 6.97 ± 0.16 | 1.20 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansikkala, T.; Patanen, M.; Kärkönen, A.; Korpinen, R.; Pranovich, A.; Ohigashi, T.; Swaraj, S.; Seitsonen, J.; Ruokolainen, J.; Huttula, M.; et al. Lignans in Knotwood of Norway Spruce: Localisation with Soft X-ray Microscopy and Scanning Transmission Electron Microscopy with Energy Dispersive X-ray Spectroscopy. Molecules 2020, 25, 2997. https://doi.org/10.3390/molecules25132997
Mansikkala T, Patanen M, Kärkönen A, Korpinen R, Pranovich A, Ohigashi T, Swaraj S, Seitsonen J, Ruokolainen J, Huttula M, et al. Lignans in Knotwood of Norway Spruce: Localisation with Soft X-ray Microscopy and Scanning Transmission Electron Microscopy with Energy Dispersive X-ray Spectroscopy. Molecules. 2020; 25(13):2997. https://doi.org/10.3390/molecules25132997
Chicago/Turabian StyleMansikkala, Tuomas, Minna Patanen, Anna Kärkönen, Risto Korpinen, Andrey Pranovich, Takuji Ohigashi, Sufal Swaraj, Jani Seitsonen, Janne Ruokolainen, Marko Huttula, and et al. 2020. "Lignans in Knotwood of Norway Spruce: Localisation with Soft X-ray Microscopy and Scanning Transmission Electron Microscopy with Energy Dispersive X-ray Spectroscopy" Molecules 25, no. 13: 2997. https://doi.org/10.3390/molecules25132997
APA StyleMansikkala, T., Patanen, M., Kärkönen, A., Korpinen, R., Pranovich, A., Ohigashi, T., Swaraj, S., Seitsonen, J., Ruokolainen, J., Huttula, M., Saranpää, P., & Piispanen, R. (2020). Lignans in Knotwood of Norway Spruce: Localisation with Soft X-ray Microscopy and Scanning Transmission Electron Microscopy with Energy Dispersive X-ray Spectroscopy. Molecules, 25(13), 2997. https://doi.org/10.3390/molecules25132997








